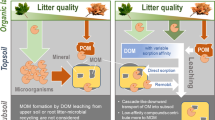
Abstract
Microbial products, largely the necromass, are key contributors to stable soil organic matter (SOM) in terrestrial systems, and microbial communities may differ in the stabilization. Plants can control microbial communities through litter quality and entry sites of plant inputs (above- or below-ground). However, whether soil mineral fractions (due to the characteristics such as soil texture) can also control the microbial communities, remains unclear. We conducted two model soil incubation experiments (E1 and E2) in order to simulate the four field soils. Materials included were plant materials, such as general plant inputs in soil systems, and four mineral materials derived from different field soils (i.e., “the four original field soils”), with their SOM removed by combustion. E1 was undertaken to simulate root exudate using oxalate and glucose, and E2 was undertaken to simulate plant litter using broad leaves, coniferous leaves, branches, and fine roots. The microbial (mainly bacterial) community structure from phospholipid fatty acid (PLFA) across E1 and E2 showed differences due to the mineral materials, and the differences after accounting for plant materials are similar to the difference in the four original field soils. Additionally, the abundance of bacterial PLFAs in E2 increased with silt and clay content and was correlated with the abundance of the bacterial PLFAs in the four original field soil. This study implies that even in the case of this experiment under such conditions as a broad variety of plant inputs, mineral fractions strongly influence bacterial communities, in a manner consistent with field soil systems.





Similar content being viewed by others
Data availability
Additional data are presented as Supplementary information.
Change history
11 October 2022
The original version of this article was revised: In this article a symbol was missing in Figure 4 due to a conversion error. The original article has been updated.
17 October 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10533-022-00982-0
References
Amann RI, Binder BJ, Olson RJ et al (1990) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56:1919–1925. https://doi.org/10.1128/aem.56.6.1919-1925.1990
Asano M, Wagai R (2014) Evidence of aggregate hierarchy at micro- to submicron scales in an allophanic andisol. Geoderma 216:62–74. https://doi.org/10.1016/j.geoderma.2013.10.005
Bååth E, Anderson TH (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem 35:955–963. https://doi.org/10.1016/S0038-0717(03)00154-8
Bardgett RD, Hobbs PJ, Frostegård Å (1996) Changes in soil fungal:bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biol Fertil Soils 22:261–264. https://doi.org/10.1007/BF00382522
Boeddinghaus RS, Marhan S, Gebala A et al (2021) The mineralosphere—interactive zone of microbial colonization and carbon use in grassland soils. Biol Fertil Soils 57:587–601
Buckeridge KM, La Rosa AF, Mason KE et al (2020) Sticky dead microbes: rapid abiotic retention of microbial necromass in soil. Soil Biol Biochem 149:107929. https://doi.org/10.1016/j.soilbio.2020.107929
Castellano MJ, Kaye JP, Lin H, Schmidt JP (2012) Linking carbon saturation concepts to nitrogen saturation and retention. Ecosystems 15:175–187. https://doi.org/10.1007/s10021-011-9501-3
Cecchi AM, Koskinen WC, Cheng HH, Haider K (2004) Sorption-desorption of phenolic acids as affected by soil properties. Biol Fertil Soils 39:235–242. https://doi.org/10.1007/s00374-003-0710-6
Clemente JS, Simpson AJ, Simpson MJ (2011) Association of specific organic matter compounds in size fractions of soils under different environmental controls. Org Geochem 42:1169–1180. https://doi.org/10.1016/j.orggeochem.2011.08.010
Cotrufo MF, Wallenstein MD, Boot CM et al (2013) The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Chang Biol 19:988–995. https://doi.org/10.1111/gcb.12113
