Abstract
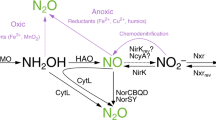
The continuous rise of atmospheric nitrous oxide (N2O) is an environmental issue of global concern. In biogeochemical studies, N2O production is commonly assumed to arise solely from enzymatic reactions in microbes and fungi. However, iron, manganese and organic compounds readily undergo redox reactions with intermediates in the nitrogen cycle that produce N2O abiotically under relevant environmental conditions at circumneutral pH. Although these abiotic N2O production pathways have been known to occur for close to a century, they are often neglected in modern ecological studies. In this Synthesis and Emerging Ideas paper, we highlight the defining characteristics, environmental controls, and isotopic signatures of abiotic reactions between nitrogen cycle intermediates (hydroxylamine, nitric oxide, and nitrite), redox-active metals (iron and manganese) and organic matter (humic and fulvic acids) that can lead to N2O production. We also discuss the emerging idea that abiotic reactions coupled to biotic processes have widespread ecological relevance and encourage consideration of abiotic production mechanisms in future biogeochemical investigations of N2O cycling.

Similar content being viewed by others
References
Adriano D (1986) Trace elements in the terrestrial environment. Springer, New York
Akiyama H, Tsuruta H (2003) Nitrous oxide, nitric oxide, and nitrogen dioxide fluxes from soils after manure and urea application. J Environ Qual 32(2):423–431
Aubert H, Pinta M (1980) Trace elements in soils. Elsevier, New York
Azam F, Muller C, Weiske A, Benckiser G, Ottow J (2002) Nitrification and denitrification as sources of atmospheric nitrous oxide-role of oxidizable carbon and applied nitrogen. Biol Fertil Soils 35(1):54–61
Babbin AR, Bianchi D, Jayakumar A, Ward BB (2015) Rapid nitrous oxide cycling in the suboxic ocean. Science 348(6239):1127–1129
Bengtsson G, Fronaeus S, Bengtsson-Kloo L (2002) The kinetics and mechanism of oxidation of hydroxylamine by iron(III). J Chem Soc, Dalton Trans 12:2548–2552
Bouwman A, Boumans L, Batjes N (2002) Modeling global annual N2O and NO emissions from fertilized fields. Global Biogeochem Cycles 16(4):28-21–28-29
Bray WC, Simpson ME, MacKenzie AA (1919) The volumetric determination of hydroxylamine. J Am Chem Soc 41(9):1363–1378
Bremner J (1997) Sources of nitrous oxide in soils. Nutr Cycl Agroecosys 49(1–3):7–16
Bremner J, Blackmer A, Waring S (1980) Formation of nitrous oxide and dinitrogen by chemical decomposition of hydroxylamine in soils. Soil Biol Biochem 12(3):263–269
Buresh RJ, Moraghan J (1976) Chemical reduction of nitrate by ferrous iron. J Environ Qual 5(3):320–325
Burns L, Stevens R, Laughlin R (1995) Determination of the simultaneous production and consumption of soil nitrite using 15N. Soil Biol Biochem 27(6):839–844
Burns L, Stevens R, Laughlin R (1996) Production of nitrite in soil by simultaneous nitrification and denitrification. Soil Biol Biochem 28(4–5):609–616
Butler JH, Gordon LI (1986a) An improved gas chromatographic method for the measurement of hydroxylamine in marine and fresh waters. Mar Chem 19(3):229–243
Butler JH, Gordon LI (1986b) Rates of nitrous oxide production in the oxidation of hydroxylamine by iron(III). Inorg Chem 25(25):4573–4577
Butler JH, Jones RD, Garber JH, Gordon LI (1987) Seasonal distributions and turnover of reduced trace gases and hydroxylamine in Yaquina Bay, Oregon. Geochim Cosmochim Acta 51(3):697–706
