Abstract
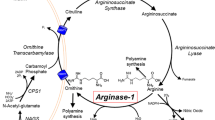
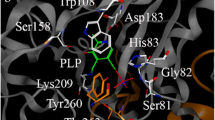
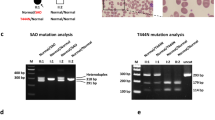
Loss of function of the urea cycle enzyme argininosuccinate lyase (ASL) is caused by mutations in the ASL gene leading to ASL deficiency (ASLD). ASLD has a broad clinical spectrum ranging from life-threatening severe neonatal to asymptomatic forms. Different levels of residual ASL activity probably contribute to the phenotypic variability but reliable expression systems allowing clinically useful conclusions are not yet available. In order to define the molecular characteristics underlying the phenotypic variability, we investigated all ASL mutations that were hitherto identified in patients with late onset or mild clinical and biochemical courses by ASL expression in human embryonic kidney 293 T cells. We found residual activities >3 % of ASL wild type (WT) in nine of 11 ASL mutations. Six ASL mutations (p.Arg95Cys, p.Ile100Thr, p.Val178Met, p.Glu189Gly, p.Val335Leu, and p.Arg379Cys) with residual activities ≥16 % of ASL WT showed no significant or less than twofold reduced Km values, but displayed thermal instability. Computational structural analysis supported the biochemical findings by revealing multiple effects including protein instability, disruption of ionic interactions and hydrogen bonds between residues in the monomeric form of the protein, and disruption of contacts between adjacent monomeric units in the ASL tetramer. These findings suggest that the clinical and biochemical course in variant forms of ASLD is associated with relevant residual levels of ASL activity as well as instability of mutant ASL proteins. Since about 30 % of known ASLD genotypes are affected by mutations studied here, ASLD should be considered as a candidate for chaperone treatment to improve mutant protein stability.




Similar content being viewed by others
References
Balmer C, Pandey AV, Rüfenacht V, Nuoffer JM, Fang P, Wong LJ, Häberle J (2014) Mutations and polymorphisms in the human argininosuccinate lyase (ASL) gene. Hum Mutat 35:27–35
Barbosa P, Cialkowski M, O’Brien WE (1991) Analysis of naturally occurring and site-directed mutations in the argininosuccinate lyase gene. J Biol Chem 266:5286–90
Bowie JU, Luthy R, Eisenberg D (1991) A method to identify protein sequences that fold into a known three-dimensional structure. Science 253:164–70
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–54
Brunetti-Pierri N, Erez A, Shchelochkov O, Craigen W, Lee B (2009) Systemic hypertension in two patients with ASL deficiency: a result of nitric oxide deficiency? Mol Genet Metab 98:195–7
Brusilow S, Horwich A (2001) Urea cycle enzymes. In: Scriver C, Beaudet A, Sly W, Valle D (eds) The metabolic & molecular bases of inherited disease, 8th edn. McGraw-Hill, New York, pp 1909–1963
Brusilow SW, Maestri NE (1996) Urea cycle disorders: diagnosis, pathophysiology, and therapy. Adv Pediatr 43:127–70
Doimo M, Trevisson E, Sartori G, Burlina A, Salviati L (2012) Yeast complementation is sufficiently sensitive to detect the residual activity of ASL alleles associated with mild forms of argininosuccinic aciduria. J Inherit Metab Dis 35:557–8
Dursun A, Sivri HS, Ozon A, Akcaören Z, Tokatly A, Koch HG, Coskun T (2008) Argininosuccinic aciduris associated with pancreatitis (abstract). J Inherit Metab Dis 31(Supp. 1):90
Engel K, Vuissoz JM, Eggimann S, Groux M, Berning C, Hu L, Klaus V, Moeslinger D, Mercimek-Mahmutoglu S, Stockler S, Wermuth B, Häberle J, Nuoffer JM (2012) Bacterial expression of mutant argininosuccinate lyase reveals imperfect correlation of in-vitro enzyme activity with clinical phenotype in argininosuccinic aciduria. J Inherit Metab Dis 35:133–40
Erez A, Nagamani SC, Lee B (2011a) Argininosuccinate lyase deficiency-argininosuccinic aciduria and beyond. Am J Med Genet C: Semin Med Genet 157:45–53
Erez A, Nagamani SC, Shchelochkov OA, Premkumar MH, Campeau PM, Chen Y, Garg HK, Li L, Mian A, Bertin TK, Black JO, Zeng H, Tang Y, Reddy AK, Summar M, O’Brien WE, Harrison DG, Mitch WE, Marini JC, Aschner JL, Bryan NS, Lee B (2011b) Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat Med 17:1619–26
