Abstract
The transition to multicellularity is perhaps the best-studied of the “major evolutionary transitions”. It has occurred independently multiple times within the eukaryotes alone, and multicellular organisms comprise virtually the entirety of Earth’s macrobiota. However, the theoretical framework used to study the major evolutionary transitions does not neatly accommodate the evolution of complex multicellularity as a process distinct from the evolution of multicellularity more generally. Here, I attempt to fill this explanatory gap. I will first give an overview of research on the major evolutionary transitions, focusing on multicellularity, and demonstrate that the theoretical framework so far utilised does not provide us with sufficient conceptual tools to explain crucial phenomena that call for explanation, such as the evolution of organs and organ systems. I will then discuss our current understanding of early metazoan evolution as paradigmatically exemplifying the evolution of complex organisation in a multicellular system, specifically regarding three core processes enabling it, namely modularisation, subfunctionalisation, and integration, allowing the provision of a general account of the evolution of complex from simple multicellularity that is potentially applicable to other such cases such as the evolution of land plants. This paves the way for a revised account of major evolutionary transitions which incorporates the evolution of complex organismal traits following the evolution of minimal autonomous reproducers while marking a shift of emphasis from reproducers to organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Evolutionary Transitions in Individuality or ETIs (Buss 1987; Michod 2000), and the closely-associated Major Transitions in Evolution or MTEs (Szathmáry and Smith 1995), are unique or rare events in the history of Life on Earth where novel units of biological organisation have evolved through the aggregation and integration of existing units (ETIs) or new mechanisms have evolved for the transfer of information across generations (MTEs). The ETI events include the evolution of the first prokaryotic cell, the first eukaryotic cell, multicellularity, and eusociality (Bourke 2011), whereas the MTEs include a larger set of events (e.g. also evolution of chromosomes and sex). ETIs, which are more hotly debated and the focus of this paper, have been characterised as occurring in three stages: formation of social groups through aggregation or non-dissociation after reproduction; maintenance through evolution of mechanisms ensuring internal cohesion; and transformation into complex, integrated individuals. Existing research has primarily focused on the first two stages of ETIs (Bourke 2011; Birch 2017), leaving an explanatory gap on the third stage: how exactly is it that biological entities evolve from relatively loose aggregates to full-fledged organisms?
This paper seeks to address this explanatory gap by drawing on early stages in the evolution of animals, especially key steps in the emergence of eumetazoans, to offer an abstract, structurally-oriented theory of organisational evolution. Early eumetazoan evolution is particularly interesting from this perspective precisely because it exemplifies the transformative stage of an ETI: non-eumetazoan animals, namely sponges and likely placozoans, lack many of the core features of eumetazoan complex organisation such as organs or organ systems, as well as the developmental mechanisms enabling them; furthermore, this episode in the evolution of animals is increasingly better-understood in light of modern palaeontological, developmental, and genomic evidence (Giribet and Edgecombe 2019).
The outline of the paper is as follows. I will begin with briefly expounding the notion of an ETI in the context of the Multilevel Selection framework and offer more detail on the three stages mentioned above along with existing examples from the literature, followed by emphasising the meaning and significance of the explanatory gap surrounding the third stage. I will then draw on a theory of organisational (alternatively “hierarchical”) complexity (McShea 2001, 2002) and its instantiation in early animal evolution in an attempt to fill the aforementioned explanatory gap, arguing that the transformative stage has, at least in the case of animal evolution, occurred via three interrelated processes: modularisation, subfunctionalisation, and integration. These evolutionary processes inject a dose of structuralist thinking into the otherwise heavily functionalist literature on evolutionary transitions (Griesemer 2006). I will then pinpoint the importance of another equally important aspect of the transformative stage, namely the evolution of novel developmental mechanisms underlying organisational complexity. I will then briefly discuss how all this can help us better understand the evolution of organismality and agency in animals and potentially elsewhere, all the while not appealing to “progressionist”Footnote 1 notions. I will conclude with a summary and prospects of this theory.
Evolutionary transitions in individuality
The modern notion of an evolutionary transition in individuality consisting of the emergence of a novel unit of organisation (such as a multicellular organism) from pre-existing lower level units (such as unicellular organisms) was first expressed in the 80s in The Evolution of Individuality (Buss 1987). This contribution, alongside the later The Major Transitions in Evolution (Maynard Smith and Szathmary 1995), bore the virtue of bringing together and attempting to explain such seemingly disparate issues as the evolution of cooperation and germ/soma segregation in light of a more overarching framework—multilevel selection (Okasha 2006).
The problem of cooperation/altruism in particular had been a matter of particularly fierce debate in the latter part of the 20th century, evident in the pervasiveness of such scientific/philosophical works as The Selfish Gene (Dawkins 2016) or Unto Others (Wilson and Sober 1998) within academia as well as the public sphere. At the core of the debate was the question of whether or not natural selection acting at the level of higher-level units (i.e. groups or superorganisms) was needed—if at all possible—to explain the evolution of adaptations that benefitted the higher-level unit, rather than the individual organisms or their genes. The salience of the question lies in the seemingly counter-intuitive observation that quite often individual organisms forego their own evolutionary interests in service to other individuals, often closely related to them or at least belonging to the same species: why should natural selection favour such altruistic behaviour? The two books cited above exemplify two diametrically opposed approaches to this problem, though both shift natural selection’s attention away from individual organisms: the former, gene-centric approach towards somewhat cryptic genes, and the latter towards loose associations or groups.
The advent of the multilevel selection framework effectively dissipated the opposition by recognising selection at multiple levels—including the genetic, organismal, and group levels—to be instrumental in bringing about adaptive change; this, in part, was facilitated by the recognition that selection at higher levels of organisation must have occurred at some point during transitions in individuality, such as the evolution of complex multicellular organisms, since it is known for a fact that both multicellular organisms and their unicellular progenitors undergo natural selection (Okasha 2006, 2022; Godfrey-Smith 2009; Birch 2017). However, the legacy of the preceding debate has since remained: the main body of the multilevel selection literature has largely focused on the evolution of basic social traits, such as physical coherence of lower-level units, internal policing, reproductive and non-reproductive division of labour at the lower level, life-cycle bottlenecks, or apoptosis (Clarke 2010; Bourke 2011). These can be collectively characterised as pertaining to the early stages of an evolutionary transition in individuality: in the case of the evolution of multicellularity, for example, these are associated with the evolution of simple multicellularity—a particularly frequent transition that has occurred multiple times in evolutionary history (Grosberg and Strathmann 2007; Rokas 2008; Calcott and Sterelny 2011). Very little has been said in the context of ETIs about further stages leading to the evolution of complex higher-level units (e.g. complex multicellular organisms).
This explanatory gap—as I will be calling it—has nevertheless been recognised by some authors, most notably by Bourke in Principles of Social Evolution (Bourke 2011; Birch 2017), where a distinction is drawn between three stages of ETIs: social group formation, maintenance, and transformation. The first stage can be achieved through the aggregation of existing units, as is observed, for instance, in the formation of the cellular slime mould Dictyostelium (Buss 1987; Bonner 2009) or the evolution of the eukaryotic cell (Maynard Smith and Szathmary 1995); or, alternatively, through lack of or incomplete dissociation of existing units during reproduction, as observed in the evolution of multicellular bacteria, fungi, plants, or animals—normally through changes in mechanisms of cell division and co-option of membrane proteins—as well as the evolution of eusocial hymenopteran colonies. The second stage is achieved through the evolution of mechanisms ensuring internal cohesion, protecting against “subversion from within” (Okasha 2006; Clarke 2010). Classic examples include the evolution of life-cycle bottlenecks, reproductive division of labour, and internal policing mechanisms—all serving to reduce genetic variation at the lower level of organisation (e.g. cells, individuals in a colony) and thereby maintain the stability of the higher-level unit.
While the third stage has—to my knowledge, at least—not been explored explicitly in such termsFootnote 2, an independent line of research provides inspiration for what it might roughly look like: this is what McShea calls “hierarchy theory” and is primarily concerned with the evolution of hierarchical organisation and complexity (McShea 1996, 2001, 2002, 2016a, b, 2017; McShea and Changizi 2003). In the following section, I will provide a brief description of some of the core ideas in this line of research, followed by applying a modified version of the theory to early (as well as later) eumetazoan evolution to show how it can fill the aforementioned explanatory gap on the third stage of social evolution.
