Abstract
Restorations of former cranberry farms (“bogs”) aim to re-establish native wetland vegetation, promote cold water habitat, and attenuate nitrogen (N) delivery to coastal waters. It is unclear, though, how elements of restoration design such as microtopography, groundwater interception, and plant communities affect N removal via denitrification. In a recently restored riparian cranberry bog with created microtopography, we compared denitrification potential, nitrous oxide (N2O) yield of denitrification (ratio of N2O:N2O + N2 gases), in situ N2O fluxes, soil chemistry, and plant communities at the highest and lowest elevations within 20 plots and at four side-channel groundwater seeps. Denitrification potential was > 2 × greater at low elevations, which had plant communities distinct from high elevations, and was positively correlated with plant species richness (Spearman’s rho = 0.43). Despite detecting high N2O yield (0.86 ± 0.16) from low elevation soils, we observed small N2O emissions in situ, suggesting minimal incomplete denitrification even in saturated depressions. Groundwater seeps had an order of magnitude higher denitrification potentials and 100–300 × greater soil NO3− concentrations than the typically saturated low elevation soils. Groundwater seeps also had high N2O yield (1.05 ± 0.15) and higher, but spatially variable, in situ N2O emissions. Our results indicate that N removal is concentrated where soils interact with NO3–rich groundwater, but other factors such as low soil carbon (C) also limit denitrification. Designing restoration features to increase groundwater residence time, particularly in low lying, species rich areas, may promote more N attenuation in restored cranberry bogs and other herbaceous riparian wetlands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To decrease threats that excess nitrogen (N) poses to waterbodies globally, wetland restoration projects typically aim to restore a suite of ecosystem functions, including N removal via denitrification (Zedler 2000; Bricker et al. 2008; Montoya et al. 2012; Cheng et al. 2020). Denitrification is the microbial reduction of nitrate (NO3−) to gaseous forms (inert dinitrogen, N2, or potent greenhouse gas nitrous oxide, N2O). Because the reaction requires an anoxic environment, wetlands are important landscape sinks for N, with denitrification as the dominant process of NO3− removal (Brinson et al. 1984; Saunders and Kalff 2001). Despite evidence that wetland restoration can increase denitrification (Wolf et al. 2011a; Ballantine et al. 2017; Dee and Tank 2020), how different restoration features, e.g., plant community, microtopography, created stream channels, alter N removal in restored wetland ecosystems remains poorly understood.
Plants affect denitrification by competing for NO3−, diffusing oxygen through their roots, and supplying organic carbon (C) as root exudates or biomass to soil microbes (Bachand and Horne 2000; Fornara and Tilman 2008; Bastviken et al. 2005; Sutton-Grier et al. 2011). In general, denitrification is higher in vegetated than unvegetated sites (Nilsson et al. 2020; Audet et al. 2021), and, in some ecosystems, plant species dominance is tightly linked to denitrification rates (Alldred and Baines 2016; Ooi et al. 2022). Highly controlled experiments also indicate that certain species (e.g., Leersia oryzoides) are correlated with higher denitrification rates (Taylor et al 2015; Speir et al. 2017; Malique et al. 2019; Brisson et al. 2020). Plant functional diversity can be related to denitrification, although the direction and magnitude of influence is largely dependent on soil characteristics like moisture, C, and N content (Sutton-Grier et al. 2011). Although species diversity is often a restoration target, examining how plant functional traits relate to ecosystem processes may promote generalization across restoration projects (Alldred and Baines 2016; Zirbel et al. 2017).
Microtopography (< 1 m vertical relief) is an important driver of wetland abiotic conditions that influence plant community composition and ecosystem processes such as denitrification (Larkin et al. 2006). Topographic heterogeneity can promote N removal by providing coupled oxic-anoxic environments so nitrification, an aerobic process, oxidizes ammonium (NH4+) to NO3−, which then fuels denitrification (Reddy and Patrick 1984; Wolf et al. 2011b). Using model simulations, Frei et al. (2012) found that microtopography created hot spots for denitrification by varying flow paths, increasing water residence time, and creating spatial separation between sites that are optimal for nitrification and denitrification. Microtopography is increasingly integrated into wetland restoration (Wolf et al. 2011a), so understanding the extent to which it alters N removal via denitrification is important for informing restoration design.
Denitrification is a spatially and temporally heterogeneous biogeochemical reaction, with elevated rates at particular locations (i.e., hot spots) and times (i.e., hot moments) (McLain et al. 2003). Bernhardt et al. (2017) argued that hot spots and hot moments create multiple categories of environmental control points. Delivery of limiting reactants can activate control points; for example, discharge of NO3−-rich groundwater may activate denitrification at the discharge site. Stream channel reconfiguration during wetland restoration could promote groundwater connectivity when new channels intercept groundwater discharge (“seeps”) (Hare et al. 2015; Harvey et al. 2019). Groundwater discharging into streams rather than more diffusely into wetlands may decrease denitrification and increase downstream N loading, but it can also provide cold water refugia for wildlife (e.g., brook trout), an objective for some restorations (Lake et al. 2007, Living Observatory 2020).
Although elevated denitrification rates reduce N loading to downstream ecosystems, they may also increase N2O emissions from restored wetlands via incomplete denitrification. With a global warming potential 298 times that of CO2, N2O emissions are an important factor when considering the outcomes of wetland restoration (Wilson et al. 2016; He et al. 2024). The N2O yield of denitrification (ratio of N2O to N2O + N2 gases) tends to be higher in acidic soils (Liu et al. 2010) and when NO3− concentrations are very high (e.g., > 15 mg/L; Moseman-Valtierra et al. 2011; Chmura et al. 2016), both of which lead to higher rates of incomplete denitrification. Restoration of former cranberry farms (“bogs”) to freshwater wetlands may increase N removal via incomplete denitrification (N2O emissions) as well as complete denitrification (N2 emissions) as they have acidic soils (pH < 6) and often NO3− rich groundwater discharge, but we lack understanding of these processes across different restoration features that are increasingly being implemented in southern New England.
Most cranberry (Vaccinium macrocarpon) farms in Massachusetts (MA), USA are located on former wetlands, and many are in coastal watersheds with impaired water quality from excess N (Howes et al. 2005). Cranberry bogs cover ~ 5400 ha of land in MA, and, in 2016, the MA Cranberry Revitalization Task Force estimated that 400 ha had been retired and up to 2400 ha were at risk of being taken out of production due to competition from other regions and uncertainty in the cranberry market (MA Department of Agricultural Resources 2016). Retired cranberry farms that are prioritized for restoration are often built around groundwater fed streams (Kennedy et al. 2020), and the groundwater of many watersheds with active and retired cranberry farms has elevated NO3− concentrations from septic systems and fertilizer use (Valiela et al. 1990; McCobb et al. 2021). Our overarching aim was to quantify the spatial patterns of N removal via denitrification and the relationships between N removal and key features of a recently restored cranberry bog complex—plant communities, created microtopography, and groundwater seeps. Specifically, we quantified: (1) relationships among denitrification potential, in situ N2O gas flux, and metrics of plant community composition, (2) the influence of relative topographic position on denitrification potential and in situ N2O gas flux, and (3) denitrification potential and in situ N2O gas flux at groundwater seeps on side-channels that fed the reconfigured stream channel.