Ficicioglu C, Mandell R, Shih VE (2009) Argininosuccinate lyase deficiency: longterm outcome of 13 patients detected by newborn screening. Mol Genet Metab 98:273–7
Flück CE, Mullis PE, Pandey AV (2009) Modeling of human P450 oxidoreductase structure by in silico mutagenesis and Md simulation. Mol Cell Endocrinol 313:17–22
Hooft RW, Vriend G, Sander C, Abola EE (1996) Errors in protein structures. Nature 381:272
Hooft RW, Sander C, Vriend G (1997) Objectively judging the quality of a protein structure from a Ramachandran plot. Comput Appl Biosci 13:425–30
Hu L, Pandey AV, Eggimann S, Rüfenacht V, Moslinger D, Nuoffer JM, Häberle J (2013) Understanding the role of argininosuccinate lyase transcript variants in the clinical and biochemical variability of the urea cycle disorder argininosuccinic aciduria. J Biol Chem 288:34599–611
Jacoby LB, Littlefield JW, Milunsky A, Shih VE, Wilroy RS Jr (1972) A microassay for argininosuccinase in cultured cells. Am J Hum Genet 24:321–4
Kleijer WJ, Garritsen VH, Linnebank M, Mooyer P, Huijmans JG, Mustonen A, Simola KO, Arslan-Kirchner M, Battini R, Briones P, Cardo E, Mandel H, Tschiedel E, Wanders RJ, Koch HG (2002) Clinical, enzymatic, and molecular genetic characterization of a biochemical variant type of argininosuccinic aciduria: prenatal and postnatal diagnosis in five unrelated families. J Inherit Metab Dis 25:399–410
Krieger E, Darden T, Nabuurs SB, Finkelstein A, Vriend G (2004) Making optimal use of empirical energy functions: force-field parameterization in crystal space. Proteins 57:678–83
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–5
Linnebank M, Tschiedel E, Häberle J, Linnebank A, Willenbring H, Kleijer WJ, Koch HG (2002) Argininosuccinate lyase (ASL) deficiency: mutation analysis in 27 patients and a completed structure of the human ASL gene. Hum Genet 111:350–9
Liu H, Elstner M, Kaxiras E, Frauenheim T, Hermans J, Yang W (2001) Quantum mechanics simulation of protein dynamics on long timescale. Proteins 44:484–9
Luthy R, Bowie JU, Eisenberg D (1992) Assessment of protein models with three-dimensional profiles. Nature 356:83–5
Mercimek-Mahmutoglu S, Moeslinger D, Häberle J, Engel K, Herle M, Strobl MW, Scheibenreiter S, Muehl A, Stockler-Ipsiroglu S (2010) Long-term outcome of patients with argininosuccinate lyase deficiency diagnosed by newborn screening in Austria. Mol Genet Metab 100:24–8
Mori T, Nagai K, Mori M, Nagao M, Imamura M, Iijima M, Kobayashi K (2002) Progressive liver fibrosis in late-onset argininosuccinate lyase deficiency. Pediatr Dev Pathol 5:597–601
O’Brien WE, Barr RH (1981) Argininosuccinate lyase: purification and characterization from human liver. Biochemistry 20:2056–60
O’Brien WE, McInnes R, Kalumuck K, Adcock M (1986) Cloning and sequence analysis of cDNA for human argininosuccinate lyase. Proc Natl Acad Sci U S A 83:7211–5
Palekar AG, Mantagos S (1981) Human liver arginiosuccinase purification and partial characterization. J Biol Chem 256:9192–4
Pandey AV, Mullis PE (2011) Molecular genetics and bioinformatics methods for diagnosis of endocrine disorders. In: Ranke MB, Mullis PE (eds) Diagnostics of endocrine function in children and adolescents, 4th edn. Karger, Basel, pp 1–21
Pandey AV, Kempna P, Hofer G, Mullis PE, Flück CE (2007) Modulation of human CYP19A1 activity by mutant NADPH P450 oxidoreductase. Mol Endocrinol 21:2579–95
Ramachandran GN, Ramakrishnan C, Sasisekharan V (1963) Stereochemistry of polypeptide chain configurations. J Mol Biol 7:95–9
Sabarinathan R, Tafer H, Seemann SE, Hofacker IL, Stadler PF, Gorodkin J (2013) RNAsnp: efficient detection of local RNA secondary structure changes induced by SNPs. Hum Mutat 34:546–56
Sampaleanu LM, Vallee F, Thompson GD, Howell PL (2001) Three-dimensional structure of the argininosuccinate lyase frequently complementing allele Q286R. Biochemistry 40:15570–80
Shulman-Peleg A, Nussinov R, Wolfson HJ (2004) Recognition of functional sites in protein structures. J Mol Biol 339:607–33