Before moving on, it is worth noting that a potential reason why the main body of the evolutionary transitions literature has not focused on this third stage is that this literature has been historically “functionalist” in outlook, sensu Griesemer (Griesemer 2006)—in short, mainly concerned with abstract theories rooted in population genetics that attempt to explain evolutionary transitions with references to adaptive effects of novel genetic mutations, without strong emphasis on structural features of organisms that could fill the gap between genetic variation and complex adaptations such as body parts, or the developmental processes that underpin these features. This functionalist outlook arguably lends itself far more naturally to earlier stages of transitions, where the relevant players form a simple hierarchy of genes and lower-level and upper-level units. What follows from here takes a complementary outlook: an emphasis on structural features occupying several intermediate levels of organisation, e.g. tissues, organs, and organ systems, and their underlying developmental processes. In doing so, it enriches the literature on evolutionary transitions by filling the aforementioned explanatory gap, provides a general theory of the evolution of novel characters, and shows the value of structuralist and processual views in evolutionary biology.
Structural and processual complexity
What kind of complexity?
There are perhaps as many notions of complexity as there are problems that are not easy to solve, each notion serving to capture why the problem is as it is. Accordingly, any notion of complexity ought to be defined in order to serve a particular purpose. There are two broad notions of complexity relevant to the present discussion: structural and processual—corresponding closely to McShea’s object and process complexity (McShea 1996). Structural complexity can in turn be categorised into vertical and horizontal complexity (hierarchical and non-hierarchical in McShea’s terms). Vertical structural complexity is taken to refer to the number of discernible levels of organisation within a system, whereas horizontal structural complexity is taken to refer to the number of types of partsFootnote 3 at each level of organisation below that of the whole system. The present discussion is concerned primarily with vertical structural complexity and vertical processual complexity, though both kinds of horizontal complexity also feature in the arguments presented. Specifically, the aim of this section is to (1) demonstrate that vertical complexity has in fact increased during the early stages of eumetazoan evolution, as well as in some cases in later eumetazoan evolution; and also to (2) show that there is a general pattern to be found in the evolution of vertical complexity resulting from three interrelated processes mentioned above: modularisation, subfunctionalisation, and integration.
Early eumetazoan evolution: a case study
Eumetazoa (Greek neologism: true animals—(Bütschli, 1910)), comprising the vast majority of living animals, were named as such to highlight their more pronounced degree of organisation compared to the remaining metazoans (= animals)—the Parazoa (Greek neologism: near-animals—(Sollas 1884)), including the simpler Porifera or sponges. Both Metazoa and Eumetazoa are now widely accepted to form monophyletic taxa (= clades), meaning that their respective members are more closely related to each other than they are to members of any other group, and therefore share each other’s evolutionary history and homologous traits. Thus, the distinction drawn between eumetazoans and non-eumetazoans enjoys a firm phylogenetic basis: the core set of traits distinguishing eumetazoans and non-eumetazoans, their unique body plan foremost, was indeed present in their last common ancestor, and absent in the last common ancestor of all animals (including sponges). In other words, the complexity found in Eumetazoa is an evolutionary novelty unique to this clade.
The significance of this fact in the present context is that it allows us to make a case study out of the transition from sponge-grade complexity to eumetazoan-grade complexity: from simple to complex multicellularity. As we shall see, at the core of this transition lies the evolution of the eumetazoan body plan, which is distinct from the sponge body plan in that it is structurally highly integrated and fixed at an early stage in development and is underpinned by a specific and highly conserved developmental mechanism, which has subsequently been utilised to give rise to organs—another feature not found in non-eumetazoan animals. These evolutionary innovations are tightly linked with the aforementioned increase in vertical structural complexity as they make up the emergence of an integrated higher-level unit from a relatively loose association of lower-level units and the emergence of two of the most prominent intermediate units of organisation—namely organs and organ systems.
It should be noted, however, that there are other senses of complexity that have arguably decreased in the transition to eumetazoan complexity: since the emergence of a new level of organisation is tightly linked to integration (as will be discussed at length below), notions of complexity that take systems with lower degrees of integration and a stronger reliance on self-organisation (e.g. ant colonies) to be more complex will likely count the evolution of eumetazoans as an example of a decrease in complexity. This is not what is meant here, as I have tried to clarify so far.
Three processes and structural complexity
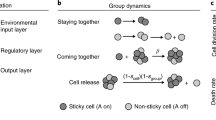
In this section, I argue that transformation of stable social groups into full-fledged organisms occurs principally via three processes. These are modularisation, integration, and subfunctionalisationFootnote 4 (Fig. 1), successively giving rise to higher vertical complexity—i.e. emergence of new, intermediate levels of organisation and consolidation of the higher level—accompanied by increases in horizontal complexity pertaining to intermediate levels: organs and organ systems in the case of multicellularity; and decreases in horizontal complexity at the lower level: loss of parts within cells in the case of multicellularity (Beklemishev 1969; McShea 2002)Footnote 5. Before proceeding, it must be pointed out that while the processes are to some degree interdependent and tend to come in particular orders (e.g. modularisation tends to precede subfunctionalisation), it will become quickly evident that, overall, they occur fairly independently of each other and often simultaneously.
Abstract representation of the three processes of the evolution of structural complexity. (a) Modularisation resulting in spatial regularity of the specialised cells (purple) in the form of rosettes (orange circle) in a colonial-grade organism. (b) Localised integration resulting in formation of pockets of specialised cells, with potential synergistic functionality. c & f. Globalised integration. d & e. Subfunctionalisation resulting in two novel cell types (red and blue), each spatially localised to perform their divergent functions. Note that other evolutionary pathways are possible but have been omitted for the sake of simplicity
Some terminological clarification is also necessary. “Specialisation” and “differentiation” are closely related terms and are used somewhat interchangeably in the literature, but here the latter is taken to refer specifically to cells becoming more specialised, and the former is taken to be the more general term, such that it can include subfunctionalisation at intermediate levels of organisation between cells and the organism. In other words, specialisation is a general process including differentiation at the cell level, and subfunctionalisation at intermediate levels.
I will now draw on early animal evolution to provide examples of these processes; note, though, that the starting point here is what I call the “colonial” stage found at the end of the maintenance stage described above: a stable social group with mechanisms of internal cohesion as well as some degree of reproductive and non-reproductive division of labour—as observed in sponges in the case of animal evolutionFootnote 6.
Modularisation
The first key step in the evolution of organism-level complexity is modularisation: the structural subdivision of the social group/colonial-grade organism into similar parts or modules, amounting to the evolution of a level of organisation. In the case of early animal evolution, this can be observed in the modular organisation of syconoid and especially leuconoid sponges, as well as that of the Ediacaran Dickinsonia and its close relatives (e.g. Yorgia, Andiva)Footnote 7—though it is at present difficult to tell if these are homologous or the result of convergent evolution, given uncertainty around the nature of their last common ancestor (Nejad Kourki 2021). Modularisation has also occurred later on in animal evolution, most notably in the evolution of segmentation in bilaterians, either independently multiple times or a single time at their base followed by multiple losses (Balavoine and Adoutte 2003; Couso 2009; Chipman 2010; Arendt 2018). Segmentation is found most prominently in the arthropods (e.g. crustaceans, insects, myriapods, chelicerates, and the extinct trilobites), annelids (polychaetes such as Platynereis, oligochaetes such as the Common Earthworm, and leeches), and chordates (e.g. vertebrates, Branchiostoma)—where individual segments are the modules of the body plan.
The transition to modular organisation could be due to various selective pressures, for example more efficient water transport in sponges, or simply a result of developmental constraints such as growth by a periodic mechanism as observed in the aforementioned extinct Ediacarans (Dunn, Liu et al. 2018, Ivantsov, Zakrevskaya et al. 2020). Either way, it results in the formation of semi-independent units of organisation intermediate between the higher and lower levels of organisation, which can then undergo structural and functional specialisation—i.e. division of labour—at an intermediate level of organisation. In other words, it paves the way for subfunctionalisation.
Before moving on to discussing subfunctionalisation, it is important to point out that modular organisation in the sense defined here could in principle be confused with colonial organisation in the case of some multicellular animals, notably corals and ectoprocts (= bryozoans). In both cases, the lower-level units are individual “polyps” equivalent to other individual organisms across eumetazoans, and the higher-level units are colonies of polyps; thus, these are examples of another ETI beyond that of the transition to multicellularity, rather than further steps in the same transition. Indeed, both ectoproct and siphonophore (close relatives of corals including the infamous Portuguese Man’o’War [Physalia]) colonies have been termed “superorganisms”, after usage of the same term in the case of eusocial insect colonies (Bourke 2011; Nielsen 2012)Footnote 8. The source of potential confusion is the occasional use in the general literature of the term “module” to refer to lower-level units in these cases.
Subfunctionalisation
Subfunctionalisation consists of the acquisition of novel structural and functional features in parts of an established or emerging systems. Thus, it can be characterised in the present context as an increase in horizontal complexity pertaining to intermediate-level units. It is clearly akin to differentiation; however, differentiation is taken here to pertain to lower-level units—i.e. cells in the transition to multicellularity and increasing numbers of their types (Arendt 2008, Arendt, Musser et al. 2016). It is worth emphasising that this distinction between differentiation and subfunctionalisation is drawn in order to explain the intuitive difference between specialisation of parts in a colonial-grade (e.g. sponge, ant colony) vs. an organism-grade system. Furthermore, while modularisation can pave the way for subfunctionalisation, there is no implication that subfunctionalisation requires modularisation to precede it. I will now turn once again to examples from animal evolution to substantiate these claimsFootnote 9.
