Abstract
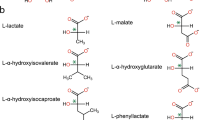
The LarA superfamily consists of nickel-dependent enzymes catalyzing racemization/epimerization reactions using a variety of α-hydroxy acids. The first-characterized LarA, a lactate racemase from Lactobacillus plantarum, led to the discovery of the nickel-pincer nucleotide (NPN) cofactor that is utilized by family members with alternative substrates, including malate racemase from Thermoanaerobacterium thermosaccharolyticum (Mar2). In this work, a higher resolution crystal structure of Mar2 was obtained with better data quality that revealed new structural and dynamic characteristics of the protein. A model of the Mar2 structure with bound cofactor and substrate was generated to uncover the common and the unique features among two distinct subgroups in the LarA superfamily. In addition, structure-guided mutational studies were used to examine the importance of residues that are modeled to interact with NPN and to explore which residues were critical for conferring specificity for malate. In particular, substitution of two residues involved in substrate binding in Mar2 to match the corresponding residues in LarA led to the acquisition of low levels of lactate racemase activity. Of additional interest, the substrate spectrum was expanded to include tartrate, an analog of malate. These new findings will help to better understand structure–function relationships of many other LarA homologs that are broadly distributed in bacterial and archaeal species.





Similar content being viewed by others
References
Battye TG, Kontogiannis L, Johnson O, Powell HR, Leslie AG (2011) iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr D Biol Crystallogr 67:271–281. https://doi.org/10.1107/S0907444910048675
DeLano WL (2002) The PyMOL molecular graphics system. http://www.pymol.org.
Desguin B, Goffin P, Viaene E, Kleerebezem M, Martin-Diaconescu V, Maroney MJ, Declercq JP, Soumillion P, Hols P (2014) Lactate racemase is a nickel-dependent enzyme activated by a widespread maturation system. Nat Commun 5:3615. https://doi.org/10.1038/ncomms4615
Desguin B, Zhang T, Soumillion P, Hols P, Hu J, Hausinger RP (2015) A tethered niacin-derived pincer complex with a nickel-carbon bond in lactate racemase. Science 349:66–69. https://doi.org/10.1126/science.aab2272
Desguin B, Soumillion P, Hols P, Hausinger RP (2016) Nickel-pincer cofactor biosynthesis involves LarB-catalyzed pyridinium carboxylation and LarE-dependent sacrificial sulfur insertion. Proc Natl Acad Sci U S A 113:5598–5603. https://doi.org/10.1073/pnas.1600486113
Desguin B, Soumillion P, Hausinger RP, Hols P (2017) Unexpected complexity in the lactate racemization system of lactic acid bacteria. FEMS Microbiol Rev 41:S71–S83. https://doi.org/10.1093/femsre/fux021
Desguin B, Fellner M, Riant O, Hu J, Hausinger RP, Hols P, Soumillion P (2018) Biosynthesis of the nickel-pincer nucleotide cofactor of lactate racemase requires a CTP-dependent cyclometallase. J Biol Chem 293:12303–12317. https://doi.org/10.1074/jbc.RA118.003741
Desguin B, Urdiain-Arraiza J, Da Costa M, Fellner M, Hu J, Hausinger RP, Desmet T, Hols P, Soumillion P (2020) Uncovering a superfamily of nickel-dependent hydroxyacid racemases and epimerases. Sci Rep 10:18123. https://doi.org/10.1038/s41598-020-74802-6
Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66:486–501. https://doi.org/10.1107/S0907444910007493
Evans P (2006) Scaling and assessment of data quality. Acta Crystallogr D Biol Crystallogr 62:72–82. https://doi.org/10.1107/S0907444905036693
Fellner M, Desguin B, Hausinger RP, Hu J (2017) Structural insights into the catalytic mechanism of a sacrificial sulfur insertase of the N-type ATP pyrophosphatase family, LarE. Proc Natl Acad Sci U S A 114:9074–9079. https://doi.org/10.1073/pnas.1704967114
Hausinger RP, Desguin B, Fellner M, Rankin JA, Hu J (2018) Nickel-pincer nucleotide cofactor. Curr Opin Chem Biol 47:18–23. https://doi.org/10.1016/j.cbpa.2018.06.019
Liebschner D et al (2019) Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr D Struct Biol 75:861–877. https://doi.org/10.1107/S2059798319011471
Madeira F et al (2019) The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res 47:W636–W641. https://doi.org/10.1093/nar/gkz268
Martinez-Luque M, Castillo F, Blasco R (2001) Assimilation of D-malate by Rhodobacter capsulatus E1F1. Curr Microbiol 43:154–157. https://doi.org/10.1007/s002840010279
Rankin JA, Mauban RC, Fellner M, Desguin B, McCracken J, Hu J, Varganov SA, Hausinger RP (2018) Lactate racemase nickel-pincer cofactor operates by a proton-coupled hydride transfer mechanism. Biochemistry 57:3244–3251. https://doi.org/10.1021/acs.biochem.8b00100
Robert X, Gouet P (2014) Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42:W320-324. https://doi.org/10.1093/nar/gku316
Acknowledgements
This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (Grant 085P1000817). This work is supported by National Science Foundation Grant CHE 1807073 (to R.P.H. and J.H.), National Institutes of Health Grant GM128959 (to R.P.H. and J.H.), and Fonds de la Recherche Scientifique (FNRS) (to B.D. and J.U.). We thank Alessia Citti for her participation in the construction of the Mar2 variants.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is dedicated to the former Editor-in-Chief of Biometals, Günther Winkelmann, on his retirement and 80th birthday.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gatreddi, S., Urdiain-Arraiza, J., Desguin, B. et al. Structural and mutational characterization of a malate racemase from the LarA superfamily. Biometals 36, 303–313 (2023). https://doi.org/10.1007/s10534-022-00372-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-022-00372-x