Methods
Site description
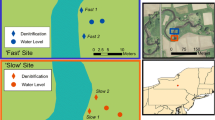
This study took place at the Coonamessett River restoration (Falmouth, MA, USA) within two former bog cells (Fig. 1). Upper (3.8 ha) and Middle (6.3 ha) Bogs were farmed for more than 100 years and were considered “flow-through” bogs with stream channels dissecting bog surfaces (Hoekstra et al. 2019). These agricultural wetlands occur in groundwater fed spring sapping valleys and have underlying peat, though Upper Bog is more uniformly underlain with peat than Middle Bog, which has patchy deposits with variable peat depth (NRCS 2016). Established cranberry farming practices involved placement of 1–5 cm of sand on bogs every 2–5 years (Sandler and DeMoranville 2008), resulting in 35 cm to > 100 cm of sand overlying the native wetland soil at this site. Both bogs were retired from farming in 2013 and restored before the 2020 growing season using practices that have been commonly employed for cranberry bog restoration in the region. Restoration included removing water control structures, filling drainage ditches, building a sinuous stream channel, adding large wood to the channel and floodplain, and creating microtopography on the bog surface. Some farm ditches were retained as small feeder creeks into the main channel. Portions of the channel banks were seeded with a native seed mix (14 species: 64% graminoids, 36% forbs) for stability. On the bog surface, seedlings of three native trees (~ 35 total seedlings/ha) and six native shrubs (~ 15 total seedlings/ha) were planted, but other plants on the former bog surface regenerated on their own without seeding.
Aerial photograph of Upper and Middle Bogs on the Coonamessett River (Falmouth, MA USA). Blue circles represent randomly located plots (9 m2) in which we located high and low elevation subplots (625 cm2), as indicated by lower, right diagram. Green triangles represent side-channel plots, which include paired 1 m2 seep and non-seep plots (top, right diagram). Black circles represent static chambers (not to scale) for measuring in situ greenhouse gas fluxes
Plot selection
Within both Upper and Middle Bogs, we established 10 randomly located, 3 × 3 m plots for vegetation surveys (Fig. 1). To examine the effects of elevational differences resulting from created microtopography, we used RTK GPS (Topcon-GR3, 5 mm vertical accuracy) to identify the highest and lowest elevation within each of the 20 plots (hereafter “elevation plots”). We used each point to center a 25 × 25 cm plot (20 per elevation class, 40 total). High plots had a mean elevation 3.43 (± 0.10) m above sea level, and low plots averaged 3.01 (± 0.09) m above sea level. We also subtracted the lowest from the highest elevation within each 3 × 3 m plot to calculate vertical relief, which ranged from 0.28 to 0.82 m and averaged 0.45 (± 0.03) m. Because this was not an experimental restoration, the abiotic conditions of high and low plots varied. Most high plots were well-drained and rarely saturated, while most low plots were at least seasonally saturated or inundated.
To investigate how actively discharging groundwater seeps alter denitrification potential and N2O emissions, we placed an additional four pairs of 1 × 1 m plots along four side-channels (hereafter “side-channel plots”; Fig. 1). In August 2022, we located groundwater seep plots by scanning side-channel feeder streams that run into the Coonamessett River with a thermal infrared camera (FLIR T540) to indicate locations of potential groundwater upwelling. Because groundwater temperature remains constant relative to surface water (Hayashi and Rosenberry 2002), infrared imaging is an effective way to locate groundwater during months when the temperature differential between groundwater and surface water is largest (Hare et al. 2015). Within two meters of seep plots, we established paired, non-seep plots on the bog surface.
Vegetation surveys
Within elevation plots (25 × 25 cm), we identified all species present in August 2021 and visually estimated their percent cover using seven cover class bins (< 1%, 1–2%, 3–5%, 6–15%, 16–25%, 26–50%, 51–75%, 76–100%) (Braun-Blanquet 1932; Walker et al. 2018).
In situ gas flux measurements
We used static chambers (Holland et al. 1999) to quantify in situ N2O soil-to-atmosphere fluxes (emissions) from different microtopographic positions. We installed opaque 10.16-cm diameter PVC static chamber collars in each elevation plot, resulting in 20 “high” and 20 “low” elevation chambers. To investigate how groundwater seeps alter N2O emissions, we installed three static chambers in each side-channel plot, resulting in 12 chambers at both groundwater seep and paired non-seep locations. All collars were installed > 7 days before gas sampling. During sampling, we placed a PVC cap fitted with a vent and septum on each collar (Holland et al. 1999; Helton et al. 2019) and collected gas samples into pre-evacuated 22 ml vials at 0, 15, 30, and 45 min. We sampled gas emissions from elevation plots over the course of 2 days in August 2021, and from side-channel plots during 1 day in August 2022. N2O concentrations were measured using a Perkin Elmer Clarus 580 gas chromatograph with an electron capture detector. The N2O flux rates were calculated as the slope of the linear regression of μg N2O-N accumulation over time per m2. To determine N2O flux, we first converted concentrations (ppm-v) to mass (μg-m3) using the ideal gas law and field measurements of barometric pressure and air temperature. We then calculated the concentration difference between sampling times for each chamber. We calculated the minimum detectable concentration difference (MDCD) following Yates et al. 2006, and fluxes below the MDCD were given a slope of zero. We included fluxes whose concentrations exceeded the MDCD and whose linear accumulation of N2O over time had an r2 > 0.85 in the final corrections for chamber volume and surface area. For fluxes above the MDCD but with an r2 < 0.85, we recalculated the flux when it was possible to improve the linear fit by dropping one or two anomalous samples (e.g., Doroski et al. 2019; Granville et al. 2021). Of 64 total flux measurements, 31 were below MDCD and set to zero, and 17 fluxes did not meet the linear criteria and were excluded from analysis.
Soil analyses
From each elevation plot, we collected three soil cores (10 cm depth, 5 cm diameter), one each for soil chemistry and microbial process rates, below ground biomass, and bulk density; these cores were kept separate. From each side-channel plot, we collected three soil cores, one from each chamber after in situ N2O flux measurement; these cores were pooled at the plot-level for measurements of soil chemistry and microbial process rates. Samples were transported back to the laboratory on ice and sieved through a 2 mm mesh screen. We dried a subset of each sample at 105 °C to determine gravimetric soil moisture and used a Costech ECS 4010 to quantify total % C and % N and the C to N molar ratio (C:N). To measure pH, we added 5 g of sieved soil to 25 ml of DI water and shook the solution on a shaker table at 200 rpm for 10 min. After allowing the mixture to equilibrate for 30 min, we measured pH using an Orion ROSS Ultra Refillable pH/ATC Triode.