Butler JH, Pequegnat JE, Gordon LI, Jones RD (1988) Cycling of methane, carbon monoxide, nitrous oxide, and hydroxylamine in a meromictic, coastal lagoon. Est Coast Shelf Sci 27(2):181–203
Carlson HK, Clark IC, Blazewicz SJ, Iavarone AT, Coates JD (2013) Fe(II) oxidation is an innate capability of nitrate-reducing bacteria that involves abiotic and biotic reactions. J Bacteriol 195(14):3260–3268
Cayuela ML, Sánchez-Monedero MA, Roig A, Hanley K, Enders A, Lehmann J (2013) Biochar and denitrification in soils: when, how much and why does biochar reduce N2O emissions? Sci Rep 3:1732
Chakraborty A, Picardal F (2013) Induction of nitrate-dependent Fe(II) oxidation by Fe(II) in Dechloromonas sp. strain UWNR4 and Acidovorax sp. strain 2AN. Appl Environ Microbiol 79(2):748–752
Chalk P, Smith C (1983) Chemodenitrification. In: Freney JR, Simpson JR (eds) Gaseous loss of nitrogen from plant-soil systems. Springer, Dordrecht, pp 65–89
Chao T-T, Kroontje W (1966) Inorganic nitrogen transformations through the oxidation and reduction of iron. Soil Sci Soc Am J 30(2):193–196
Clément J-C, Shrestha J, Ehrenfeld JG, Jaffé PR (2005) Ammonium oxidation coupled to dissimilatory reduction of iron under anaerobic conditions in wetland soils. Soil Biol Biochem 37(12):2323–2328
Coby AJ, Picardal FW (2005) Inhibition of NO −3 and NO −2 reduction by microbial Fe(III) reduction: evidence of a reaction between NO −2 and cell surface-bound Fe2+. Appl Environ Microbiol 71(9):5267–5274
Conrad R (1996) Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol Mol Biol Rev 60(4):609–640
Cooper D, Picardal F, Schimmelmann A, Coby A (2003) Chemical and biological interactions during nitrate and goethite reduction by Shewanella putrefaciens 200. Appl Environ Microbiol 69(6):3517–3525
De Bie MJ, Middelburg JJ, Starink M, Laanbroek HJ (2002) Factors controlling nitrous oxide at the microbial community and estuarine scale. Mar Ecol Prog Ser 240:1–9
Druschel GK, Baker BJ, Gihring TM, Banfield JF (2004) Acid mine drainage biogeochemistry at Iron Mountain, California. Geochem Trans 5(2):13–32
Firestone M, Davidson E (1989) Microbial basis of NO and N2O production and consumption in soil. In: Andreae M, Schimel D (eds) Life sciences research reports. Wiley, Chichester, pp 7–21
Frame CH, Casciotti K (2010) Biogeochemical controls and isotopic signatures of nitrous oxide production by a marine ammonia-oxidizing bacterium. Biogeosci Discuss 7(2):3019–3059
Frear D, Burrell R (1955) Spectrophotometric method for determining hydroxylamine reductase activity in higher plants. Anal Chem 27(10):1664–1665
Freing A, Wallace DWR, Bange HW (2012) Global oceanic production of nitrous oxide. Philos Trans R Soc B 367(1593):1245–1255
Gebhardt S, Walter S, Nausch G, Bange H (2004) Hydroxylamine (NH2OH) in the Baltic Sea. Biogeosci Discuss 1(1):709–724
Glass JB, Kretz CB, Ganesh S, Ranjan P, Seston SL, Buck KN, Morton PL, Landing WM, Moffett JW, Giovannoni SJ, Vergin KL, Stewart FJ (2015) Meta-omic signatures of microbial metal and nitrogen cycling in marine oxygen minimum zones. Front Microbiol 6:998
Glass JB, Stanton CL, Ochoa H, Taillefert M, DiChristina TJ, Klotz MG, Haslun JA, Gandhi H, Ostrom NE (in review) An alternative pathway for nitrous oxide production in the ocean. Nat Geosci
Harper WF, Takeuchi Y, Riya S, Hosomi M, Terada A (2015) Novel abiotic reactions increase nitrous oxide production during partial nitrification: modeling and experiments. Chem Eng J 281:1017–1023
Heil J, Wolf B, Brüggemann N, Emmenegger L, Tuzson B, Vereecken H, Mohn J (2014) Site-specific 15N isotopic signatures of abiotically produced N2O. Geochim Cosmochim Acta 139:72–82