Shulman-Peleg A, Nussinov R, Wolfson HJ (2005) SiteEngines: recognition and comparison of binding sites and protein-protein interfaces. Nucleic Acids Res 33:W337–41
Smith RE, Lovell SC, Burke DF, Montalvao RW, Blundell TL (2007) Andante: reducing side-chain rotamer search space during comparative modeling using environment-specific substitution probabilities. Bioinformatics 23:1099–105
Solitare GB, Shih VE, Nelligan DJ, Dolan TF Jr (1969) Argininosuccinic aciduria: clinical, biochemical, anatomical and neuropathological observations. J Ment Defic Res 13:153–70
Tanaka T, Nagao M, Mori T, Tsutsumi H (2002) A novel stop codon mutation (X465Y) in the argininosuccinate lyase gene in a patient with argininosuccinic aciduria. Tohoku J Exp Med 198:119–24
Todd S, McGill JR, McCombs JL, Moore CM, Weider I, Naylor SL (1989) cDNA sequence, interspecies comparison, and gene mapping analysis of argininosuccinate lyase. Genomics 4:53–9
Tomlinson S, Westall RG (1960) Argininosuccinase activity in brain tissue. Nature 188:235–6
Tomlinson S, Westall RG (1964) Argininosuccinic aciduria. Argininosuccinase and arginase in human blood cells. Clin Sci 26:261–9
Topham CM, Srinivasan N, Blundell TL (1997) Prediction of the stability of protein mutants based on structural environment-dependent amino acid substitution and propensity tables. Protein Eng 10:7–21
Trevisson E, Salviati L, Baldoin MC, Toldo I, Casarin A, Sacconi S, Cesaro L, Basso G, Burlina AB (2007) Argininosuccinate lyase deficiency: mutational spectrum in Italian patients and identification of a novel ASL pseudogene. Hum Mutat 28:694–702
Trevisson E, Burlina A, Doimo M, Pertegato V, Casarin A, Cesaro L, Navas P, Basso G, Sartori G, Salviati L (2009) Functional complementation in yeast allows molecular characterization of missense argininosuccinate lyase mutations. J Biol Chem 284:28926–34
Vallee F, Turner MA, Lindley PL, Howell PL (1999) Crystal structure of an inactive duck delta II crystallin mutant with bound argininosuccinate. Biochemistry 38:2425–34
Vriend G (1990) WHAT IF: a molecular modeling and drug design program. J Mol Graph 8(52–6):29
Walker DC, McCloskey DA, Simard LR, McInnes RR (1990) Molecular analysis of human argininosuccinate lyase: mutant characterization and alternative splicing of the coding region. Proc Natl Acad Sci U S A 87:9625–9
Walker DC, Christodoulou J, Craig HJ, Simard LR, Ploder L, Howell PL, McInnes RR (1997) Intragenic complementation at the human argininosuccinate lyase locus. Identification of the major complementing alleles. J Biol Chem 272:6777–83
Worth CL, Bickerton GR, Schreyer A, Forman JR, Cheng TM, Lee S, Gong S, Burke DF, Blundell TL (2007) A structural bioinformatics approach to the analysis of nonsynonymous single nucleotide polymorphisms (nsSNPs) and their relation to disease. J Bioinforma Comput Biol 5:1297–318
Yu B, Thompson GD, Yip P, Howell PL, Davidson AR (2001) Mechanisms for intragenic complementation at the human argininosuccinate lyase locus. Biochemistry 40:15581–90
Zimmermann A, Bachmann C, Baumgartner R (1986) Severe liver fibrosis in argininosuccinic aciduria. Arch Pathol Lab Med 110:136–40
Acknowledgments
The authors are grateful for the technical assistance provided by the late M. Groux, Bern.
Funding
This work was supported by the Swiss National Science Foundation [grants No. 310030_127184/1 and 310030_153196/1 to JH and 310031_134926 to AVP] and a grant from Schweizerische Mobiliar Genossenschaft Jubiläumsstiftung to AVP.
Compliance with Ethics Guidelines
ᅟ
Conflict of interest
None.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Carlo Dionisi-Vici
Jean-Marc Nuoffer and Johannes Häberle contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 41235 kb)
Rights and permissions
About this article
Cite this article
Hu, L., Pandey, A.V., Balmer, C. et al. Unstable argininosuccinate lyase in variant forms of the urea cycle disorder argininosuccinic aciduria. J Inherit Metab Dis 38, 815–827 (2015). https://doi.org/10.1007/s10545-014-9807-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-014-9807-3