To extract soil NH4+ and NO3−, we mixed 2.5 g of sieved soil with 25 ml of 2N KCl in 50 ml tubes. After vortexing for five seconds, we shook the tubes at 200 rpm for 30 min. After allowing the mixture to settle, we filtered the supernatant through Whatman 589/1 filters that were pre-rinsed with 2 M KCl followed by DI water and dried in an oven at 38 °C. We used colorimetric determination on a Gallery™ Discrete Analyzer to quantify KCl-extractable NO3− (by enzymatic reduction following Campbell et al. 2004) and NH4+ (EPA Method 350.1 Rev 2). Along with measuring soil-extractable NO3− and NH4+ at the time of collection, we measured N mineralization and net nitrification from the elevation plot samples by incubating field moist soil samples for 14 days at room temperature in flasks exposed to room air, covered with parafilm, while maintaining field moisture (Yavitt et al. 2018). After 14 days, we collected a second set of KCl-extractable NO3− and NH4+ samples. Nitrogen mineralization was calculated as the difference in the sum of NH4+ and NO3− concentrations between the two extractions, and net nitrification was the difference between NO3− concentrations in the first and second extractions. To estimate bulk density, we dried a soil core at 105 °C and divided dry mass by core volume (g/cm3).
Denitrification potential
We measured denitrification potential using the denitrification enzyme activity (DEA) assay method (Groffman et al. 1999), which uses acetylene gas to induce incomplete denitrification and stop the reaction at N2O. We placed 10 g of sieved, field-moist soil from each subplot in 125 ml Erlenmeyer flasks and amended soil samples with 20 ml of a solution containing glucose, KNO3, and chloramphenicol to provide excess C, excess NO3−, and to inhibit synthesis of new denitrifying enzymes, respectively. Flasks were sealed, evacuated of air, flushed with N2, and acetylene gas was added to the headspace. N2O concentrations from DEA assays were measured at 0, 30, 60, and 90 min, and production rate was calculated as the slope of the N2O accumulation in the headspace over time per gram of dry soil. With an acetylene block, the N2O measured is assumed to include both N2 that would be produced from complete denitrification and N2O from incomplete denitrification. We also conducted DEA assays using the same method but without adding acetylene for the 20 low plots and all side-channel samples. Without acetylene, the denitrification reaction can proceed to N2, and the only N2O measured is the product of incomplete denitrification. N2O yield is N2O produced by a DEA assay with no acetylene divided by N2O produced by a DEA assay on the same soil with acetylene (Granville et al. 2021). As with in situ flux samples, we measured N2O concentrations on the Perkin Elmer Clarus 580 gas chromatograph, calculated concentration differences to determine the fluxes that exceed the MDCD and considered samples with a linear accumulation with an r2 > 0.75 (94% of fluxes had an r2 > 0.85). For fluxes above the MDCD but with an r2 < 0.75, we recalculated the flux when we could improve the linear fit by dropping one or two anomalous samples. Of 58 total flux measurements, 10 were below MDCD and set to zero, and three did not meet the linear criteria and were excluded from analysis.
Denitrification enzyme activity assays do not measure actual denitrification in the field but rather create ideal conditions for denitrification in the laboratory. The anoxic environment of DEA assays inhibits nitrification and potentially underestimates denitrification in low-NO3− systems where linked nitrification–denitrification is important (Groffman 2006). Nevertheless, DEAs are a useful tool for comparing many samples across space or time (Groffman et al. 2006), but our application does not allow for accurate annual site-scale estimates of N removal.
Plant community metrics
In each elevation plot, we measured above and below ground biomass and used root porosity as an index of how the vegetation may impact redox conditions via rhizospheric oxidation (after McGill et al. 2010 and Sutton-Grier et al. 2011). To determine above ground biomass, we clipped all the vegetation at the soil surface in each 25 × 25 cm plot. To estimate below ground biomass, we washed sediment from roots and rhizomes from 196 cm3 cores over a 1-mm mesh sieve. All biomass was dried at 60 °C until constant mass. A subset of field fresh roots was used to estimate root porosity using the pycnometer method (Jensen et al. 1969).
Data analysis
Data analyses were conducted using R 4.1.2 (R Core Team 2022). We used analysis of variance (ANOVA) or Kruskal–Wallis tests (when parametric model assumptions were violated) to evaluate denitrification potential, soil chemistry parameters, and plant community traits between high vs. low elevations and seeps vs. non-seep locations. In the elevation plots, pH, species richness, and log-transformed gravimetric soil moisture met assumptions of normality and equal variance and were compared between low and high elevation plots using ANOVA. The remaining variables were compared with Kruskal–Wallis tests. In side-channel plots, we compared the pH, gravimetric soil moisture, C:N, NH4+ (µg N/g dry soil) (square root transformed), NO3− (µg N/g dry soil), and in situ N2O flux (µg N/m2/hr) of seeps and non-seeps using ANOVA.
For elevation plot data, we used Spearman rank comparison tests to test relationships between denitrification potential and soil and vegetation characteristics and 3 × 3 m plot-level vertical relief. We then included all characteristics with significant correlations in a generalized linear model with a quasipoisson distribution to examine a multi-parameter model for denitrification potential. For side-channel data, we used Spearman rank comparison tests to test relationships between denitrification potential and soil characteristics. To test for an interactive effect between soil moisture and NH4+ or NO3− in seeps vs. non-seeps, we used two generalized linear models with quasipoisson distributions. We used paired Wilcoxon tests to test the difference between denitrification potential and N2O production for the low elevation plots and the side-channel plots. We used a Friedman test to compare in situ N2O fluxes at seeps and non-seeps, and we compared fluxes with denitrification potential and soil chemistry parameters using Pearson correlation tests. We then included all characteristics with significant correlations to in situ flux as fixed effects in a linear mixed effects model with in situ flux as a response variable and block as a random effect.
We used the indicspecies package in R (De Cáceres et al. 2023) to conduct an indicator species analysis to identify plant species that were likely to occur in only high or low relative elevations. We also ran an indicator species analysis for denitrification potential, which required us to group plots into high, low, and zero denitrification potential categories. In this analysis, high denitrification potentials were greater than 10 ng N/hr/g dry soil. To evaluate the probability of associations with our a priori groups, we ran 999 random permutations using the multipatt function.
To further examine plant community composition between high and low plots, we used non-metric multidimensional scaling (NMDS) ordination to visualize trends in the species composition. We constructed a Bray–Curtis dissimilarity matrix for species percent cover (using mid-points of each cover class). We used the metaMDS function in the vegan package in R (Oksanen et al. 2020) with 50 randomized runs and three final axis iterations to determine a solution with a stress value < 0.15. To test for differences between plant communities among elevation groups, we completed a permutational multivariate analysis of variance (PERMANOVA) using the adonis2 function with 999 random permutations after using the betadisper function to ensure the two groups (high and low) had similar dispersions (Oksanen et al. 2020).
Results
Denitrification potential & N2O yield: microtopography
Denitrification potential was more than 2 × greater in soils collected from low (8.39 ± 2.70 ng N/hr/g dry soil) than high (3.40 ± 1.00 ng N/hr/g dry soil) elevations (Kruskal–Wallis (K–W) chi-squared (χ2) = 4.05, p-value = 0.04) (Fig. 2). N2O yield from low elevation soils averaged 0.86 (± 0.16); we did not calculate N2O yield for high elevation soils because denitrification potentials were very low. We did not detect differences in N2O production from DEA assays with (8.39 ± 2.70 ng N/hr/g dry soil) or without (7.40 ± 1.71 ng N/hr/g dry soil) an acetylene block, suggesting predominance of incomplete denitrification.
