Abstract
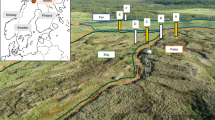
Iron (oxyhydr)oxides strongly adsorb phosphate and limit its bioavailability, but interactions between phosphate and various Fe (oxyhydr)oxides are poorly constrained in natural systems. An in-situ incubation experiment was conducted to explore Fe (oxyhydr)oxide transformation and effects on phosphate sorption in soils with contrasting saturation and redox conditions. Synthetic Fe (oxyhydr)oxides (ferrihydrite, goethite and hematite) were coated onto quartz sand and either pre-sorbed with phosphate or left phosphate-free. The oxide-coated sands were mixed with natural organic matter, enclosed in mesh bags, and buried in and around a vernal pond for up to 12 weeks. Redox conditions were stable and oxic in the upland soils surrounding the vernal pond but largely shifted from Fe reducing to Fe oxidizing in the lowland soils within the vernal pond as it dried during the summer. Iron (oxyhydr)oxides lost more Fe (− 41% ± 10%) and P (− 43 ± 11%) when incubated in the redox-dynamic lowlands compared to the uplands (− 18% ± 5% Fe and − 24 ± 8% P). Averaged across both uplands and lowlands, Fe losses from crystalline goethite and hematite (− 38% ± 6%) were unexpectedly higher than losses from short range ordered ferrihydrite (− 12% ± 10%). We attribute losses of Fe and associated P from goethite and hematite to colloid detachment and dispersion but losses from ferrihydrite to reductive dissolution. Iron losses were partially offset by retention of solubilized Fe as organic-bound Fe(III). Iron (oxyhydr)oxides that persisted during the incubation retained or even gained P, indicating low amounts of phosphate sorption from solution. These results demonstrate that hydrologic variability and Fe (oxyhydr)oxide mineralogy impact Fe mobilization pathways that may regulate phosphate bioavailability.






Similar content being viewed by others
Data availability
All datasets used in this manuscript are provided in tables in the main text or supporting information.
References
Aiken GR, Hsu-Kim H, Ryan JN (2011) Influence of dissolved organic matter on the environmental fate of metals, nanoparticles, and colloids. Environ Sci Technol 45(8):3196–3201
Ajmal Z, Muhmood A, Usman M, Kizito S, Lu J, Dong R, Wu S (2018) Phosphate removal from aqueous solution using iron oxides: Adsorption, desorption and regeneration characteristics. J Colloid Interf Sci 528:145–155
Bache BW, Williams EG (1971) A phosphate sorption index for soils. J Soil Sci 22:289–301
Balint R, Said-Pullicino D, Ajmone-Marsan F (2015) Copper dynamics under alternating redox conditions is influenced by soil properties and contamination source. J Contam Hydrol 173:83–91
Barczok M, Smith C, Di Domenico N, Kinsman-Costello L, Herndon E (2023) Variability in soil redox response to seasonal flooding in a vernal pond. Front Environ Sci Biogeochem Dyn. https://doi.org/10.3389/fenvs.2023.1114814
Bhattacharyya A, Schmidt MP, Stavitski E, Martínez CE (2018) Iron speciation in peats: chemical and spectroscopic evidence for the co-occurrence of ferric and ferrous iron in organic complexes and mineral precipitates. Org Geochem 115:124–137
Blackwood CB, Smemo KA, Kershner MW, Feinstein LM, Valverde-Barrantes OJ (2013) Dexay of ecosystem differences and decoupling of tree community-soil environment relationships at ecotones. Ecol Monogr 83(3):403–417
Borch T, Masue Y, Kukkadapu RK, Fendorf S (2007) Phosphate imposed limitations on biological reduction and alteration of ferrihydrite. Environ Sci Technol 41:166–172