Segments and appendages
As briefly mentioned above, the evolution of modules of different kinds, including segments and the more broadly-defined metameres (Couso 2009) is often followed by the subfunctionalisation of individual modules, amounting to division of labour at the intermediate level of organisation. The evolution of arthropods and vertebrates exemplify this process: within the arthropods, most body segments in both the extinct trilobites and the living myriapods (= centipedes and millipedes) are more or less undifferentiated, with the notable exception of segments bearing eyes, antennae, mouthparts, or the heart. Crustaceans, on the other hand, have several different kinds of segment, each with their own specialised appendages (as well as some with specialised internal organs); these include (in malacostracan crustaceans) thoracic segments bearing antennae, eyes, mouthparts, maxillipeds, chelipeds, and walking legs, and abdominal segments bearing swimming legs and the tail (uropods and telson). Insects and arachnids follow a broadly similar pattern, and so do vertebrates: the simplest chordates (the phylum containing vertebrates) with the most conserved body plan (e.g. Branchiostoma) parallel the simpler arthropods in that most (though, again, not all) of their segments are also more or less undifferentiated—notable exceptions being segments bearing gill slits. Later-diverging clades, such as cartilaginous or bony fishes, have more differentiated segments with more diverse functions and bearing more diverse parts: fins, opercula of gills, gill arches, and jaws are well-known examples (Nielsen 2012). Furthermore, tetrapod segments have ribs and vertebrae of varying morphology—the ribless abdominal segments of mammals and the prominent sternal keels of birds are notable examples; and the same holds for their serially homologous yet distinct appendages—the forelimbs and the hindlimbs—capable of taking up a diverse array of functions including walking, running, swimming, flying, and object manipulation.
Digestive organs and hearts
The evolution of complex digestive systems with multiple organs in various taxa exemplifies subfunctionalisation not necessarily as tightly linked to modularisation as the specialisation of segments and appendages. The degree of subfunctionalisation in the digestive systems of eumetazoan taxa is highly variable (Annunziata, Andrikou et al. 2019), ranging from a simple cavity with little to no spatial separation of digestive and absorptive functions (e.g. cnidarians, xenacoelomorphs), to the highly subfunctionalised digestive tracts of vertebrates and insects consisting of various organs (e.g. stomach, intestine, crop, etc.), efficient enough to break down food into macromolecules and eliminate the need for phagocytosis (Steinmetz 2019), as well as similarly subfunctionalised (yet highly diverse) ones such as those of many molluscs (Lobo-da-Cunha 2019). While a comprehensive description and analysis of the evolution of digestive system subfunctionalisation is far beyond the scope of this paper, it is at least clear that the maximum level of subfunctionalisation has increased over the course of animal evolution, likely in order to cope with the advent of predation and phytophagy from the early Cambrian onwards (Conway Morris 2003, Sperling, Frieder et al. 2013, Budd and Jensen 2017).
Another similar example involves the evolution of hearts via the functional specialisation of part(s) of the circulatory system for pumping blood or haemolymph (Sohal, Nghiem et al. 2001, Xavier-Neto, Davidson et al. 2010, Jensen, Wang et al. 2013, Göpel and Wirkner 2018). As with the evolution of organs as part of the digestive system, the emergence of hearts does not strictly require previous modularisation, though in some cases (e.g. annelids, vertebrates) previous modularisation does play a role.
Integration
Integration is here quite broadly construed as an evolutionary process whereby previously novel intermediate-level parts are formed out of the association of lower-level or lower-intermediate-level parts and is a core component in increasing vertical structural complexity. It is closely related to the concept of individuation, sometimes contrasted with modularisation as the latter involves the formation of several similar units out of a single whole that may be well-integrated or relatively loosely-integrated (Winther 2001; Schlosser and Wagner 2004; Wagner 2014, DiFrisco, Love et al. 2020). However, integration is distinct from individuation in that it is more broadly encompassing and includes such phenomena as the evolution of organ systems and the entire body in addition to the evolution of organs or body regions happening through individuation. Integration can be relatively localised, resulting in individuated structures such as organs or body regions; or it could be more globalised (i.e. affect the entire body) and result in the formation of an integrated body plan or entire organ systems. Thus, though not all of the following examples neatly fall into the local/global categorisation, some do exemplify one end of the spectrum rather than the other. Some are also tightly linked to modularisation and subfunctionalisation: modularisation often precedes integration, and subfunctionalisation and integration co-occur in some cases (e.g. evolution of the heart). Finally, it is important to note that integration is normally built “on top of” differentiation—that it comprises, in other words, of specialisation at a level higher than that of individual cells—and thereby the following examples mostly consist of differentiated cells of various types being integrated together in organs and organ systems.
Body plans and body regions
The defining feature of eumetazoans is their possession of an integrated body plan that comes in an astounding variety of shapes and sizes, ranging from the sac-like, radial or quasi-radial body plan of ctenophores and cnidarians to the worm-like, bilateral body plan of most bilaterians, highly derived bilateral body plans of arthropods, molluscs, and vertebrates, and even the divergent body plan of adult echinoderms and tunicates (Nielsen 2012). Though the exact evolutionary relationship between the cnidarian, ctenophore, and bilaterian body plans remains a matter of debate (Genikhovich and Technau 2017, Nielsen, Brunet et al. 2018, Lebedeva, Aman et al. 2021), it is generally accepted that they are on the whole homologous—i.e. the integrated eumetazoan body plan underpinned by Wnt and BMP signalling and spatially specified by homeobox gene expression was present, in some form, in the last common eumetazoan ancestor, and absent in the sponges (though homology with the placozoan body plan seems plausible (DuBuc, Ryan et al. 2019)). The eumetazoan body plan is also distinct from sponge morphology in that it contains (with some exceptions such as tapeworms) a digestive system—either a cavity as in cnidarians or a tract as in most bilaterians—which represents specialisation and later integration of cells performing digestive and absorptive functions into an organ system and associated organs (the latter through subfunctionalisation; see above).
Overall, the evolution of the eumetazoan body plan through distinct developmental genetic mechanisms marks a shift away from the colonial/modularFootnote 10 sponge morphology where cells are sufficiently independent to reaggregate after complete dissociation, giving sponges spectacular regenerative abilities (Eerkes-Medrano, Feehan et al. 2015), and towards greater interdependence between cells, decreased regenerative abilities in larger organism and smaller dependence of body morphology on environmental factors. This has in turn laid the groundwork for the evolution of integrated tissues, organs, and organ systems.
A similar evolutionary process is the evolution of body regions, often on a pre-existing modular body plan. Some examples include tagmatisation in arthropods (Zrzavý and Štys 1997; Balavoine and Adoutte 2003; Couso 2009), the tripartite coelom of chordates and hemichordates (Giribet and Edgecombe 2020), and the emergence of the head in disparate bilaterians (Vinther, Parry et al. 2017, Nanglu and Caron 2018, Aldea, Subirana et al. 2019, Chipman and Edgecombe 2019). The evolution of body regions, as paradigmatically exemplified by tagmatisation in insects giving rise to the head, thorax, and abdomen marks the integration of modules into units with overlapping functions and facilitates the subfunctionalisation of the modules; e.g. specialisation of the head for sensation and ingestion, the thorax for locomotion, and the abdomen for food absorption, reproduction, waste excretion, etc. It also marks the evolution of intermediate-level units of organisation.
Circulatory systems
In smaller animals, or more generally animals with a very high surface area to volume ratio (e.g. sponges, tapeworms), individual cells are capable of exchanging nutrients and gases with their environment and sending signals (e.g. hormones) to one another via simple diffusion or cell-level processes such as facilitated diffusion or active transport. Nevertheless, increasing size results in decreasing surface area to volume ratio, rendering such processes insufficient for the transfer of nutrients and gases as well as intercellular signals between cells and the environment and between themselves difficult to achieve, in turn necessitating the evolution of systems of internal transport or circulatory systems (Ruppert and Carle 1983). Circulatory systems play a crucial enabling role in the integration of animal bodies by allowing the transfer of nutrients, gases, and signals over long distances through a single, interconnected system. As mentioned before, their emergence is often followed by specialised contractile parts later evolving into hearts, thereby also paving the way for subfunctionalisation.
Metanephridia and kidneys
Besides exchanging nutrients and gases, animals also need to excrete their metabolic waste into the environment. Once again, animals with the highest surface area to volume ratio achieve this via diffusion alone. Most animals use specialised cells to achieve this, either in very small proto-organs called protonephridia (e.g. as found in turbellarian flatworms), or in larger, more integrated collections of cells formed into organs called metanephridia or paradigmatically in kidneys (Bartolomaeus and Ax 1992). The evolution of metanephridia and kidneys from oligocytic protonephridia exemplifies organ-level integration and the emergence of specialised intermediate-level parts in animals.