No other pool or flux of N differed between high and low elevations (Table 1). Across all plots, NO3− concentrations in collected soils were three orders of magnitude lower than soil NH4+, and NO3− was only detectable in 10% of samples while all samples had detectable NH4+. Net nitrification, which occurred in 31% of samples, was an order of magnitude lower than net mineralization, which occurred in 69% of samples. Of the 10 other soil and plant community metrics we quantified, only three differed among high and low elevations (Table 1). Gravimetric soil moisture (F = 9.74, p < 0.001) and pH (F = 4.15, p < 0.05) were greater in low than high elevations. Bulk density was greater at higher elevations (KW χ2 = 6.82, p-value = 0.009).
Plant species richness and Shannon diversity index were strongly positively correlated with each other (Spearman’s rho = 0.73, p < 0.0001) and had similar relationships with denitrification potential, so we only report species richness here. Denitrification potential was positively correlated with plant species richness and negatively correlated with net nitrification, although these relationships were driven by a relatively small number of plots (Fig. 3). The two plots with the highest denitrification potential did not have other notable differentiating characteristics. Both were close, but not the closest plots, to either a side channel or the main stem. No other soil or plant community metrics correlated with denitrification potential, nor did absolute elevation above sea level (m) or 3 × 3 m plot-level vertical relief.
Across high and low wetland elevations (a) Plant species richness (number of species per 625 cm2 subplot) was positively correlated with denitrification potential, and (b) net nitrification (μg N/g dry soil) was negatively correlated with denitrification potential, based on Spearman correlation tests
Although species richness did not differ between high and low elevations, plant community composition was distinct between elevations (F = 2.15, p = 0.001) (Fig. 4). Additionally, indicator species analysis identified two species associated with high elevations that were different from the five species associated with low elevations (Table 2). Neither high elevation indicator species were obligate wetland species (ACOE 2021), but all five low elevation indicator species were. We did not find indicator species of denitrification potential based on categorical groupings.
NMDS ordination of plant community composition (k = 3, stress = 0.13) using a Bray–Curtis distance matrix suggests separation between high and low elevations along axis 1. Each point represents the composition of one subplot; points closer together in ordination space have more similar species composition. Site centroids with one standard deviation are shown as ellipses and shaded by relative elevation. Vector represents direction of increasing denitrification potential within the ordination space
Restoration feature: groundwater seeps
Denitrification potential was more than 34 × higher in soils collected from seep (64.24 ± 24.04 ng N/hr/g dry soil) than paired non-seep locations (1.87 ± 0.67 ng N/hr/g dry soil) (K–W χ2 = 5.33, p = 0.02) (Fig. 5). Seeps also tended to have higher (but not significantly so) N2O yield 1.05 ± (0.15) than non-seeps (0.65 ± 0.37). Denitrification potential was positively correlated with soil moisture and pH, and both parameters were significantly higher in seeps (soil moisture: 0.37 ± 0.04, pH: 6.06 ± 0.15) than non-seeps (soil moisture: 0.11 ± 0.01, pH: 5.51 ± 0.12). Molar C:N of seeps (27.6 ± 4.2) and non-seeps (25.3 ± 1.9) did not differ and was not correlated with denitrification potential.
Soil-extractable NO3− did not differ between seeps (2.35 ± 0.57 µg N/g dry soil) and non-seeps (1.45 ± 0.32 µg N/g dry soil), but side-channel locations had higher NO3− than the high and low elevations (KW χ2 = 31.57, p < 0.0001). Seeps (4.15 ± 0.35 µg N/g dry soil) had nearly 3 × the soil NH4+ of non-seeps (1.40 ± 0.14 µg N/g dry soil) (F = 52.38, p < 0.0001). Non-seeps had lower soil NH4+ than the high and low elevations on the former bog platform (F = 6.44, p < 0.01). NH4+ in side-channel soils was also positively correlated with denitrification potential (Fig. 6), and soil NO3− was marginally positively correlated with denitrification potential (Spearman’s rho = 0.67, p < 0.09). In a model including soil moisture, NO3−, NH4+, and pH as explanatory variables, only NH4+ was a significant predictor of denitrification potential (t = 4.05, p < 0.03), and we saw no interaction between soil moisture and NO3− or NH4+.
In situ N2O emissions
We observed very limited in situ N2O emissions on the former bog platform, regardless of topographic position. Of the 40 high and low elevation locations from which we sampled N2O emissions, six were excluded from analysis. Only four (12%) locations had N2O emissions, one from a high and three from low elevations. Only two of the four locations with N2O emissions had detectable denitrification potentials in our laboratory assays (5.40 and 15.60 ng N/hr/g dry soil), and both locations had high N2O yield. Further analysis of relationships among in situ flux, denitrification potential, and plant community traits was not possible.
In situ N2O emissions were also low in seep and non-seep locations, but N2O emissions were detected in 76% of seep and non-seep locations. Eight of the 13 (61.5%) detectable N2O fluxes were in seeps. Despite mean fluxes that were more than 10 × greater (and median fluxes 5 × greater) from seeps (mean: 51.18 ± 16.96 µg-N/m2/hr; median: 20.97 µg-N/m2/hr) than non-seeps (mean: 4.90 ± 2.41 µg-N/m2/hr; median: 4.20 µg-N/m2/hr), they were only marginally different from each other (Friedman chi-squared = 3, p = 0.08). We observed spatial heterogeneity in N2O fluxes, suggesting very localized emissions. At three seeps, two or three replicate chambers had significant N2O emissions. At all four non-seeps, we detected N2O emissions in one of the three replicate chambers.
There was a positive correlation between in situ N2O emissions and denitrification potential (Spearman’s rho = 0.67, p < 0.02), soil NH4+ (Spearman’s rho = 0.93, p < 0.003), and soil moisture (Spearman’s rho = 0.93, p < 0.003). When combined in a linear mixed effects model, though, only soil moisture was a significant predictor of in situ emissions. Soil temperature during in situ flux sampling was not significantly correlated with fluxes, and because soil temperature was negatively correlated with soil moisture (R2 = − 0.73, p < 0.05), we did not include it as a covariate in the model.
Discussion
Removing excess N via denitrification is a common goal of wetland restoration, but understanding patterns of denitrification is challenging because it is a spatially and temporally heterogeneous process that requires specific conditions, often leading to hot spots and hot moments (McClain et al. 2003). We examined the relationship between design elements, including microtopography, vegetation, and groundwater connectivity, and N removal via denitrification to advance the practice of wetland restoration of former agricultural cranberry bogs. Our data showed that while relative topographic position and plant communities were related to denitrification potential across the bog platform, potential denitrification rates at groundwater seeps were an order of magnitude higher, likely because of high NO3− availability in discharging groundwater. Groundwater seeps were hot spots for N removal via denitrification (i.e., they had substantially higher potential denitrification rates relative to the surrounding matrix), but potential denitrification rates were still low relative to reference wetlands in the region (Ballantine et al. 2017) and appeared to be constrained by other factors, including limited organic C availability in sandy surface soils.