Brooks SC, Taylor DL, Jardine PM (1996) Reactive transport of EDTA-complexed cobalt in the presence of ferrihydrite. Geochim Cosmochim Acta 60:1899–1908
Bryce C, Blackwell N, Schmidt C, Otte J, Huang Y, Kleindienst S, Tomaszewski E, Schad M, Warter V, Peng C, Byrne JM, Kappler A (2018) Microbial anaerobic Fe(II) oxidation—ecology, mechanisms and environmental implications. Environ Microbiol 20(10):3462–3483
Caraco N, Cole J, Likens GE (1990) A comparison of phosphate immobilization in sediments of freshwater and coastal marine systems. Biogeochemistry 9:277–290
Chacón N, Silver WL, Dubinsky EA, Cusack DF (2006) Iron reduction and soil phosphorus solubilization in humid tropical forests soils: the roles of labile carbon pools and an electron shuttle compound. Biogeochemistry 78:67–84
Chapin FS, Matson PA, Mooney HA, Vitousek PM (2002) Principles of terrestrial ecosystem ecology. Springer, New York
Chen C, Thompson A (2018) Ferrous iron oxidation under varying pO2 levels: the effect of Fe(III)/Al(III) oxide minerals and organic matter. Environ Sci Technol 52:597–606
Colombo C, Palumbo G, He J-Z, Pinton R, Cesco S (2014) Review on iron availability in soil: interaction of Fe minerals, plants, and microbes. J Soil Sediment 14(3):538–548
Cornell RM, Schwertmann U (2003) The iron oxides: structure, properties, reactions, occurrences and uses. Wiley, Weinheim
Coward EK, Thompson AT, Plante AF (2017) Iron-mediated mineralogical control of organic matter accumulation in tropical soils. Geoderma 306:206–216
Essington ME (2015) Soil and water chemistry: an integrative approach. CRC Press, Boca Raton
Gálvez N, Barrón V, Torrent J (1999) Effect of phosphate on the crystallization of hematite, goethite, and lepidocrocite from ferrihydrite. Clay Clay Miner 47(3):304–311
Giannetta B, Plaza C, Siebecker MG, Aquilanti G, Vischetti C, Plaisier JR, Juanco M, Sparks DL, Zaccone C (2020) Iron speciation in organic matter fractions isolated from soils amended with biochar and organic fertilizers. Environ Sci Technol 54:5093–5101
Gerke J, Hermann R (1992) Adsorption of orthophosphate to humic–Fe-complexes and to amorphous Fe-oxide. Zeitschrift Für Pflanzenernährung Und Bodenkunde 155(3):233–236
Gu C, Dam T, Hart SC, Turner BL, Chadwick OA, Berhe AA, Hu Y, Zhu M (2020) Quantifying uncertainties in sequential chemical extraction of soil phosphorus using XANES spectroscopy. Environ Sci Technol 54:2257–2267
Guzman G, Alcantara E, Barron V, Torrent J (1994) Phytoavailability of phosphate adsorbed on ferrihydrite, hematite, and goethite. Plant Soil 159:219–225
Handler RM, Frierdich AJ, Johnson CM, Rosso KM, Beard BL, Wang C, Latta DE, Neumann A, Pasakarnis T, Premaratne WAPJ, Scherer MM (2014) Fe(II)-catalyzed recrystallization of goethite revisited. Environ Sci Technol 48:11302–11311
Hansel CM, Benner SG, Neiss J, Dohnalkova A, Kukkadapu RK, Fendorf S (2003) Secondary mineralization pathways induced by dissimilatory iron reduction of ferrihydrite under advective flow. Geochim Cosmochim Acta 67:2977–2992
Hansel CM, Benner SG, Fendorf S (2005) Competing Fe(II)-induced mineralization pathways of ferrihydrite. Environ Sci Technol 39:7147–7153
Herndon E, AlBashaireh A, Singer D, Chowdhury TR, Gu B, Graham D (2017) Influence of iron redox cycling on organo-mineral associations in arctic tundra soil. Geochim Cosmochim Acta 207:210–231