Ganglia, sensory organs, brains, and the CNS
The earliest-diverging animals, namely sponges and placozoans (though the latter may be secondarily-simplified eumetazoans; see (Laumer, Gruber-Vodicka et al. 2018)), lack neurons or sensory organs (Nickel 2010), with intercellular signalling and signal reception from the environment occurring through most cells; though placozoans likely have some specialised sensory cells (Varoqueaux, Williams et al. 2018). Virtually all eumetazoans have neurons and sensory cells, and many possess sensory organs of some complexity (examples abound, but a few well-known ones are statocysts, rhopalia, numerous types of eyes, mechanosensory organs such as ears, tympana, and lateral line organs, chemosensory tentacles, antennae, and noses) and varying levels of nervous system centralisation such as nerve nets, nerve rings, single or multiple nerve cords, modularised nerve cords with partially-integrated ganglia, or highly-integrated collections of ganglia—i.e. brains—are found across eumetazoans (Arendt, Tosches et al. 2016, Martín-Durán, Pang et al. 2018). The evolution of sensory organs and nervous systems (as well as the closely-associated endocrine systems) via integration of neurons and sensory cells has likely played a major role in the evolution of large, complex animals by allowing communication between cells far away from each other and thereby affecting one another physiologically and developmentally through intracellular signalling pathways, in contrast to the predominant autocrine and paracrine signalling observed in sponges and placozoans.
Muscles and limbs
Muscle cells are another character present in eumetazoans but absent in non-eumetazoans. Though cellular contraction does occur in sponges (Nickel 2004; Elliott and Leys 2007, Nickel, Scheer et al. 2011, Bond 2013) and placozoans (Armon, Bull et al. 2018), it is achieved without using specialised cells for doing so; in other words, there is no cell-level differentiation of contractile function. Among eumetazoans, ctenophores and cnidarians possess smooth and occasionally striated, more specialised, muscle cells as part of the epithelium (epitheliomuscular cells) but lack integrated muscle tissues (Steinmetz, Kraus et al. 2012). On the other hand, many bilaterians possess sheets of mesodermally-derived smooth and striated muscle tissues arranged in sheets in diverse orientations, underlying either locomotion or movement of food within the digestive cavity or tract (Mackie and Singla 1987; Hochberg and Litvaitis 2000, Gschwentner, Mueller et al. 2003, Hochberg, O’Brien et al. 2010, Meyer-Wachsmuth, Raikova et al. 2013), representing a level of integration of muscles above the cellular level. Furthermore, some bilaterians, notably numerous arthropods, molluscs, and vertebrates, have evolved specialised organs—i.e. limbs—through the integration of muscle and skeletal tissues that aid in the locomotion of the whole body as well as the manipulation of objects. Organised, integrated muscle tissues and especially limbs exemplify the evolution of intermediate-level structures bearing organism-level adaptative functionsFootnote 11.
Germ line
Within the ETI framework outlined above, a distinction is often made between reproductive and non-reproductive division of labour (Godfrey-Smith 2009; Bourke 2011). This is primarily because reproductive division of labour—instantiated in the evolution of multicellularity as the emergence of germ lines (i.e. specialised gamete-producing cell lineages) and in the evolution of eusociality as the emergence of worker and queen castes—enables the transfer of fitness from the lower to the higher level (Buss 1987; Michod and Nedelcu 2003; Michod 2005)Footnote 12, marking the emergence of adaptiveness at the higher level. There are varying levels of germ-line specification and sequestration in the animal kingdom: sponge germ cells are not sequestered and are instead differentiated primarily from the pluripotent archaeocytes (Funayama 2008), whereas the spatially disparate cnidarian germ cells are derived from more differentiated stem cells (Nieuwkoop and Sutasurya 1981). Germ lines are ancestrally present in bilaterians, though there have been multiple losses (Extavour 2007); furthermore, they are often organised in specialised organ-level structures, namely testes and ovaries (Leonard and Córdoba-Aguilar 2010). The evolution of testes and ovaries in animals marks further integration of germ cells from their non-sequestered state in cnidarians into integrated germ lines supported by auxiliary tissues in individuated organs at intermediate levels of organisation.
Limits and merits of the three processes theory
Though there are many traits whose evolution fits fairly neatly into the three-process paradigm described here, there are other traits of organisms that do not. For example, less-differentiated cells (e.g. fibroblasts, stem cells, etc.) are conspicuously present and widely scattered in the bodies of animals, and their evolution cannot be straightforwardly explained in terms of any of the present three processes. Another example concerns skeletons: while the evolution of internal or external skeletons does bear the mark of integration, it is more the integration of the extracellular matrix and only partly that of the cells producing it. In a similar vein, lungs evolve as an enlargement of a part of the embryonic digestive tract (Swarr and Morrisey 2015), and their evolution is therefore ascribable to subfunctionalisation only by a stretch. In other cases, entire sets of related modules undergo through major changes in functions and are therefore not straightforwardly cases of subfunctionalisation, such as in the case of the evolution of fish fins to tetrapod limbs. The takeaway message is that the three-process paradigm proposed here is an explanatory model with inevitable idealisations and therefore exceptions. Though it is not an attempt at providing a full account of the evolution of metazoan traits, it does serve two related purposes, as mentioned earlier. Firstly, it provides a general theory of character evolution that, on the one hand, unifies the evolution of a large set of otherwise apparently contingent characters, and on the other hand avoids simple recourse to genetic mutations as the prime cause of the emergence of characters. Secondly, it incorporates well-known cases of the evolution of metazoan characters into the ETI framework by showing how the evolution of these complex, adaptive, organism-level traits characterises the emergence of vertical structural complexity in animal evolution.
From individuals to organisms: a shift of emphasis
One potential issue arising from the discussion so far is that the three processes presented here often give rise to traits that do not squarely fit within the context of ETIs. Take, for example, the evolution of wings from forelimbs previously used for walking via a gradual process primarily involving subfunctionalisation. Unlike, say, the evolution of policing mechanisms, this particular evolutionary process seems explanatorily unrelated to the evolution of individuality at a higher level, as it is not involved in the transfer of fitness from the lower to the higher level. Somewhat similarly, the evolution of circulatory systems in relatively large organisms can simply be explained by reference to evolutionary pressures relating to size itself, rather than being directly related to the evolution of higher-level individuals, despite playing a role in increasing interdependence between parts of the higher-level unit.
One response to this problem is to admit that while the processes picked out here underpin the evolution of some traits that are clearly implicated in ETIs to varying extents (e.g. germ lines and centralised nervous systems), they also inevitably underpin the evolution of other traits that do not—at least in no straightforward way. While this response evades the full force of the criticism, it also loses much of the steam of the view expounded in this paper. In other words, this response effectively relegates our three processes to a partial theory of character evolution, and renders them only tangentially relevant to ETIs.
Another superficially similar yet substantially stronger response is as follows: while one must admit that many traits underpinned by our three processes do not fit neatly within the ETI framework, this ought to be deemed a limitation of this framework. As discussed above, this framework has left us with an explanatory gap with respect to dealing with the transformation of social groups/evolution of complex organisation. I have tried to argue that one viable way of filling this explanatory gap is to identify evolutionary processes that give rise to organismal complexity. By doing so, I have effectively extended the framework beyond its original formulation and into incorporating explanations of the evolution of organismal traits more generally. In other words, recognising the importance of the evolution of complex organisation marks a shift of emphasis from evolutionary individuals to organismsFootnote 13 in the context of ETIs, which makes for a more comprehensive framework than the current one. In short, the response is that we ought not to restrict ourselves to the evolution of what is strictly implicated in ETIs in a strict sense, and instead incorporate them into a broader framework seeking to explain the evolution of organisms.
Nevertheless, it still seems that the evolution of some traits underpinned by the three processes—paradigmatically wings—remains somewhat irrelevant: while the evolution of circulatory system results in a more integrated organism with higher interdependence between parts, the evolution of wings does not obviously achieve anything of the sort. However, with increasing subfunctionalisation the organism ends up relying more heavily on the subfunctionalised parts: while a crab missing a pair or two of limbs might still be able to walk fairly well, an insect missing its wings will not be able to fly. In other words, even the evolution of wings might contribute to interdependence of parts via making them indispensable. Nevertheless, it at least intuitively seems that traits like wings are somewhat more distantly related to the evolution of complex organisation, and very indirectly related to ETIs. Neither of these are problematic: not all traits have to be strongly implicated in the framework being developed here. It is sufficient that (1) our three processes unify (by abstraction) the evolution of a broad class of organismal traits, (2) most of these traits are implicated to some extent in the evolution of complex organisation, and (3) ideally, some of them are more directly related to ETIs more strictly construed.