Heil J, Liu S, Vereecken H, Brüggemann N (2015) Abiotic nitrous oxide production from hydroxylamine in soils and their dependence on soil properties. Soil Biol Biochem 84:107–115
Huang S, Jaffé P (2015) Characterization of incubation experiments and development of an enrichment culture capable of ammonium oxidation under iron-reducing conditions. Biogeosciences 12(3):769–779
IPCC (2007) Climate change 2007: mitigation of climate change. Cambridge University Press, Cambridge
IPCC (2014) Climate change 2014: mitigiation of climate change. Cambridge University Press, Cambridge
Jenni S, Mohn J, Emmenegger L, Udert KM (2012) Temperature dependence and interferences of NO and N2O microelectrodes used in wastewater treatment. Environ Sci Technol 46(4):2257–2266
Johnson KS, Gordon RM, Coale KH (1997) What controls dissolved iron concentrations in the world ocean? Mar Chem 57(3–4):137–161
Jones LC, Peters B, Lezama Pacheco JS, Casciotti KL, Fendorf S (2015) Stable isotopes and iron oxide mineral products as markers of chemodenitrification. Environ Sci Technol 49(6):3444–3452
Jung M-Y, Well R, Min D, Giesemann A, Park S-J, Kim J-G, Kim S-J, Rhee S-K (2014) Isotopic signatures of N2O produced by ammonia-oxidizing archaea from soils. ISME J 8(5):1115–1125
Kampschreur MJ, Kleerebezem R, de Vet WW, van Loosdrecht M (2011) Reduced iron induced nitric oxide and nitrous oxide emission. Water Res 45(18):5945–5952
Klüglein N, Kappler A (2013) Abiotic oxidation of Fe(II) by reactive nitrogen species in cultures of the nitrate-reducing Fe(II) oxidizer Acidovorax sp. BoFeN1–questioning the existence of enzymatic Fe(II) oxidation. Geobiology 11(2):180–190
Kock A, Bange HW (2013) Nitrite removal improves hydroxylamine analysis in aqueous solution by conversion with iron (III). Environ Chem 10(1):64–71
Lam P, Jensen MM, Kock A, Lettmann KA, Plancherel Y, Lavik G, Bange HW, Kuypers MM (2011) Origin and fate of the secondary nitrite maximum in the Arabian Sea. Biogeosciences 8(1565–1577):375
Latimer (1952) The oxidation states of the elements and their potentials in aqueous solution, 2nd edn. Prentice-Hall, Englewood Cliffs
Law C (2008) Predicting and monitoring the effects of large-scale ocean iron fertilization on marine trace gas emissions. Mar Ecol Prog Ser 364:283–288
Law C, Ling R (2001) Nitrous oxide flux and response to increased iron availability in the Antarctic circumpolar current. Deep Sea Res II 48(11):2509–2527
Learman D, Voelker B, Vazquez-Rodriguez A, Hansel C (2011a) Formation of manganese oxides by bacterially generated superoxide. Nat Geosci 4(2):95–98
Learman D, Wankel S, Webb S, Martinez N, Madden A, Hansel C (2011b) Coupled biotic–abiotic Mn(II) oxidation pathway mediates the formation and structural evolution of biogenic Mn oxides. Geochem Cosmochim Acta 75(20):6048–6063
Learman DR, Voelker BM, Madden AS, Hansel CM (2013) Constraints on superoxide mediated formation of manganese oxides. Front Microbiol 4:262
Li L, Yinghua P, Qitu W, Xiuru Z, Zhengao L (1988) Investigation of amorphous ferric oxide acting as an electron acceptor in the oxidation of NH4 + under anaerobic condition. Acta Pedol Sin 25(2):184–190
Linn D, Doran J (1984) Effect of water filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Sci Soc Am J 48(6):1267–1272
Liu S, Vereecken H, Brüggemann N (2014) A highly sensitive method for the determination of hydroxylamine in soils. Geoderma 232:117–122
Lomas MW, Lipschultz F (2006) Forming the primary nitrite maximum: nitrifiers or phytoplankton? Limnol Oceangr 51(5):2453–2467