Dümig A, Häusler W, Steffens M, Kögel-Knabner I (2012) Clay fractions from a soil chronosequence after glacier retreat reveal the initial evolution of organo-mineral associations. Geochim Cosmochim Acta 85:1–18. https://doi.org/10.1016/j.gca.2012.01.046
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA 103:626–631. https://doi.org/10.1073/pnas.0507535103
Fierer N, Jackson JA, Vilgalys R, Jackson RB (2005) Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microbiol 71:4117–4120. https://doi.org/10.1128/AEM.71.7.4117-4120.2005
Fox TR, Comerford NB (1990) Low-molecular-weight organic acids in selected forest soils of the Southeastern USA. Soil Sci Soc Am J 54:1139–1144. https://doi.org/10.2136/sssaj1990.03615995005400040037x
Frostegard A, Tunlid A, Baath E (1993) Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl Environ Microbiol 59:3605–3617
Fujii K, Hayakawa C, Van Hees PAW et al (2010) Biodegradation of low molecular weight organic compounds and their contribution to heterotrophic soil respiration in three Japanese forest soils. Plant Soil 334:475–489. https://doi.org/10.1007/s11104-010-0398-y
Garrison-Johnston MT, Mika PG, Miller DL, et al (2007) Ash cap influences on site productivity and fertilizer response in forests of the Inland Northwest. In: Page-Dumroese D, Miller R, Mital J, et al. (eds) Volcanic-Ash-Derived Forest Soils of the Inland Northwest: Properties and Implications for Management and Restoration. 9-10 November 2005. Coeur d’Alene, ID. Proceedings RMRS-P-44, (pp. 137–163)
Griffiths BS, Ritz K, Ebblewhite N, Dobson G (1999) Soil microbial community structure: Effects of substrate loading rates. Soil Biol Biochem 31:145–153
Guan N, Liu L (2020) Microbial response to acid stress: mechanisms and applications. Appl Microbiol Biotechnol 104:51–65. https://doi.org/10.1007/s00253-019-10226-1
Hansson K, Kleja DB, Kalbitz K, Larsson H (2010) Amounts of carbon mineralised and leached as DOC during decomposition of Norway spruce needles and fine roots. Soil Biol Biochem 42:178–185. https://doi.org/10.1016/j.soilbio.2009.10.013
Heckman K, Welty-Bernard A, Vazquez-Ortega A et al (2013) The influence of goethite and gibbsite on soluble nutrient dynamics and microbial community composition. Biogeochemistry 112:179–195. https://doi.org/10.1007/s10533-012-9715-2
Hemingway JD, Rothman DH, Grant KE et al (2019) Mineral protection regulates long-term global preservation of natural organic carbon. Nature 570:228–231. https://doi.org/10.1038/s41586-019-1280-6
Hobbie SE (2015) Plant species effects on nutrient cycling: revisiting litter feedbacks. Trends Ecol Evol 30:357–363. https://doi.org/10.1016/j.tree.2015.03.015
Hobbie SE, Oleksyn J, Eissenstat DM, Reich PB (2010) Fine root decomposition rates do not mirror those of leaf litter among temperate tree species. Oecologia 162:505–513. https://doi.org/10.1007/s00442-009-1479-6
Högberg MN (2006) Discrepancies between ergosterol and the phospholipid fatty acid 18:2ω6,9 as biomarkers for fungi in boreal forest soils. Soil Biol Biochem 38:3431–3435. https://doi.org/10.1016/j.soilbio.2006.06.002
Imagawa M, Touch N, Nakashita S, Hibino T (2011) Burning characteristic of organic matter deposited on sea bottom sediment. J Japan Soc Civ Eng Ser B (Coastal Eng) 67:I_1156-I_1160. https://doi.org/10.2208/kaigan.67.i_1156
Iwaoka C, Imada S, Taniguchi T et al (2018) The impacts of soil fertility and salinity on soil nitrogen dynamics mediated by the soil microbial community beneath the halophytic Shrub Tamarisk. Microb Ecol 75:985–996. https://doi.org/10.1007/s00248-017-1090-z
Jardine PM, Weber NL, McCarthy JF (1989) Mechanisms of dissolved organic carbon adsorption on soil. Soil Sci Soc Am J 53:1378–1385. https://doi.org/10.2136/sssaj1989.03615995005300050013x
Jilling A, Keiluweit M, Contosta AR et al (2018) Minerals in the rhizosphere: overlooked mediators of soil nitrogen availability to plants and microbes. Biogeochemistry 139:103–122. https://doi.org/10.1007/s10533-018-0459-5
Jones DL (1998) Organic acids in the rhizosphere—a critical review. Plant Soil 205:25–44. https://doi.org/10.1023/A:1004356007312