Linkages between microtopography, vegetation, and denitrification potential
Created microtopography in restored wetlands can result in soil conditions that increase denitrification potential (Wolf et al. 2011b) but creating microtopography is resource intensive. We found that lower elevations had more than 2 × greater denitrification potential and were wetter than high elevations, but we found no relationship between 3 × 3 m plot-scale vertical relief and denitrification potential, so the optimal magnitude of topographic variation to promote N removal via denitrification in restoration design is still unclear. Soil moisture at the time of soil collection was not directly correlated with denitrification potential, but greater cover of wetland obligate species at low elevations strongly suggested that soil was more frequently inundated at lower elevations.
Despite elevated NO3− in groundwater of the Coonamessett River watershed (Valiela et al. 1990), we found low extractable soil NO3− concentrations and relatively low rates of potential denitrification across the bog platform that indicated groundwater was not discharging at these locations. Soil-extractable NO3− was below detection in 88% of our samples, which is common in wetlands due to high plant uptake during the growing season and reduced conditions that favor denitrification and limit nitrification (Jacinthe et al. 2012; Helton et al. 2015). At other restored cranberry bogs, high NO3− concentrations in groundwater were localized and only detected at groundwater springs or seeps (Redder et al. 2021; Watts et al. 2023). Similarly, we found soil collected from seeps had higher NO3− concentrations (> 260×) and denitrification potentials (> 7×) than soils from the bog platform, indicating that seeps were denitrification hot spots where localized NO3− availability led to higher potential for denitrification.
Plant communities of restored cranberry bogs may be useful indicators of denitrification. Although the correlation was noisy, we found plant species richness to be positively correlated with denitrification potential. While we cannot definitively separate the effect of species richness from other environmental factors, species richness was not highly correlated to the abiotic factors we measured. We could not determine whether species assemblages worked together to enhance denitrification (complementarity) or if the presence of more species made the presence of specific species that enhance denitrification more likely (selection), but we did not find any species that were significant indicators of high denitrification potential. In a meta-analysis of biodiversity and ecosystem functioning, Brisson et al. (2020) also saw a positive relationship between species richness and denitrification, and they found more evidence of selection than complementarity. In the absence of evidence of specific species effects on denitrification, species richness is a relatively easy characteristic to measure, making it a potentially useful indicator of denitrification in restored wetlands.
The presence of Leersia oryzoides is an example of a species that might enhance denitrification; it is an abundant early successional wetland species (Farnsworth and Meyerson 2003) associated with higher denitrification potential in agricultural ditches (Tyler et al. 2012, Taylor et al. 2015; Speir et al. 2017) and was indicative of low elevations in our restored wetland. Our soils had uniformly low C levels (all but three samples < 3% C) and no relationship between soil C and L. oryzoides; however, L. oryzoides has high leaf biomass turnover that may increase supplies of organic C for denitrifying bacteria (Weisner et al. 1994; Farnsworth and Meyerson 2003), suggesting that species that rapidly increase soil organic matter immediately post-restoration may enhance denitrification.
High N2O yield but low in situ N2O emissions
N2O yield (N2O:(N2O + N2)) both from bog platform and side-channel plots ranged between 0.6 and 1.0, suggesting a large portion of in situ denitrification was incomplete and produced N2O rather than N2. Our soils had pH levels on the low end of the optimal range (5.5–6) for denitrification, which may have promoted the relatively high N2O yields (Liu et al. 2010), yet we found no correlation between pH and N2O yield. Across wetland systems, water table height is a driver of N2O emissions with higher emissions occurring during drier periods (Zou et al. 2022). It is possible that soil oxygen in wetter locations was low enough for incomplete denitrification but too high for complete denitrification, resulting in high N2O yields (Wrage et al. 2001), though limitations associated with DEA methodology preclude our ability to make conclusions about variability in soil hypoxia. Although we did not detect elevated levels of net nitrification, N2O as a byproduct of nitrification is another plausible explanation for some N2O production as lower elevations had much more soil NH4+ than NO3−, and seeps also had comparable NH4+ concentrations.
In situ N2O emissions told a different story than N2O yield. Although potential differences in abiotic conditions between years limited interpretation of in situ N2O emissions from a single sampling campaign, our data suggest N2O emissions varied among restoration features. Where in situ N2O emissions were detectable, particularly at seeps, they were similar in magnitude to those from undisturbed riparian wetlands in agricultural catchments and salt marshes (Audet et al. 2014; Rosentreter et al. 2021). We are unaware of other estimates of in situ emissions from former or restored cranberry farms. Groundwater seeps comprise a relatively small amount of the wetland area, and emissions were generally low or undetected away from the seeps. There are limitations of extrapolation from one site, but many cranberry bogs that have been restored or are in the planning phases have similar groundwater chemistry and restoration design. Additional sampling across space and time would be helpful to augment our initial finding that restoring wetlands on former cranberry bogs does not result in large increases in N2O emissions.
Patterns of denitrification in situ over space and time
We quantified how microbial activity, soil chemistry, and vegetation varied across space, yet short-term temporal trends in denitrification may also be important. Denitrification rates can vary within (Granville et al. 2021) and between (Wang et al. 2018) seasons, but we expect that groundwater seep locations are likely to remain hot spots over time, though the magnitude of N removal may fluctuate.
We measured denitrification potential and in situ N2O emissions in August during the second and third growing seasons after restoration. Wetland restoration can increase denitrification potential, but soil functions are often slow to develop, and rates rarely return to reference wetland levels in the short-term (Moreno-Mateos et al. 2012, Moreno-Mateos et al. 2015, Ballentine et al. 2017). Investigating short-term N removal response is valuable for understanding drivers of restoration trajectories, but longer-term monitoring is needed to inform rates of recovery.
Although soils at this site mostly had higher NH4+ than NO3− and may be candidates for more linked nitrification–denitrification than we detected, we detected minimal nitrification, including in locations with relatively high NH4+ and low soil moisture. Low nitrification rates were also measured in other active, retired, and restored cranberry bogs (Stackpoole et al. 2008; Ballantine et al. 2017; Kennedy et al. 2020). At sites such as the Coonamessett River where managers are interested in the potential of restored wetlands to attenuate N, mechanistic studies based on DEAs could be paired with mass balance studies to quantify N loads above and below restored wetlands.