Another valid and complementary way to approach this issue is to emphasise the relative importance of integration (especially global integration) among the three processes in the consolidation of ETIs, because of its role in increasing interdependence of parts. Increasing interdependence (or loss of independence) is a feature of ETIs that is present both in the evolution of mechanisms of group maintenance as discussed above, as well as in the evolution of structural organism-level traits brought about by the process of integration; thus, increasing interdependence serves as a conceptual bridge between the standard talk on evolutionary transitions mostly focusing on formation and maintenance of groups, and the later stage of group transformation emphasised in this paper. Highlighting this conceptual bridge serves to show that despite the inherent differences in research objectives between standard ETI research and the present work, they are nevertheless bound together through their shared emphasis on interdependence as a key feature of ETIs (Fig. 2).
Diagram representing the relationships between stages of ETIs, increases in interdependence of parts at the lower level, emergence and consolidation of the higher level, and evolution of intermediate-level parts. Though the emergence and initial consolidation of the higher level has been the main concern of the ETI literature, and the evolution of intermediate levels the main concern of this paper, they are nevertheless related through their combined role in increasing interdependence of parts over the course of a transition.
Processual complexity
The evolution of metazoan structural complexity reflects a similarly hierarchical or quasi-hierarchical trend in increasing processual complexity; i.e. the evolution of more complex developmental processes underlying the structural traits. This includes increasing numbers of genes and gene familiesFootnote 14 (Rokas 2008; Paps and Holland 2018, Paps and Holland 2018)—though decreases have been comparably pervasive and crucial (Guijarro-Clarke, Holland et al. 2020)—gene-regulatory networks or GRNs (Davidson and Erwin 2006; Davidson 2010), and key morphogenetic developmental mechanisms such as epithelial-mesenchymal transition or EMT (Pérez-Pomares and Muñoz‐Chápuli 2002). In the present framework, the number of genes and intracellular pathways and networks (standing for genetic interactions and closely associated with number of cells) represents horizontal processual complexity at the cell level, whereas the hierarchically or quasi-hierarchically organised intercellular processes, either GRNs or physical morphogenetic interactions (DiFrisco 2021), represent intermediate levels of processual organisation in animals and thereby both horizontal complexity at the intermediate levels and vertical complexity of the organism. Crucially, increasing processual and structural complexity are causally linked: novel developmental processes are required for the evolution of novel structural traits at different levels of organisation including tissues, organs, and organ systems of various kinds as presented above. This is concordant with the recently proposed concept of character identity mechanisms (ChIMs), which are meant to explain the evolutionary stability of the identity of such characters despite variation in their respective states (Wagner 2014, DiFrisco, Love et al. 2020). Selective pressures for the evolution of structures with novel functional capacities likely plays a role in stabilising the processes underpinning the structures. Furthermore, the three-process paradigm presented here can also be applied to the evolution of processual complexity. While a detailed discussion is far beyond the scope of this paper, one particular mechanism shows the utility of this approach and the quasi-independence of the evolution of processual and structural complexity: 3D spatial specification using various signalling gradients, notably Wnt and BMP.
Spatial specification: a ubiquitous process
The Wnt and BMP (part of the TGF-β superfamily) signalling pathways are among the most ubiquitously utilised mechanisms in animal development (Nusse, Brown et al. 1991, Huelsken and Behrens 2002, Kestler and Kühl 2008, Weiss and Attisano 2013), often interacting with each other as well as a host of other well-studied pathways—e.g. Notch/Delta (Kopan 2012), FGF (Goetz and Mohammadi 2013), and SHH (Choudhry, Rikani et al. 2014). They are involved in the spatial specification of multiple tissues, organs, and organ systems across metazoans including lungs (Bellusci, Henderson et al. 1996, Pepicelli, Lewis et al. 1998, Horowitz and Simons 2008), tear glands (Dean, Miller et al. 2005), teeth (O’Connell, Ho et al. 2012), the inner ear (Riccomagno, Takada et al. 2005), the thymus gland (Swann, Happe et al. 2017), kidneys (Kuure, Vuolteenaho et al. 2000), the liver (Suksaweang, Lin et al. 2004), hair (Plikus, Mayer et al. 2008), skeleton (Kanzler, Foreman et al. 2000, Wan and Cao 2005, Li and Cao 2006), digits (Raspopovic, Marcon et al. 2014), limbs (Kawakami, Ishikawa et al. 1996, Shubin, Tabin et al. 1997, Zou, Choe et al. 1997, Nulsen and Nagy 1999, Church and Francis-West 2004, Geetha-Loganathan, Nimmagadda et al. 2008, Tarazona, Lopez et al. 2019), and the extracellular matrix (Schultz, Bennett et al. 2014); virtually in all cases, the aforementioned pathways and networks are involved in spatial specification in close relation to tissue growth.
Interestingly, Wnt and BMP pathways are also together utilised in the specification of eumetazoan body plans (Niehrs 2010, Genikhovich, Fried et al. 2015, Genikhovich and Technau 2017, Wijesena, Simmons et al. 2017, DuBuc, Stephenson et al. 2018, Nielsen, Brunet et al. 2018, Lebedeva, Aman et al. 2021), and independently of each other (BMP in placozoans and Wnt in sponges) in specifying the placozoan body plan (DuBuc, Ryan et al. 2019), sponge embryogenesis (Adamska, Degnan et al. 2007, Reid, Matveev et al. 2018) and formation of the aquiferous system and oscula (Lapébie, Gazave et al. 2009, Windsor and Leys 2010, Kozin, Borisenko et al. 2019). Thus, given the role of BMP and Wnt pathways in specifying the ancestral eumetazoan body plan and the absence of any organs in the last common eumetazoan ancestor, it is highly plausible that body plan specification was the primary function of the integrated Wnt/BMP network, and that it was later co-opted and subsequently modified many times in the evolution of the traits mentioned above. This hypothesised evolutionary process fits fairly neatly with the three-process paradigm: integration of formerly more independent BMP and Wnt pathways results in a novel network with a novel function—probably originally specifying the eumetazoan body plan—followed by repeated co-option resulting in a spatiotemporally modular deployment of the network in animal development alongside the subfunctionalisation of each specific variant of the network, with various degrees of integration with other signalling pathways and a host of transcription factors, especially the homeobox family (Arendt 2018, DuBuc, Stephenson et al. 2018, He, Del Viso et al. 2018).
Interestingly, a recent paper (DiFrisco and Wagner 2022) presents a similar view of body plans in eumetazoans, whereby certain conserved developmental mechanisms (body plan identity mechanisms or BpIMs) similar in causal profile to character identity mechanisms are taken to underpin at least the vertebrate and the insect body plans. Interestingly, the authors take body plans to be a contingent feature of some and not necessarily all eumetazoan taxa, rather than taking the eumetazoan body plan as a single character identity. Nor do they infer the plausible evolutionary relationship between BpIMs as ChIMs, as I have done here. Given the high degree of convergence between the two views, a unified theory integrating both seems like a highly desirable research aim to be pursued in the future.
Finally, it must once again be noted that this view elucidates only one aspect of a highly multifaceted and complex series of evolutionary events and is by no means intended as a comprehensive explanation thereof; nevertheless, it does demonstrate the potential explanatory value of the three processes in elucidating the evolution of complexity in animals.
Complexity and agency
A particularly interesting and hotly debated feature of ETIs, explicitly or implicitly, is their relationship with teleological notions such as intentionality, agency, goal-directedness, and autonomy (Rosslenbroich 2014; McShea 2016a, b; Okasha 2018). It is generally acknowledged that organisms behave as agents, via the selective reinforcement of instinctive behaviours or evolution of deliberative decision-making mechanisms, both through the evolution of complex specialised information-processing systems, with the paradigmatic example being the animal nervous system. As discussed above, the evolution of the nervous and endocrine systems in animals in multiple steps, including the evolution of neurons, nerve nets, ganglia, endocrine glands, and the CNS has enabled efficient—and rapid, in the case of the former—communication between cells often far apart from one another. Furthermore, from the present perspective, the evolution of highly centralised nervous systems marks a transition from collective agency (Misselhorn 2015) where, roughly speaking, agents form groups and make collective decisions, as observed in ants or sponge cells—to what I will here call integrated agency where individual agents make decisions autonomously. The evolution of integrated from collective agency in turn reflects growing interdependence and shared fate between lower-level units, concomitant with the evolution of specialisation at the lower and intermediate levels of organisation and the consolidation of mechanisms directly responsible for maintaining internal cohesion (e.g. policing). Thus, a close investigation of the relationship between structural complexity and type of agency beyond the speculation presented in this section promises to enhance the explanatory scope and power of this framework.