Herndon EM, Kinsman-Costello L, Duroe KA, Mills J, Kane ES, Sebestyen SD, Thompson AA, Wullschleger SD (2019) Iron (oxyhydr)oxides serve as phosphate traps in tundra and boreal peat soils. J Geophys Res Biogeogr 124:227–246
Hochella M, Lower SK, Maurice PA, Penn RL, Sahai N, Sparks DL, Twining BS (2008) Nanominerals, mineral nanoparticles, and earth systems. Science 319:1631–1635
Ilg K, Dominik P, Kaupenjohann M, Siemens J (2008) Phosphorus-induced mobilization of colloids: model systems and soils. Eur J Soil Sci 59:233–246
Kappler A, Bryce C, Mansor M, Lueder U, Byrne JM, Swanner ED (2021) An evolving view on biogeochemical cycling of iron. Nat Rev Microbiol 19:360–374
Karlsson T, Persson P (2010) Coordination chemistry and hydrolysis of Fe(III) in a peat humic acid studied by X-ray absorption spectroscopy. Geochim Cosmochim Acta 74:30–40
Khare N, Hesterberg D, Martin JD (2005) XANES investigation of phosphate sorption in single and binary systems of iron and aluminum oxide minerals. Environ Sci Technol 39:2152–2160
Kiczka M, Wiederhold JG, Frommer J, Voegelin A, Kraemer SM, Bourdon B, Kretzschmar R (2011) Iron speciation and isotope fractionation during silicate weathering and soil formation in an alpine glacier forefield chronosequence. Geochim Cosmochim Acta 75:5559–5573
Kögel-Knabner I, Amelung W, Cao Z, Fiedler S, Frenzel P, Jahn R, Kalbitz K, Kölbl A, Schloter M (2010) Biogeochemistry of paddy soils. Geoderma 157:1–14
Lalonde K, Mucci A, Ouellet A, Gélinas Y (2012) Preservation of organic matter in sediments promoted by iron. Nature 483(7388):198–200
Lambers H, Chapin FS, Pons TL (2008) Plant physiological ecology, vol 2. Springer, New York, pp 11–99
Larsen O, Postma D (2000) Kinetics of reductive bulk dissolution of lepidocrocite, ferrihydrite, and goethite. Geochim Cosmochim Acta 65:1367–1379
Lin Y, Bhattacharyya A, Campbell AN, Nico PS, Pett-Ridge J, Silver WL (2018) Phosphorus fractionation responds to dynamic redox conditions in a humid tropical forest soil. J Geophys Res Biogeogr 123:3016–3027
Liptzin D, Silver WL (2009) Effects of carbon additions on iron reduction and phosphorus availability in a humid tropical soil. Soil Biol Biochem 41:1696–1702
Mallet M, Barthélémy K, Ruby C, Renard A, Naille S (2013) Investigation of phosphate adsorption onto ferrihydrite by X-ray photoelectron spectroscopy. J Colloid Interfaces Sci 407:95–101
Mikutta C, Mikutta R, Bonneville S, Wagner F, Voegelin A, Christl I, Kretzschmar R (2008) Synthetic coprecipitates of exopolysaccharides and ferrihydrite, Part I: characterization. Geochim Cosmochim Acta 72:1111–1127
Moormann FR, van Breemen N (1978) Rice: soil, water, land. International Rice Research Institute, Los Baños
Mortimer CH (1941) The exchange of dissolved substances between mud and water in lakes. J Ecol 29(2):280–329
National Oceanic Atmospheric Agency/National Centers for Environmental Information (2021) Climate data Online. Subset used 1998–2018. Accessed 27 July 2021
Newville M (2013) Larch: an analysis package for XAFS and related spectroscopies. J Phys Conf Ser 430:012007
Newville M, Boyanov BI, Sayers DE (1999) Estimation of uncertainties in XAFS data. J Synchrotron Rad 6:264–265
O’Day PA, Rivera N Jr, Root R, Carroll SA (2004) X-ray absorption spectroscopic study of Fe reference compounds for the analysis of natural sediments. Am Mineral 89(4):572–585
O’Loughlin EJ, Boyanov MI, Gorski CA, Scherer MM, Kemner KM (2021) Effects of Fe(III) oxide mineralogy and phosphate on Fe(II) secondary mineral formation during microbial iron reduction. Minerals 11:149