Restoration design and N removal
While accurate predictions of N removal via denitrification at our study site were not possible given the constraints of our methods (DEAs) and sampling design, we can use our measured rates on the former bog platform and at the seeps to estimate the order of magnitude of N removal on the bogs. At our study site, the stream area (main channel and side channels with 2-m buffers) occupied ~ 10% of the total restored area. If we assume that 10–50% of the stream area (1–5% of total area) has the denitrification capacity of seeps, it accounted for 13–40% of total potential denitrification in the restored area. Estimated N removal via denitrification of 14–20 kg N/yr was, however, much less than 1% of the inorganic N exported (~ 6,000 kgN/yr) through the restored reach of the Coonamessett River, estimated from surface water NH4+ and NO3− concentrations and river discharge (Neill 2022). Even if the entire site had the denitrification potential rate of seeps, total N removal by denitrification would be less than 5% of exported inorganic N. Denitrification potentials measured from seeps were also an order of magnitude lower than those measured by Ballantine et al. (2017) in a wetland with organic-rich soils that served as a natural reference to restored cranberry bogs (1342 ± 331 ng N/hr/g dry soil), so it is possible that denitrification in the restored wetlands will increase over time. Although denitrification occurs in locations other than the platform and at groundwater seeps, the data suggest that cranberry bog wetland restoration alone, even when located in an area of active groundwater seepage, is likely to remove only a relatively small portion of total watershed N loading.
In cranberry bog restoration sites more generally, increasing the area of seeps may make incremental gains in N removal via denitrification, but dramatically increasing denitrification in watersheds with high-NO3− groundwater might require changes in restoration design. Creating lower elevation microsites, particularly those with higher species richness and contact with groundwater, could help produce anoxic locations for accumulation of organic matter. While there are still questions about how denitrification might increase over time as wetland soils accumulate more organic matter, incorporating design elements that intersect groundwater flow paths, substantially increase residence time of discharged groundwater, and increase surface area of soil-groundwater interaction by distributing water outside of principal stream channels could be viable strategies to increase N removal. At the same time, these design features would likely be at odds with creating fish habitat and lowering stream temperature. Increasing the area of rewetted, anoxic soil could also increase CH4 emissions, and a recently restored cranberry bog had elevated CH4 emissions relative to an older restored site, an unrestored, retired farm, and a reference wetland (Bartolucci et al. 2021). A meta-analysis by Zou et al (2022) found, though, that keeping the water table of rewetted wetlands near the soil surface minimized CH4 and N2O emissions. Both coastal and inland wetland restorations commonly have N removal goals, and better understanding how restoration features affect denitrification can help us learn about recovery trajectories of biogeochemical cycles and inform future restoration design.
Data availability
All data used in this manuscript will be publicly available upon acceptance in the Zenodo database.
References
Alldred M, Baines SB (2016) Effects of Wetland plants on denitrification rates: a meta-analysis. Ecol Appl 26:676–685. https://doi.org/10.1890/14-1525/suppinfo
Audet J, Hoffmann CC, Andersen PM et al (2014) Nitrous oxide fluxes in undisturbed riparian wetlands located in agricultural catchments: emission, uptake and controlling factors. Soil Biol Biochem 68:291–299. https://doi.org/10.1016/j.soilbio.2013.10.011
Audet J, Olsen TM, Elsborg T et al (2021) Influence of plant habitats on denitrification in lowland agricultural streams. J Environ Manag. https://doi.org/10.1016/j.jenvman.2021.112193
Bachand PAM, Horne AJ (2000) Denitrification in constructed free-water surface wetlands: II. Effects of vegetation and temperature. Ecol Eng 14:17–32
Ballantine KA, Anderson TR, Pierce EA, Groffman PM (2017) Restoration of denitrification in agricultural wetlands. Ecol Eng 106:570–577. https://doi.org/10.1016/j.ecoleng.2017.06.033
Bartolucci NN, Anderson TR, Ballantine KA (2021) Restoration of retired agricultural land to wetland mitigates greenhouse gas emissions. Restor Ecol 29:1–9. https://doi.org/10.1111/rec.13314
Bastviken SK, Eriksson PG, Premrov A, Tonderski K (2005) Potential denitrification in wetland sediments with different plant species detritus. Ecol Eng 25:183–190. https://doi.org/10.1016/j.ecoleng.2005.04.013
Bernhardt ES, Blaszczak JR, Ficken CD et al (2017) Control points in ecosystems: moving beyond the hot spot hot moment concept. Ecosystems 20:665–682. https://doi.org/10.1007/s10021-016-0103-y
Braun-Blanquet J (1932) Plant sociology: the study of plant communities, 1st edn. McGraw-Hill, London
Bricker SB, Longstaff B, Dennison W et al (2008) Effects of nutrient enrichment in the nation’s estuaries: a decade of change. Harmful Algae 8:21–32. https://doi.org/10.1016/j.hal.2008.08.028
Brinson MM, Bradshaw DH, Kane ES (1984) Nutrient assimilative capacity of an alluvial floodplain swamp. J Appl Ecol 21:1041–1057
Brisson J, Rodriguez M, Martin CA, Proulx R (2020) Plant diversity effect on water quality in wetlands: a meta-analysis based on experimental systems. Ecol Appl 30:1–12. https://doi.org/10.1002/eap.2074
Campbell WH, Kinnunen-Skidmore T, Brodeur-Campbell MJ, Campbell ER (2004) New and improved nitrate reductase for enzymatic nitrate analysis. Am Lab 36:12–12
Cheng FY, Van Meter KJ, Byrnes DK, Basu NB (2020) Maximizing US nitrate removal through wetland protection and restoration. Nature 588:625–630. https://doi.org/10.1038/s41586-020-03042-5
Chmura GL, Kellman L, Van Ardenne L, Guntenspergen GR (2016) Greenhouse gas fluxes from salt marshes exposed to chronic nutrient enrichment. PLoS ONE 11:1–13. https://doi.org/10.1371/journal.pone.0149937
De Cáceres M, Jansen F, Dell N (2023) Associations between species and groups of sites: indices and statistical inference. Ecology 90(12):3566–3574
Dee MM, Tank JL (2020) Inundation time mediates denitrification end products and carbon limitation in constructed floodplains of an agricultural stream. Biogeochemistry 149:141–158. https://doi.org/10.1007/s10533-020-00670-x