Summary and prospects
To summarise, the explanatory gap pertaining to the transformation of stable social groups into full-fledged individual organisms has been addressed here with the evolution of intermediate levels of organisation—i.e. tissues, organs, and organ systems—via the three processes of modularisation, subfunctionalisation, and integration, giving rise to complex and adaptive organism-level traits without appeal to any “progressionist” notions. I have used the evolution of complexity in eumetazoans as a paradigmatic case of the emergence of complex from simple multicellularity. Here, the evolution of the eumetazoan body plan in particular plays a crucial role by providing a template for integration that has likely been utilised innumerable times in the evolution of the nigh-unfathomable diversity of metazoan organisation. It should be borne in mind that the early steps in eumetazoan body plan evolution are still hotly-debated; a particularly salient point of debate for the present discussion is whether eumetazoans evolved from highly modularised organisms fossilised as the Ediacaran Biota such as Dickinsonia and retained their modularity one way or another (Gold, Runnegar et al. 2015, Dunn, Liu et al. 2018) or that the last common ancestor of eumetazoans was in fact not particularly modular, in accordance with more traditional hypotheses (Nielsen 2012). Either way, the importance of integration and modularisation in shaping eumetazoan complexity remains untouched; though, how exactly and in what order the aforementioned processes operated remains an open question.
It is also worth re-emphasising that this model does not aim to capture all aspects of the evolution of complex metazoan characters and is only intended as a means of incorporating the evolution of these characters into the ETI framework. Furthermore, this incorporation and consequent extension of the ETI framework is potentially applicable to cases other than animal evolution: the most obvious such cases are the evolution of complex multicellularity in fungi, red and brown algae, and plants; members of all groups possess specialised tissues and organs and some degree of modular organisation, subfunctionalisation, and integration. Their evolution is therefore prima facie analysable under the present framework. Flowering plants are a prime candidate for this kind of analysis: they are highly modular, consisting of multiple hierarchically-organised levels and modularised, subfunctionalised parts including various cell types, tissue types, and a vast array of types of leaves that sometimes display local integration in the form of flowers, which are underpinned by conserved developmental mechanisms (Bowman, Smyth et al. 2012). The inclusion of plants, as well as other complex multicellular organisms, could also serve to highlight the unique global integration of the eumetazoan body plan.
The same holds for the evolution of eusociality, particularly in cases where complex traits are present: Physalia (Portuguese Man’o’War) with its specialised “tentacles” bearing different types of zooids with specialised functions (dactylozooids, gastrozooids, and gonozooids) is a prime example (Webb, Wallwork et al. 1975). The evolution of the eukaryotic cell is another fairly obvious candidate, where there are clear intermediate levels of organisation between genesFootnote 15 and the whole cell—though some processes at work in that transition do not fit too well with the present framework, most notably endosymbiosis (Margulis and Chapman 1998); this is probably in line with Queller’s distinction between fraternal and egalitarian transitions (Queller 1997) and the framework presented here being primarily focused on the former and endosymbiosis being a feature of the latter. Less directly, the framework is potentially applicable to sociocultural evolution in humans, where analogous levels of organisation such as tribes, organisations, and states have arisen in response to larger size of societies and ensuing group-level challenges (Hodgson and Knudsen 2010; Waring and Wood 2021), though a comprehensive discussion must be left for future work. Overall, this framework has the potential to play an enhancing part in a generalised theory of evolutionary transitions, if such a theory is ever to come by (Okasha 2022). Finally, it also demonstrates the value of going beyond the analysis of organismal traits as functions of genes and their evolution in terms of genetic evolution, and instead recognising the importance of structural, processual, and agential perspectives (Griesemer 2006) in studying the evolution of Life on Earth.
Data availability
N/A.
Code availability
N/A.
Notes
Broadly understood as notions under which the major transitions marks some sort of inevitable progress in evolution; see (Okasha 2022).
This, of course is an idealisation: while there are discernibly different cell types in a multicellular organism, for example, there are also intermediate types. It is nevertheless a useful idealisation precisely because the types are sufficiently discernible as well as explanatory. Where this is not so, degree of differentiation among parts serves as an equally useful and sufficiently similar concept.
Briefly put, evidence suggests that during the transition to multicellularity, the number of parts within cells actually decreases on average due to specialisation. For an extreme exemplification, compare any self-sufficient ciliate with the haemoglobin-packed mammalian erythrocytes.
Coloniality is sometimes used differently in the literature; e.g. (Blackstone and Jasker 2003).
Though the inclusion of these fossils in the animal kingdom has long been a matter of contention, there is mounting support for it (Bobrovskiy, Hope et al. 2018, Dunn, Liu et al. 2018).
Whether the term “superorganism” is a useful one at all is a matter of contention; see (Okasha 2022).
Any reader familiar with genetics will recognise that subfunctionalisation in this sense and its importance has also long been recognised in the case of genes, with the only difference being that it is normally preceded by duplication rather than modularisation.
Modularity is best ascribed to sponges with leuconoid and (especially) syconoid organisation, rather than the much simpler asconoids.
Notably, Arnellos and Keijzer (Arnellos and Keijzer 2019) present a broadly similar discussion on the evolution of complexity in animals centred around the evolution of muscular locomotory systems.
Or, in other terms, the transition from multilevel-selection type 1 to multilevel-selection type 2. In the former, the fitness of the group is simply the aggregate of the fitnesses of lower-level units, whereas in the latter the higher-level unit (or group) reproduces independently of the lower-level units and therefore its fitness is not simply the aggregate of the fitness of its component units (Okasha 2006).
While genes and gene families are not processes per se, the larger their numbers the larger the expected number of interactions between them: the former are thus used as a proxy for the latter.
Assuming genes are the appropriate lower-level structural units, rather than chromosomes. If organelles were the appropriate lower-level units, the applicability of this framework would be undermined.
References
Adamska M et al (2007) “Wnt and TGF-β expression in the sponge Amphimedon queenslandica and the origin of metazoan embryonic patterning.“PloS one2(10)
Aldea D et al (2019) Genetic regulation of amphioxus somitogenesis informs the evolution of the vertebrate head mesoderm. Nat Ecol Evol 3(8):1233–1240
Annunziata R et al (2019) Development and evolution of gut structures: from molecules to function. Cell Tissue Res 377(3):445–458
Arendt D (2008) The evolution of cell types in animals: emerging principles from molecular studies. Nat Rev Genet 9(11):868–882
Arendt D (2018) Hox genes and body segmentation. Science 361(6409):1310–1311
Arendt D et al (2016a) The origin and evolution of cell types. Nat Rev Genet 17(12):744–757
Arendt D et al (2016b) From nerve net to nerve ring, nerve cord and brain—evolution of the nervous system. Nat Rev Neurosci 17(1):61–72
Armon S et al (2018) “Ultrafast epithelial contractions provide insights into contraction speed limits and tissue integrity.“ Proceedings of the National Academy of Sciences 115(44): E10333-E10341
Arnellos A, Keijzer F (2019) Bodily complexity: Integrated multicellular organizations for contraction-based motility. Front Physiol 10:1268
Balavoine G, Adoutte A (2003) The segmented Urbilateria: a testable scenario. Integr Comp Biol 43(1):137–147
Bartolomaeus T, Ax P (1992) Protonephridia and Metanephridia-their relation within the Bilateria. J Zoological Syst Evolutionary Res 30(1):21–45
Beklemishev W (1969) “Principles of comparative anatomy of invertebrates.“ Promorphology
Bellusci S et al (1996) “Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. " Dev 122(6):1693–1702