Paludan C, Jensen HS (1995) Sequential extraction of phosphorus in freshwater wetland and lake sediment: Significance of humic acids. Wetlands 4:365–373
Patrick WH Jr, DeLaune RD (1977) Chemical and biological redox systems affecting nutrient availability in the coastal wetlands. Geosci Man 18(13):137
Pedersen HD, Postma D, Jakobsen R, Larsen O (2005) Fast transformation of iron oxyhydroxides by the catalytic action of aqueous Fe(II). Geochim Cosmochim Acta 69:3967–3977
Ravel B, Newville M (2005) ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J Synchrotron Radiat 12(4):537–541
Reed SC, Yang X, Thornton PE (2015) Incorporating phosphorus cycling into global modeling efforts: a worthwhile, tractable endeavor. New Phytol 208:324–329
Roden EE, Edmonds J (1997) Phosphate mobilization in iron-rich anaerobic sediments: microbial Fe(III) oxide reduction versus iron-sulfide formation. Arch Hydrobiol 139(3):347–378
Rusch B, Hanna K, Humbert B (2010) Coating of quartz silica with iron oxides: characterization and surface reactivity of iron coating phases. Colloid Surface A 353:172–180
Ruttenberg KC, Sulak DJ (2011) Sorption and desorption of dissolved organic phosphorus onto iron (oxyhydr)oxides in seawater. Geochim Cosmochim Acta 75:4095–4112
Ryan JN, Gschwend PM (1994) Effect of solution chemistry on clay colloid release from an iron oxide-coated aquifer sand. Environ Sci Technol 28:1717–1726
Schaefer MV, Gorski CA, Scherer MM (2011) Spectroscopic evidence for interfacial Fe(II)–Fe(III) electron transfer in clay mineral. Environ Sci Technol 45:540–545
Scheidegger A, Borkovec M, Sticher H (1993) Coating of silica sand with goethite: preparation and analytical identification. Geoderma 58:43–65
Schwertmann U, Cornell RM (2000) Iron oxides in the laboratory: preparation and characterization, 2nd completely rev. and extended edn. Wiley-VCH, Weinheim
Sun J, Mailloux BJ, Chillrud SN, van Geen A, Thompson A, Bostick BC (2018) Simultaneously quantifying ferrihydrite and goethite in natural sediments using the method of standard additions with X-ray absorption spectroscopy. Chem Geol 476:248–259
Sjöstedt C, Persson I, Hesterberg D, Kleja DB, Borg H, Gustafsson JP (2013) Iron speciation in soft-water lakes and soils as determined by EXAFS spectroscopy and geochemical modelling. Geochim Cosmochim Acta 105:172–186
Slomp CP, Van der Gaast SJ, Van Raaphorst W (1996) Phosphorus binding by poorly crystalline iron oxides in North Sea sediments. Mar Chem 52(1):55–73
Sundman A, Karlsson T, Laudon H, Persson P (2014) XAS study of iron speciation in soils and waters from a boreal catchment. Chem Geol 364:93–102
Sundman A, Karlsson T, Sjöberg S, Persson P (2016) Impact of iron-organic matter complexes on aqueous phosphate concentrations. Chem Geol 426:109–117
Tessier A, Rapin F, Carignan R (1985) Trace metals in oxic lake sediments: possible adsorption onto iron oxyhydroxides. Geochim Cosmochim Acta 49(1):183–194
Thamdrup B (2000) Bacterial manganese and iron reduction in aquatic sediments. Adv Microb Ecol 16:41–84
Thompson A, Chadwick OA, Rancourt DG, Chorover J (2006) Iron-oxide crystallinity increases during soil redox oscillations. Geochim Cosmochim Acta 70(7):1710–1727
Thompson A, Rancourt DG, Chadwick OA, Chorover J (2011) Iron solid-phase differentiation along a redox gradient in basaltic soils. Geochim Cosmochim Acta 75:119–133