Jones DL, Hodge A, Kuzyakov Y (2004) Plant and mycorrhizal regulation of rhizodeposition. New Phytol 163:459–480. https://doi.org/10.1111/j.1469-8137.2004.01130.x
Kaiser K, Zech W (2000) Sorption of dissolved organic nitrogen by acid subsoil horizons and individual mineral phases. Eur J Soil Sci 51:403–411. https://doi.org/10.1046/j.1365-2389.2000.00320.x
Kallenbach CM, Frey SD, Grandy AS (2016) Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat Commun 7:13630. https://doi.org/10.1038/ncomms13630
Kandeler E, Tscherko D, Bruce KD et al (2000) Structure and function of the soil microbial community in microhabitats of a heavy metal polluted soil. Biol Fertil Soils 32:390–400. https://doi.org/10.1007/s003740000268
Keiluweit M, Bougoure JJ, Nico PS et al (2015) Mineral protection of soil carbon counteracted by root exudates. Nat Clim Chang 5:588–595. https://doi.org/10.1038/nclimate2580
Kochian LV (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46:237–260. https://doi.org/10.1146/annurev.pp.46.060195.001321
Kopittke PM, Hernandez-Soriano MC, Dalal RC et al (2018) Nitrogen-rich microbial products provide new organo-mineral associations for the stabilization of soil organic matter. Glob Chang Biol 24:1762–1770. https://doi.org/10.1111/gcb.14009
Lavallee JM, Soong JL, Cotrufo MF (2020) Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob Chang Biol 26:261–273. https://doi.org/10.1111/gcb.14859
Lechtenfeld OJ, Hertkorn N, Shen Y et al (2015) Marine sequestration of carbon in bacterial metabolites. Nat Commun 6:6711. https://doi.org/10.1038/ncomms7711
Lehmann J, Kleber M (2015) The contentious nature of soil organic matter. Nature 528:60–68. https://doi.org/10.1038/nature16069
Liang C, Balser TC (2011) Microbial production of recalcitrant organic matter in global soils: implications for productivity and climate policy. Nat Rev Microbiol 9:75. https://doi.org/10.1038/nrmicro2386-c1
Liang C, Schimel JP, Jastrow JD (2017) The importance of anabolism in microbial control over soil carbon storage. Nat Microbiol 2:17105. https://doi.org/10.1038/nmicrobiol.2017.105
Liang C, Amelung W, Lehmann J, Kästner M (2019) Quantitative assessment of microbial necromass contribution to soil organic matter. Glob Chang Biol 25:3578–3590. https://doi.org/10.1111/gcb.14781
Ludwig M, Achtenhagen J, Miltner A et al (2015) Microbial contribution to SOM quantity and quality in density fractions of temperate arable soils. Soil Biol Biochem 81:311–322. https://doi.org/10.1016/j.soilbio.2014.12.002
Ma C, Xiong Y, Li L, Guo D (2016) Root and leaf decomposition become decoupled over time: implications for below-and above-ground relationships. Funct Ecol 30:1239–1246. https://doi.org/10.1111/1365-2435.12619
Manzoni S, Taylor P, Richter A et al (2012) Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol 196:79–91. https://doi.org/10.1111/j.1469-8137.2012.04225.x
Mikutta R, Kleber M, Kaiser K, Jahn R (2005) Review: organic matter removal from soils using hydrogen peroxide, sodium hypochlorite, and disodium peroxodisulfate. Soil Sci Soc Am J 69:120–135. https://doi.org/10.2136/sssaj2005.0120
Mikutta R, Kaiser K, Dörr N et al (2010) Mineralogical impact on organic nitrogen across a long-term soil chronosequence (0.3–4100kyr). Geochim Cosmochim Acta 74:2142–2164. https://doi.org/10.1016/j.gca.2010.01.006
Mikutta R, Turner S, Schippers A et al (2019) Microbial and abiotic controls on mineral-associated organic matter in soil profiles along an ecosystem gradient. Sci Rep 9:1–9. https://doi.org/10.1038/s41598-019-46501-4
Moinet GYK, Hunt JE, Kirschbaum MUF et al (2018) The temperature sensitivity of soil organic matter decomposition is constrained by microbial access to substrates. Soil Biol Biochem 116:333–339. https://doi.org/10.1016/j.soilbio.2017.10.031
Mooshammer M, Wanek W, Hämmerle I et al (2014) Adjustment of microbial nitrogen use efficiency to carbon: nitrogen imbalances regulates soil nitrogen cycling. Nat Commun 5:1–7. https://doi.org/10.1038/ncomms4694