Birch J (2017) The philosophy of social evolution. Oxford University Press
Blackstone NW, Jasker BD (2003) Phylogenetic considerations of clonality, coloniality, and mode of germline development in animals. J Experimental Zool Part B: Mol Dev Evol 297(1):35–47
Bobrovskiy I et al (2018) Ancient steroids establish the Ediacaran fossil Dickinsonia as one of the earliest animals. Science 361(6408):1246–1249
Bock WJ, Von Wahlert G (1965) “Adaptation and the form-function complex.“ Evolution: 269–299
Bond C (2013) “Locomotion and contraction in an asconoid calcareous sponge”. Invertebr Biol 132(4):283–290
Bonner JT (2009) First signals. Princeton University Press
Bourke AF (2011) Principles of social evolution. Oxford University Press
Bowman JL et al (2012) “The ABC model of flower development: then and now " Development 139(22):4095–4098
Budd GE (2006) “On the origin and evolution of major morphological characters”. Biol Rev 81(4):609–628
Budd GE, Jensen S (2017) “The origin of the animals and a ‘Savannah’hypothesis for early bilaterian evolution. " Biol Reviews 92(1):446–473
Buss LW (1987) The evolution of individuality. Princeton University Press
Calcott B, Sterelny K (2011) The major transitions in evolution revisited. MIT Press
Chipman AD (2010) of ancestral gene regulatory networks " BioEssays 32(1):60–70"Parallel evolution of segmentation by co-option
Chipman AD, Edgecombe GD (2019) “Developing an integrated understanding of the evolution of arthropod segmentation using fossils and evo-devo.“ Proceedings of the Royal Society B 286(1912): 20191881
Choudhry Z et al (2014) Sonic hedgehog signalling pathway: a complex network. Annals of neurosciences 21(1):28
Church VL, Francis-West P (2004) Wnt signalling during limb development. Int J Dev Biol 46(7):927–936
Clarke E (2010) “The problem of biological individuality”. Biol Theory 5(4):312–325
Conway Morris S (2003) “The Cambrian “explosion” of metazoans.“Origination of organismal form:13–32
Couso JP (2009) Segmentation, metamerism and the Cambrian explosion. Int J Dev Biol 53(8–9–10):1305–1316
Davidson EH (2010) Emerging properties of animal gene regulatory networks. Nature 468(7326):911–920
Davidson EH, Erwin DH (2006) “Gene regulatory networks and the evolution of animal body plans. " Sci 311(5762):796–800
Dawkins R (2016) The selfish gene. Oxford university press
Dean CH et al (2005) Canonical Wnt signaling negatively regulates branching morphogenesis of the lung and lacrimal gland. Dev Biol 286(1):270–286
DiFrisco J (2021) “Integrating composition and process in levels of developmental evolution. " Levels of organization in the biological sciences
DiFrisco J et al (2020) Character identity mechanisms: a conceptual model for comparative-mechanistic biology. Biology & Philosophy 35(4):1–32
DiFrisco J, Wagner GP (2022) “Body Plan Identity. A Mechanistic Model.“ Preprints
DuBuc TQ et al (2019) ““Dorsal–Ventral” Genes Are Part of an Ancient Axial Patterning System: Evidence from Trichoplax adhaerens (Placozoa). " Mol biology Evol 36(5):966–973
DuBuc TQ et al (2018) “Hox and Wnt pattern the primary body axis of an anthozoan cnidarian before gastrulation. " Nat Commun 9(1):1–12
Dunn FS et al (2018) “Ediacaran Dev biology " Biol Reviews 93(2):914–932
Eerkes-Medrano D et al (2015) Sponge cell aggregation: checkpoints in development indicate a high level of organismal complexity. Invertebr Biol 134(1):1–18
Elliott GR, Leys SP (2007) Coordinated contractions effectively expel water from the aquiferous system of a freshwater sponge. J Exp Biol 210(21):3736–3748
Extavour CG (2007) Evolution of the bilaterian germ line: lineage origin and modulation of specification mechanisms. Integr Comp Biol 47(5):770–785
Funayama N (2008) Stem cell system of sponge.Stem cells, Springer:17–35
Geetha-Loganathan P et al (2008) “Wnt Signal limb organogenesis " Organogenesis 4(2):109–115
Genikhovich G et al (2015) Axis patterning by BMPs: cnidarian network reveals evolutionary constraints. Cell Rep 10(10):1646–1654
Genikhovich G, Technau U (2017) “On the evolution of bilaterality " Development 144(19):3392–3404
Giribet G, Edgecombe GD (2019) “Perspectives in Animal Phylogeny and Evolution”: A decade later. University of Padova Press
Giribet G, Edgecombe GD (2020) 14. Deuterostomia. The Invertebrate Tree of Life. Princeton University Press, pp 102–107
Godfrey-Smith P (2009) Darwinian populations and natural selection. Oxford University Press
Goetz R, Mohammadi M (2013) Exploring mechanisms of FGF signalling through the lens of structural biology. Nat Rev Mol Cell Biol 14(3):166–180
Gold DA et al (2015) Ancestral state reconstruction of ontogeny supports a bilaterian affinity for Dickinsonia. Evol Dev 17(6):315–324
Göpel T, Wirkner CS (2018) “Morphological description, character conceptualization and the reconstruction of ancestral states exemplified by the evolution of arthropod hearts”. PLoS ONE 13(9):e0201702
Gregory WK (1935) ‘Williston’s law’relating to the evolution of skull bones in the vertebrates. Am J Phys Anthropol 20(2):123–152
Griesemer J (2006) 8 Genetics from an Evolutionary Process Perspective. Genes in development,Duke University Press:199–237
Grosberg RK, Strathmann RR (2007) “The evolution of multicellularity: a minor major transition?“ Annu. Rev Ecol Evol Syst 38:621–654
Gschwentner R et al (2003) “Unique patterns of longitudinal body-wall musculature in the Acoela (Plathelminthes): the ventral musculature of Convolutriloba longifissura. " Zoomorphology 122(2):87–94
Guijarro-Clarke C et al (2020) Widespread patterns of gene loss in the evolution of the animal kingdom. Nat Ecol Evol 4(4):519–523
He S et al (2018) “An axial Hox code controls tissue segmentation and body patterning in Nematostella vectensis. " Sci 361(6409):1377–1380
Hochberg R, Litvaitis MK (2000) “Functional morphology of the muscles in Philodina sp.(Rotifera:. Bdelloidea) " Hydrobiologia 432(1–3):57–64
Hochberg R et al (2010) “Behavior, metamorphosis, and muscular organization of the predatory rotifer Acyclus inquietus (Rotifera, Monogononta). " Invertebrate Biology 129(3):210–219
Hodgson GM, Knudsen T (2010) Darwin’s conjecture: The search for general principles of social and economic evolution. University of Chicago Press
Horowitz A, Simons M (2008) “Branching morphogenesis " Circulation research 103(8):784–795
Huelsken J, Behrens J (2002) The Wnt signalling pathway. J Cell Sci 115(21):3977–3978
Ivantsov A et al (2020) Intravital damage to the body of Dickinsonia (Metazoa of the late Ediacaran). J Paleontol 94(6):1019–1033
Jensen B et al (2013) “Evolution and development of the building plan of the vertebrate heart.“ Biochimica et Biophysica Acta (BBA)-Molecular. Cell Res 1833(4):783–794
Kanzler B et al (2000) “BMP signaling is essential for development of skeletogenic and neurogenic cranial neural crest. " Dev 127(5):1095–1104
Kawakami Y et al (1996) BMP signaling during bone pattern determination in the developing limb. Development 122(11):3557–3566
Kestler HA, Kühl M (2008) “From individual Wnt pathways towards a Wnt signalling network”. Philosophical Trans Royal Soc B: Biol Sci 363(1495):1333–1347
Kopan R (2012) “Notch signaling”. Cold Spring Harb Perspect Biol 4(10):a011213
Kozin VV et al (2019) “Establishment of the Axial Polarity and Cell Fate in Metazoa via Canonical Wnt Signaling: New Insights from Sponges and Annelids”. Biology Bull 46(1):14–25
Kuure S et al (2000) Kidney morphogenesis: cellular and molecular regulation. Mech Dev 92(1):31–45
Lapébie P et al (2009) “WNT/β-catenin signalling and epithelial patterning in the homoscleromorph sponge Oscarella.“PloS one4(6)
Laumer CE et al (2018) “Support for a clade of Placozoa and Cnidaria in genes with minimal compositional bias. " Elife 7:e36278
Lebedeva T et al (2021) “Cnidarian-bilaterian comparison reveals the ancestral regulatory logic of the β-catenin dependent axial patterning”. Nat Commun 12(1):4032
Leonard J, Córdoba-Aguilar A (2010) The evolution of primary sexual characters in animals. Oxford University Press
Li X, Cao X (2006) BMP signaling and skeletogenesis. Ann N Y Acad Sci 1068(1):26–40
Lobo-da-Cunha A (2019) Structure and function of the digestive system in molluscs. Cell Tissue Res 377(3):475–503
Mackie G, Singla C (1987) “Impulse propagation and contraction in the tunic of a compound ascidian”. Biol Bull 173(1):188–204
Margulis L, Chapman MJ (1998) Endosymbioses: cyclical and permanent in evolution. Trends Microbiol 6(9):342–345
Martín-Durán JM et al (2018) Convergent evolution of bilaterian nerve cords. Nature 553(7686):45–50
Maynard Smith J, Szathmary E (1995) The major transitions in evolution. Oxford University Press
McShea DW (1996) “Meatzoan Complexity and Evolution: is there a trend?“ Evolution 50
McShea DW (2001) “The hierarchical structure of organisms: a scale and documentation of a trend in the maximum. " Paleobiology 27(2):405–423
McShea DW (2002) “A complexity drain on cells in the evolution of multicellularity. " Evol 56(3):441–452