Tomaszewski EJ, Cronk SS, Gorski CA, Ginder-Vogel M (2016) The role of dissolved Fe(II) concentration in the mineralogical evolution of Fe (hydr)oxides during redox cycling. Chem Geol 438:163–170
USGCRP (2018) Impacts, risks, and adaptation in the United States. In: Reidmiller DR, Avery CW, Easterling DR, Kunkel KE, Lewis KLM, Maycock TK, Stewart BC (eds) Fourth National Climate Assessment, vol II. Global Change Research Program, Washington. https://doi.org/10.7930/NCA4.2018
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen–phosphorus interactions. Ecol Appl 20(1):5–15
Vogelsang V, Fiedler S, Jahn R, Kaiser K (2016) In-situ transformation of iron-bearing minerals in marshland-derived paddy subsoil. Eur J Soil Sci 67:676–685
Wang Y, Shen Z, Niu J, Liu R (2009) Adsorption of phosphorus on sediments from the Three-Gorges Reservoir (China) and the relation with sediment compositions. J Hazard Mater 162:92–98
Weber KA, Achenbach LA, Coates JD (2006) Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4:752–764
Westheimer FH (1987) Why nature chose phosphates. Science 235(4793):1173–1178
Williams AGB, Scherer MM (2004) Spectroscopic evidence for Fe(II)–Fe(III) electron transfer at the iron oxide–water interface. Environ Sci Technol 38:4782–4790
Winkler P, Kaiser K, Thompson A, Kalbitz K, Fiedler S, Jahn R (2018) Contrasting evolution of iron phase composition in soils exposed to redox fluctuations. Geochim Cosmochim Acta 235:89–102
Yang H, Liptzin D (2015) High potential for iron reduction in upland soils. Ecology 96:2015–2020
Acknowledgements
We would like to thank Shannon Joseph for field and lab work assistance, as well as Lindsey Yazbek, Raihan Chowdhury, and Mallory Klein for help with mesh bag assembly.
Funding
This work was supported by grants from the National Science Foundation (EAR-1609027 and OPP-2006194) and the Kent State Environmental Science and Design Research Initiative to Herndon and Kinsman-Costello. Portions of this work were performed at GeoSoilEnviroCARS (Sector 13) and 12-BMB (Sector 12), Advanced Photon Source (APS), Argonne National Laboratory. GeoSoilEnviroCARS is supported by the National Science Foundation – Earth Sciences (EAR-1128799) and Department of Energy – GeoSciences (DE-FG02-94ER14466). Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
MB developed methodologies, conducted the experiments, performed data analyses, and wrote the manuscript. CS and ND contributed to conceptualization, methodology, and experimentation. LKC contributed to conceptualization, funding acquisition, and review and editing of the manuscript. DS contributed to project supervision and review and editing of the manuscript. EH provided funding, supervised the project, and contributed to conceptualization, methodology, and writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no financial or non-financial conflicts of interest.
Additional information
Responsible Editor: Jeffrey A. Bird
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript has been authored in part by UT-Battelle, LLC, under contract DE-AC05-00OR22725 with the US Department of Energy (DOE). The publisher acknowledges the US government license to provide public access under the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barczok, M., Smith, C., Di Domenico, N. et al. Influence of contrasting redox conditions on iron (oxyhydr)oxide transformation and associated phosphate sorption. Biogeochemistry 166, 87–107 (2023). https://doi.org/10.1007/s10533-023-01094-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-023-01094-z