Doroski AA, Helton AM, Vadas TM (2019) Denitrification potential and carbon mineralization in restored and unrestored coastal wetland soils across an urban landscape. Wetlands. https://doi.org/10.1007/s13157-019-01128-z
Farnsworth EJ, Meyerson LA (2003) Comparative ecophysiology of four wetland plant species along a continuum of invasiveness. Wetlands 23:750–762. https://doi.org/10.1672/0277-5212(2003)023[0750:CEOFWP]2.0.CO;2
Fornara DA, Tilman D (2008) Plant functional composition influences rates of soil carbon and nitrogen accumulation. J Ecol 96:314–322. https://doi.org/10.1111/j.1365-2745.2007.01345.x
Frei S, Knorr KH, Peiffer S, Fleckenstein JH (2012) Surface micro-topography causes hot spots of biogeochemical activity in wetland systems: a virtual modeling experiment. J Geophys Res Biogeosci 117:1–18. https://doi.org/10.1029/2012JG002012
Granville KE, Ooi SK, Koenig LE et al (2021) Seasonal patterns of denitrification and N2O production in a southern New England salt marsh. Wetlands. https://doi.org/10.1007/s13157-021-01393-x
Groffman PM, Holland E, Myrold DD et al (1999) Denitrification. In: Roberston GP, Coleman DC, Sollins P, Bledsoe CS (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 272–288
Groffman PM, Altabet MA, Bohlke JK et al (2006) Methods for measuring denitrification: diverse approaches to a difficult problem. Ecol Appl 16:2091–2122
Hare DK, Briggs MA, Rosenberry DO et al (2015) A comparison of thermal infrared to fiber-optic distributed temperature sensing for evaluation of groundwater discharge to surface water. J Hydrol 530:153–166. https://doi.org/10.1016/j.jhydrol.2015.09.059
Harvey MC, Hare DK, Hackman A et al (2019) Evaluation of stream and wetland restoration using UAS-based thermal infrared mapping. Water (switzerland) 11:1–13. https://doi.org/10.3390/w11081568
Hayashi M, Rosenberry DO (2002) Effects of ground water exchange on the hydrology and ecology of surface water. Ground Water 4:309–316
He T, Ding W, Cheng X et al (2024) Meta-analysis shows the impacts of ecological restoration on greenhouse gas emissions. Nat Commun 15:1–14. https://doi.org/10.1038/s41467-024-46991-5
Helton AM, Ardón M, Bernhardt ES (2015) Thermodynamic constraints on the utility of ecological stoichiometry for explaining global biogeochemical patterns. Ecol Lett 18:1049–1056. https://doi.org/10.1111/ele.12487
Helton AM, Ardón M, Bernhardt ES (2019) Hydrologic context alters greenhouse gas feedbacks of coastal wetland salinization. Ecosystems 22:1108–1125. https://doi.org/10.1007/s10021-018-0325-2
Hoekstra BR, Neill C, Kennedy CD (2019) Trends in the Massachusetts cranberry industry create opportunities for the restoration of cultivated riparian wetlands. Restor Ecol. https://doi.org/10.1111/rec.13037
Holland E, Roberston GP, Greenberg J et al (1999) Soil CO2, N2O, CH4 exchange. In: Roberston GP, Coleman DC, Bledsoe CS, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 185–201
Howes B, Ramsey JS, Kelley SW et al (2005) Linked watershed-embayment model to determine critical nitrogen loading thresholds for great/perch pond, green pond, and Bournes pong, Falmouth. Massachusetts, Boston
Jacinthe PA, Bills JS, Tedesco LP, Barr RC (2012) Nitrous oxide emission from riparian buffers in relation to vegetation and flood frequency. J Environ Qual 41:95–105. https://doi.org/10.2134/jeq2011.0308
Jensen CR, Luxmoore RJ, VanGundy SD, Stolzy LH (1969) Root air space measurements by a pycnometer method1. Agron J 61:474–475. https://doi.org/10.2134/agronj1969.00021962006100030045x
Kennedy CD, Buda AR, Bryant RB (2020) Amounts, forms, and management of nitrogen and phosphorus export from agricultural peatlands. Hydrol Process 34:1768–1781. https://doi.org/10.1002/hyp.13671
Lake PS, Bond N, Reich P (2007) Linking ecological theory with stream restoration. Freshw Biol 52:597–615. https://doi.org/10.1111/j.1365-2427.2006.01709.x
Larkin D, Vivian-smith G, Zedler JB (2006) Topographic heterogeneity theory and ecological restoration. In: Falk DA, Palmer MA, Zedler JB (eds) Foundations of restoration ecology. Island Press, Washington, D.C., pp 142–164
Liu B, Mørkved PT, Frostegård Å, Bakken LR (2010) Denitrification gene pools, transcription and kinetics of NO, N2O and N2 production as affected by soil pH. FEMS Microbiol Ecol 72:407–417. https://doi.org/10.1111/j.1574-6941.2010.00856.x
Living Observatory (2020) Learning from the restoration of wetlands on cranberry farmland: preliminary benefits assessment. Plymouth, MA
Malique F, Ke P, Boettcher J et al (2019) Plant and soil effects on denitrification potential in agricultural soils. Plant Soil 439:459–474. https://doi.org/10.1007/s11104-019-04038-5
Massachusetts Department of Agricultural Resources (2016) The Massachusetts cranberry revitalization task force final report
McClain ME, Boyer EW, Dent CL et al (2003) Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6:301–312. https://doi.org/10.1007/s10021-003-0161-9
McCobb TD, Barbaro JR, LeBlanc DR, and Belaval M (2021) Evaluating the effects of replacing septic systems with municipal sewers on groundwater quality in a densely developed coastal neighborhood, Falmouth, Massachusetts, 2016–19: U.S. Geological Survey Scientific Investigations Report 2021–5130, p 39, https://doi.org/10.3133/sir20215130.
McGill BM, Sutton-Grier AE, Wright JP (2010) Plant trait diversity buffers variability in denitrification potential over changes in season and soil conditions. PLoS ONE. https://doi.org/10.1371/journal.pone.0011618
Montoya D, Rogers L, Memmott J (2012) Emerging perspectives in the restoration of biodiversity-based ecosystem services. Trends Ecol Evol 27:666–672. https://doi.org/10.1016/j.tree.2012.07.004
Moreno-Mateos D, Power ME, Comín FA, Yockteng R (2012) Structural and functional loss in restored wetland ecosystems. PLoS Biol. https://doi.org/10.1371/journal.pbio.1001247
Moreno-Mateos D, Meli P, Vara-Rodríguez MI, Aronson J (2015) Ecosystem response to interventions: lessons from restored and created wetland ecosystems. J Appl Ecol 52:1528–1537. https://doi.org/10.1111/1365-2664.12518
Moseman-Valtierra S, Gonzalez R, Kroeger KD et al (2011) Short-term nitrogen additions can shift a coastal wetland from a sink to a source of N2O. Atmos Environ 45:4390–4397. https://doi.org/10.1016/j.atmosenv.2011.05.046
Neill C (2022) Data report on nitrogen monitoring and dynamics of the Coonamessett River, May 2018 to April 2022. Report to the Town of Falmouth Water Quality Management Committee. December 22, 2023.