McShea DW (2016a) Hierarchy: The source of teleology in evolution. Evolutionary Theory, University of Chicago Press: 86–102
McShea DW (2016b) “Three trends in the history of life: an evolutionary syndrome”. Evol Biol 43(4):531–542
McShea DW (2017) “Evolution of complexity.“ Evolutionary Developmental Biology: A Reference Guide: 1–11
McShea DW, Changizi MA (2003) Three puzzles in hierarchical evolution. Integr Comp Biol 43(1):74–81
Meyer-Wachsmuth I, Nemertodermatida et al (2013)Acoelomorpha).“ Zoomorphology132(3):239–252
Michod RE (2000) Darwinian dynamics: evolutionary transitions in fitness and individuality. Princeton University Press
Michod RE (2005) On the transfer of fitness from the cell to the multicellular organism. Biol Philos 20(5):967–987
Michod RE, Nedelcu AM (2003) On the reorganization of fitness during evolutionary transitions in individuality. Integr Comp Biol 43(1):64–73
Misselhorn C (2015) Collective agency and cooperation in natural and artificial systems. Collective agency and cooperation in natural and artificial systems, Springer: 3–24
Nanglu K, Caron J-B (2018) “A new Burgess Shale polychaete and the origin of the annelid head revisited”. Curr Biol 28(2):319–326e311
Nejad Kourki A (2021) Evolution of the Eumetazoan Body Plan: Conceptual challenges, Ediacaran Fossils, and Organismal Complexity. School of Biological Sciences, University of Bristol. PhD
Nickel M (2004) Kinetics and rhythm of body contractions in the sponge Tethya wilhelma (Porifera: Demospongiae). J Exp Biol 207(26):4515–4524
Nickel M (2010) “Evolutionary emergence of synaptic nervous systems: what can we learn from the non-synaptic, nerveless Porifera?“. Invertebr Biol 129(1):1–16
Nickel M et al (2011) The contractile sponge epithelium sensu lato–body contraction of the demosponge Tethya wilhelma is mediated by the pinacoderm. J Exp Biol 214(10):1692–1698
Niehrs C (2010) “On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. " Dev 137(6):845–857
Nielsen C (2012) Animal evolution: interrelationships of the living phyla. Oxford University Press on Demand
Nielsen C et al (2018) Evolution of the bilaterian mouth and anus. Nat Ecol Evol 2(9):1358–1376
Nieuwkoop PD, Sutasurya LA (1981) Primordial germ cells in the invertebrates: from epigenesis to preformation. CUP Archive
Nulsen C, Nagy L (1999) The role of wingless in the development of multibranched crustacean limbs. Dev Genes Evol 209(6):340–348
Nusse R et al (1991) A new nomenclature for int-1 and related genes: the Wnt gene family. Cell 64(2):231–231
O’Connell DJ et al (2012) A Wnt-bmp feedback circuit controls intertissue signaling dynamics in tooth organogenesis. Sci Signal 5(206):ra4–ra4
Okasha S (2006) Evolution and the levels of selection. Oxford University Press
Okasha S (2018) Agents and goals in evolution. Oxford University Press
Okasha S (2022) “The Major Transitions in Evolution—A Philosophy-of-Science Perspective.“Frontiers in Ecology and Evolution10
Paps J (2018) What makes an animal? The molecular quest for the origin of the animal kingdom. Integr Comp Biol 58(4):654–665
Paps J, Holland PW (2018) Reconstruction of the ancestral metazoan genome reveals an increase in genomic novelty. Nat Commun 9(1):1–8
Pepicelli CV et al (1998) Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol 8(19):1083–1086
Pérez-Pomares JM, Muñoz‐Chápuli R (2002) “Epithelial–mesenchymal transitions: a mesodermal cell strategy for evolutive innovation in metazoans”. Anat Record: Official Publication Am Association Anatomists 268(3):343–351
Plikus MV et al (2008) Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 451(7176):340–344
Pradeu T (2011) The limits of the self: immunology and biological identity. Oxford University Press
Pradeu T (2016) The many faces of biological individuality. Springer 31:761–773
Queller DC (1997) Cooperators since life began. University of Chicago Press
Raspopovic J et al (2014) “Digit patterning is controlled by a Bmp-Sox9-Wnt Turing network modulated by morphogen gradients. " Sci 345(6196):566–570
Reid PJW et al (2018) Wnt signaling and polarity in freshwater sponges. BMC Evol Biol 18(1):1–14
Riccomagno MM et al (2005) Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev 19(13):1612–1623
Rokas A (2008) The origins of multicellularity and the early history of the genetic toolkit for animal development. Annu Rev Genet 42:235–251
Rosslenbroich B (2014) On the origin of autonomy: A new look at the major transitions in evolution. Springer
Ruppert E, Carle KJ (1983) “Morphology of metazoan circulatory systems " Zoomorphology 103(3):193–208
Schlosser G, Wagner GP (2004) Modularity in development and evolution. University of Chicago Press
Schultz RD et al (2014) “Regulation of extracellular matrix organization by BMP signaling in Caenorhabditis elegans.“PloS one9(7)
Shubin N et al (1997) Fossils, genes and the evolution of animal limbs. Nature 388(6643):639–648
Sohal DS et al (2001) Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circul Res 89(1):20–25
Sollas W (1884) Memoirs: On the Development of Halisarca lobularis (O. Schmidt). J Cell Sci 2(96):603–621
Sperling EA et al (2013) “Oxygen, ecology, and the Cambrian radiation of animals.“ Proceedings of the National Academy of Sciences 110(33): 13446–13451
Steinmetz PR (2019) “A non-bilaterian perspective on the development and evolution of animal digestive systems.“ Cell and tissue research: 1–19
Steinmetz PR et al (2012) Independent evolution of striated muscles in cnidarians and bilaterians. Nature 487(7406):231–234
Suksaweang S et al (2004) “Morphogenesis of chicken liver: identification of localized growth zones and the role of β-catenin/Wnt in size regulation”. Dev Biol 266(1):109–122
Swann JB et al (2017) Elevated levels of Wnt signaling disrupt thymus morphogenesis and function. Sci Rep 7(1):1–15
Swarr DT, Morrisey EE (2015) Lung endoderm morphogenesis: gasping for form and function. Annu Rev Cell Dev Biol 31:553–573
Szathmáry E, Smith JM (1995) “The major evolutionary transitions " Nature 374(6519):227–232
Tarazona OA et al (2019) “Evolution of limb development in cephalopod mollusks " Elife 8:e43828
Thornhill RH, Ussery DW (2000) A classification of possible routes of Darwinian evolution. J Theor Biol 203(2):111–116
Varoqueaux F et al (2018) High cell diversity and complex peptidergic signaling underlie placozoan behavior. Curr Biol 28(21):3495–3501e3492
Vinther J et al (2017) Ancestral morphology of crown-group molluscs revealed by a new Ordovician stem aculiferan. Nature 542(7642):471–474
Wagner GP (2014) Homology, genes, and evolutionary innovation. princeton university press
Wan M, Cao X (2005) BMP signaling in skeletal development. Biochem Biophys Res Commun 328(3):651–657
Waring TM, Wood ZT (2021) “Long-term gene–culture coevolution and the human evolutionary transition.“ Proceedings of the Royal Society B 288(1952): 20210538
Webb JE et al (1975) Guide to invertebrate animals. Macmillan International Higher Education
Weiss A, Attisano L (2013) “The TGFbeta superfamily signaling pathway”. Wiley Interdisciplinary Reviews: Developmental Biology 2(1):47–63
Wijesena N et al (2017) “Antagonistic BMP–cWNT signaling in the cnidarian Nematostella vectensis reveals insight into the evolution of mesoderm.“ Proceedings of the National Academy of Sciences 114(28): E5608-E5615
Wilson DS, Sober E (1998) “Unto Others " Harvard University Press Carnbridge 9:95–112
Windsor PJ, Leys SP (2010) Wnt signaling and induction in the sponge aquiferous system: evidence for an ancient origin of the organizer. Evol Dev 12(5):484–493
Winther RG (2001) Varieties of modules: kinds, levels, origins, and behaviors. J Exp Zool 291(2):116–129
Xavier-Neto J et al (2010) Evolutionary origins of hearts. Heart development and regeneration, Elsevier: 3–45
Zou H et al (1997) BMP signaling and vertebrate limb development. Cold Spring Harbor symposia on quantitative biology, Cold Spring Harbor Laboratory Press
Zrzavý J, Štys P (1997) The basic body plan of arthropods: insights from evolutionary morphology and developmental biology. J Evol Biol 10(3):353–367
Acknowledgements
I would like to thank Professor Daniel W. McShea (Duke), Professor Timothy Lewens (Cambridge), Professor Samir Okasha (Bristol), Dr James DiFrisco (Leuven), and members of Dr Marta Halina’s research group (Cambridge) for invaluable feedback on this paper.
Funding
This paper was originally drafted as a chapter of my PhD thesis. The PhD was undertaken at the University of Bristol and was partially funded by the university’s fee waiver scholarship for international students.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
N/A.
Ethics approval
N/A.
Consent to participate
N/A.
Consent for publication
N/A.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nejad Kourki, A. The evolution of complex multicellularity in animals. Biol Philos 37, 43 (2022). https://doi.org/10.1007/s10539-022-09870-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10539-022-09870-1