Muyzer G, De Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700. https://doi.org/10.1128/aem.59.3.695-700.1993
Oldfield EE, Crowther TW, Bradford MA (2018) Substrate identity and amount overwhelm temperature effects on soil carbon formation. Soil Biol Biochem 124:218–226. https://doi.org/10.1016/j.soilbio.2018.06.014
Or D, Smets BF, Wraith JM et al (2007) Physical constraints affecting bacterial habitats and activity in unsaturated porous media—a review. Adv Water Resour 30:1505–1527. https://doi.org/10.1016/j.advwatres.2006.05.025
Orwin KH, Dickie IA, Holdaway R, Wood JR (2018) A comparison of the ability of PLFA and 16S rRNA gene metabarcoding to resolve soil community change and predict ecosystem functions. Soil Biol Biochem 117:27–35. https://doi.org/10.1016/j.soilbio.2017.10.036
Poll C, Thiede A, Wermbter N et al (2003) Micro-scale distribution of microorganisms and microbial enzyme activities in a soil with long-term organic amendment. Eur J Soil Sci 54:715–724. https://doi.org/10.1046/j.1351-0754.2003.0569.x
Pronk GJ, Heister K, Ding GC et al (2012) Development of biogeochemical interfaces in an artificial soil incubation experiment; aggregation and formation of organo-mineral associations. Geoderma 189–190:585–594. https://doi.org/10.1016/j.geoderma.2012.05.020
Quideau SA, McIntosh ACS, Norris CE et al (2016) Extraction and analysis of microbial phospholipid fatty acids in soils. J vis Exp 114:54360. https://doi.org/10.3791/54360
Ranjard L, Richaume A (2001) Quantitative and qualitative microscale distribution of bacteria in soil. Res Microbiol 152:707–716. https://doi.org/10.1016/S0923-2508(01)01251-7
Rasmussen C, Heckman K, Wieder WR et al (2018) Beyond clay: towards an improved set of variables for predicting soil organic matter content. Biogeochemistry 137:297–306. https://doi.org/10.1007/s10533-018-0424-3
Schmidt MWI, Torn MS, Abiven S et al (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56. https://doi.org/10.1038/nature10386
Schrumpf M, Kaiser K, Guggenberger G et al (2013) Storage and stability of organic carbon in soils as related to depth, occlusion within aggregates, and attachment to minerals. Biogeosciences 10:1675–1691. https://doi.org/10.5194/bg-10-1675-2013
Schurig C, Smittenberg RH, Berger J et al (2013) Microbial cell-envelope fragments and the formation of soil organic matter: a case study from a glacier forefield. Biogeochemistry 113:595–612. https://doi.org/10.1007/s10533-012-9791-3
Shahbaz M, Kuzyakov Y, Sanaullah M et al (2017) Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: mechanisms and thresholds. Biol Fertil Soils 53:287–301. https://doi.org/10.1007/s00374-016-1174-9
Shoji S, Nanzyo M, Dahlgren RA (1993) Volcanic ash soils Genesis properties and utilization, vol 21. Elsevier Science, Amsterdam
Soares M, Rousk J (2019) Microbial growth and carbon use efficiency in soil: links to fungal-bacterial dominance, SOC-quality and stoichiometry. Soil Biol Biochem 131:195–205. https://doi.org/10.1016/j.soilbio.2019.01.010
Sokol NW, Bradford MA (2019) Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat Geosci 12:46–53. https://doi.org/10.1038/s41561-018-0258-6
Sokol NW, Sanderman J, Bradford MA (2019) Pathways of mineral-associated soil organic matter formation: integrating the role of plant carbon source, chemistry, and point of entry. Glob Chang Biol 25:12–24. https://doi.org/10.1111/gcb.14482
Tatsumi C, Taniguchi T, Du S et al (2019) The steps in the soil nitrogen transformation process vary along an aridity gradient via changes in the microbial community. Biogeochemistry 144:15–29. https://doi.org/10.1007/s10533-019-00569-2
Toju H, Tanabe AS, Yamamoto S, Sato H (2012) High-coverage ITS primers for the DNA-based identification of ascomycetes and basidiomycetes in environmental samples. PLoS ONE. https://doi.org/10.1371/journal.pone.0040863
Urakawa R, Shibata H, Kuroiwa M et al (2014) Effects of freeze-thaw cycles resulting from winter climate change on soil nitrogen cycling in ten temperate forest ecosystems throughout the Japanese archipelago. Soil Biol Biochem 74:82–94. https://doi.org/10.1016/j.soilbio.2014.02.022