Nilsson JE, Liess A, Ehde PM, Weisner SEB (2020) Mature wetland ecosystems remove nitrogen equally well regardless of initial planting. Sci Total Environ 716:1–6. https://doi.org/10.1016/j.scitotenv.2020.137002
NRCS (2016) Ground penetrating radar (GPR) survey of Upper and Middle Coonamessett River Bogs in Falmouth, MA. West Wareham, MA
Oksanen AJ, Blanchet FG, Friendly M, et al (2020) vegan: Community Ecology Package
Ooi SK, Barry A, Lawrence BA et al (2022) Vegetation zones as indicators of denitrification potential in salt marshes. Ecol Appl 32:1–16. https://doi.org/10.1002/eap.2630
R Core Team (2022) R: a language and environment for statistical computing
Redder BW, Kennedy CD, Buda AR et al (2021) Groundwater contributions of flow and nitrogen in a headwater agricultural watershed. Hydrol Process 35:1–15. https://doi.org/10.1002/hyp.14179
Reddy KR, Patrick WH (1984) Nitrogen transformations and loss in flooded soils and sediments. Crit Rev Environ Control 13:273–309
Rosentreter JA, Al-Haj AN, Fulweiler RW, Williamson P (2021) Methane and nitrous oxide emissions complicate coastal blue carbon assessments. Global Biogeochem Cycles 35:1–8. https://doi.org/10.1029/2020GB006858
Sandler H, DeMoranville C (2008) Cranberry production: a guide for Massachusetts. UMass Amherst Cranberry Stn 37
Saunders DL, Kalff J (2001) Nitrogen retention in wetlands, lakes and rivers. Hydrobiologia 443:205–212. https://doi.org/10.1023/A:1017506914063
Speir SL, Taylor JM, Scott JT (2017) Seasonal differences in relationships between nitrate concentration and denitrification rates in ditch sediments vegetated with rice cutgrass. J Environ Qual 46:1500–1509. https://doi.org/10.2134/jeq2016.11.0450
Stackpoole SM, Workmaster BAA, Jackson RD, Kosola KR (2008) Nitrogen conservation strategies of cranberry plants and ericoid mycorrhizal fungi in an agroecosystem. Soil Biol Biochem 40:2736–2742. https://doi.org/10.1016/j.soilbio.2008.07.017
Sutton-Grier AE, Wright JP, McGill BM, Richardson C (2011) Environmental conditions influence the plant functional diversity effect on potential denitrification. PLoS ONE 6:1–9. https://doi.org/10.1371/journal.pone.0016584
Taylor JM, Moore MT, Scott JT (2015) Contrasting nutrient mitigation and denitrification potential of agricultural drainage environments with different emergent aquatic macrophytes. J Environ Qual 44:1304–1314. https://doi.org/10.2134/jeq2014.10.0448
Tyler HL, Moore MT, Locke MA (2012) Influence of three aquatic macrophytes on mitigation of nitrogen species from agricultural runoff. Water Air Soil Pollut 223:3227–3236. https://doi.org/10.1007/s11270-012-1104-x
United States Army Corps of Engineers (2021) National Wetland Plant List, version 3.4. Hanover, NH.
Valiela I, Costa J, Foreman K et al (1990) Transport of groundwater-borne nutrients from watersheds and their effects on coastal waters. Biogeochemistry 10:177–197
Walker DA, Daniëls FJA, Matveyeva NV et al (2018) Circumpolar arctic vegetation classification. Phytocoenologia 48:181–201. https://doi.org/10.1127/phyto/2017/0192
Wang X, Hu M, Ren H et al (2018) Seasonal variations of nitrous oxide fluxes and soil denitrification rates in subtropical freshwater and brackish tidal marshes of the Min River estuary. Sci Total Environ 616–617:1404–1413. https://doi.org/10.1016/j.scitotenv.2017.10.175
Watts CL, Hatch CE, Wicks R (2023) Mapping groundwater discharge seeps by thermal UAS imaging on a wetland restoration site. Front Environ Sci 10:1–16. https://doi.org/10.3389/fenvs.2022.946565
Weisner SEB, Eriksson PG, Graneli W, Leonardson L (1994) Influence of macrophytes on nitrate removal in wetlands. Ambio 23:363–366
Wilson D, Farrell CA, Fallon D et al (2016) Multiyear greenhouse gas balances at a rewetted temperate peatland. Glob Chang Biol 22:4080–4095. https://doi.org/10.1111/gcb.13325
Wolf KL, Ahn C, Noe GB (2011a) Development of soil properties and nitrogen cycling in created wetlands. Wetlands 31:699–712. https://doi.org/10.1007/s13157-011-0185-4
Wolf KL, Ahn C, Noe GB (2011b) Microtopography enhances nitrogen cycling and removal in created mitigation wetlands. Ecol Eng 37:1398–1406. https://doi.org/10.1016/j.ecoleng.2011.03.013
Wrage N, Velthof GL, Van Beusichem ML, Oenema O (2001) Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem 33:1723–1732. https://doi.org/10.1016/S0038-0717(01)00096-7
Yates TT, Si BC, Farrell RE, Pennock DJ (2006) Probability distribution and spatial dependence of nitrous oxide emission. Soil Sci Soc Am J 70:753–762. https://doi.org/10.2136/sssaj2005.0214
Yavitt JB, Burtis JC, Smemo KA, Welsch M (2018) Plot-scale spatial variability of methane, respiration, and net nitrogen mineralization in muck-soil wetlands across a land use gradient. Geoderma 315:11–19. https://doi.org/10.1016/j.geoderma.2017.11.038
Zedler JB (2000) Progress in wetland restoration ecology. Trends Ecol Evol 15:402–407. https://doi.org/10.1016/S0169-5347(00)01959-5
Zirbel CR, Bassett T, Grman E, Brudvig LA (2017) Plant functional traits and environmental conditions shape community assembly and ecosystem functioning during restoration. J Appl Ecol 54:1070–1079. https://doi.org/10.1111/1365-2664.12885
Zou J, Ziegler AD, Chen D et al (2022) Rewetting global wetlands effectively reduces major greenhouse gas emissions. Nat Geosci 15:627–632. https://doi.org/10.1038/s41561-022-00989-0
Acknowledgements
We thank Mark Asch, Madeline Kollegger, Andrew Tienken, and Nicholas Nelson for their assistance in the field and laboratory. We thank two anonymous reviewers for feedback that helped improve the manuscript. Inter-Fluve, Inc. provided restoration design and planting plans as well as access to a RTK-GPS unit. We thank the Town of Falmouth, MA for permitting research activities on the restored Coonamessett River Bogs. This work was supported by the USDA NIFA Hatch program (grant #1020626) as well as the Garden Club of America Fellowship in Ecological Restoration and the Phipps Conservancy and Botanical Gardens Botany-in-Action Fellowship.
Funding
This work was supported by the USDA NIFA Hatch program (grant #1020626) as well as the Garden Club of America Fellowship in Ecological Restoration and the Phipps Conservancy and Botanical Gardens Botany-in-Action Fellowship.
Author information
Authors and Affiliations
Contributions
All authors, Sarah M. Klionsky, Christopher Neill, Ashley M. Helton, and Beth Lawrence, contributed to the study conception and design. Material preparation, data collection and analysis were performed by Sarah M. Klionsky, Beth Lawrence, and Ashley M. Helton. The first draft of the manuscript was written by Sarah M. Klionsky, and all authors commented on multiple versions of the manuscript. All authors read and approved the final manuscript. Beth Lawrence and Christopher Neill were co-Principal Investigators on the USDA NIFA Hatch grant.
Corresponding author
Ethics declarations
Competing interests
SMK occasionally works on an hourly basis for Inter-Fluve, Inc., the ecological engineering firm that designed the wetland restoration project studied here, but SMK received no compensation for work on the study site.
Additional information
Responsible Editor: Ishi Buffam.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klionsky, S.M., Neill, C., Helton, A.M. et al. Groundwater seeps are hot spots of denitrification and N2O emissions in a restored wetland. Biogeochemistry (2024). https://doi.org/10.1007/s10533-024-01156-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10533-024-01156-w