Uroz S, Kelly LC, Turpault MP et al (2015) The mineralosphere concept: mineralogical control of the distribution and function of mineral-associated bacterial communities. Trends Microbiol 23:751–762. https://doi.org/10.1016/j.tim.2015.10.004
Van Hees PAW, Jones DL, Finlay R et al (2005) The carbon we do not see—the impact of low molecular weight compounds on carbon dynamics and respiration in forest soils: a review. Soil Biol Biochem 37:1–13. https://doi.org/10.1016/j.soilbio.2004.06.010
Vieira S, Sikorski J, Gebala A et al (2020) Bacterial colonization of minerals in grassland soils is selective and highly dynamic. Environ Microbiol 22:917–933. https://doi.org/10.1111/1462-2920.14751
Wardle DA (1992) A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol Rev Camb Philos Soc 67:321–358. https://doi.org/10.1111/j.1469-185X.1992.tb00728.x
Wardle DA (2004) Ecological Linkages Between Aboveground and Belowground Biota. Science 304:1629–1633. https://doi.org/10.1126/science.1094875
Waring BG, Averill C, Hawkes CV (2013) Differences in fungal and bacterial physiology alter soil carbon and nitrogen cycling: insights from meta-analysis and theoretical models. Ecol Lett 16:887–894. https://doi.org/10.1111/ele.12125
Whitman T, Neurath R, Perera A et al (2018) Microbial community assembly differs across minerals in a rhizosphere microcosm. Environ Microbiol 20:4444–4460. https://doi.org/10.1111/1462-2920.14366
Yokobe T, Hyodo F, Tokuchi N (2018) Seasonal effects on microbial community structure and nitrogen dynamics in temperate forest soil. Forests 9:153. https://doi.org/10.3390/f9030153
Yokobe T, Hyodo F, Tokuchi N (2020) Volcanic deposits affect soil nitrogen dynamics and fungal–bacterial dominance in temperate forests. Soil Biol Biochem 150:108011. https://doi.org/10.1016/j.soilbio.2020.108011
Acknowledgements
We thank Takahito Yoshioka and Shunsuke Matsuoka for helpful comments, and Soyoka Makino and Masataka Nakayama for assistance with laboratory work. Thanks to the anonymous reviewers and the editor for their helpful comments and suggestions regarding the manuscript.
Funding
This study was supported by Field Science Education and Research Center, Kyoto University.
Author information
Authors and Affiliations
Contributions
TY: conceived the project; TY and NT: contributed to the fieldwork; TY: conducted the incubation experiments and analyzed the data; FH: contributed to PLFA analysis; TY: wrote the manuscript; all authors contributed to reviewing and editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of competing interests to report.
Additional information
Responsible Editor: Steven J. Hall.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: In this article a symbol was missing in Figure 4 due to a conversion error. The original article has been updated.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
See Fig. 6.
Relationships between PLFAs and quantitative PCR of fungi and bacteria, and between total PLFA and DNA content in E1 and E2. The two fungal indicators were significantly correlated in all the samples (P = 0.0062), and neither was significantly correlated in EM1 (P = 0.4464) nor in EM2 (P = 0.5723). The bacterial indicators were significantly correlated in all the samples (P < 0.0001), and significantly correlated both in EM1 (P = 0.0201) and in EM2 (P = 0.0002). The total PLFA content was not significantly correlated with the DNA content in all the samples (P = 0.3509), but significantly correlated in EM1 (P = 0.0120) and in EM2 (P = 0.0196). V volcanic mineral material; NV non-volcanic mineral material
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yokobe, T., Hyodo, F., Tateno, R. et al. Soil mineral fraction influences the bacterial abundance: evidence from a mineral and plant materials incubation study. Biogeochemistry 161, 273–287 (2022). https://doi.org/10.1007/s10533-022-00978-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-022-00978-w