Lovley DR (1991) Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev 55(2):259
Ludwig J, Meixner FX, Vogel B, Förstner J (2001) Soil-air exchange of nitric oxide: an overview of processes, environmental factors, and modeling studies. Biogeochemistry 52(3):225–257
Luther G (2010) The role of one-and two-electron transfer reactions in forming thermodynamically unstable intermediates as barriers in multi-electron redox reactions. Aquat Geochem 16(3):395–420
Luther G, Popp J (2002) Kinetics of the abiotic reduction of polymeric manganese dioxide by nitrite: an anaerobic nitrification reaction. Aquat Geochem 8(1):15–36
Luther G, Sundby B, Lewis B, Brendel P, Silverberg N (1997) Interactions of manganese with the nitrogen cycle: alternative pathways to dinitrogen. Geochim Cosmochim Acta 61(19):4043–4052
Macko S, Ostrom N (1994) Pollution studies using stable isotopes. In: Michener R, Lajtha K (eds) Stable isotopes in ecology and environmental science. Blackwell Scientific, Oxford
Madison AS, Tebo BM, Mucci A, Sundby B, Luther GW (2013) Abundant porewater Mn(III) is a major component of the sedimentary redox system. Science 341(6148):875–878
McKenney D, Lazar C, Findlay W (1990) Kinetics of the nitrite to nitric oxide reaction in peat. Soil Sci Soc Am J 54(1):106–112
McNamara N, Black H, Beresford N, Parekh N (2003) Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Appl Soil Ecol 24(2):117–132
Medinets S, Skiba U, Rennenberg H, Butterbach-Bahl K (2015) A review of soil NO transformation: associated processes and possible physiological significance on organisms. Soil Biol Biochem 80:92–117
Melton ED, Swanner ED, Behrens S, Schmidt C, Kappler A (2014) The interplay of microbially mediated and abiotic reactions in the biogeochemical Fe cycle. Nat Rev Microbiol 12:797–808
Minami K, Fukushi S (1986) Emission of nitrous oxide from a well-aerated andosol treated with nitrite and hydroxylamine. Soil Sci Plant Nutr 32(2):233–237
Moffett JW, Goepfert TJ, Naqvi SWA (2007) Reduced iron associated with secondary nitrite maxima in the Arabian Sea. Deep Sea Res 54:1341–1349
Montzka S, Dlugokencky E, Butler J (2011) Non-CO2 greenhouse gases and climate change. Nature 476(7358):43–50
Moraghan J, Buresh R (1977) Chemical reduction of nitrite and nitrous oxide by ferrous iron. Soil Sci Soc Am J 41(1):47–50
Murray A, Kenig F, Fritsen C, McKay C, Cawley K, Edwards R, Kuhn E, McKnight D, Ostrom N, Peng V (2012) Microbial life at −13 °C in the brine of an ice-sealed Antarctic lake. Proc Natl Acad Sci 109(50):20626–20631
Naqvi S, Bange HW, Farías L, Monteiro P, Scranton M, Zhang J (2010) Marine hypoxia/anoxia as a source of CH4 and N2O. Biogeosciences 7(7):2159–2190
Nelson D, Bremner J (1969) Factors affecting chemical transformations of nitrite in soils. Soil Biol Biochem 1(3):229–239
Nelson D, Bremner J (1970a) Gaseous products of nitrite decomposition in soils. Soil Biol Biochem 2(3):203–204
Nelson D, Bremner J (1970b) Role of soil minerals and metallic cations in nitrite decomposition and chemodenitrification in soils. Soil Biol Biochem 2(1):1–8
Ostrom NE, Ostrom PH (2012) The isotopomers of nitrous oxide: analytical considerations and application to resolution of microbial production pathways. In: Baskaran M (ed) Handbook of environmental isotope geochemistry. Springer, Dordrecht, pp 453–476
Ostrom NE, Russ ME, Popp B, Rust TM, Karl DM (2000) Mechanisms of nitrous oxide production in the subtropical North Pacific based on determinations of the isotopic abundances of nitrous oxide and di-oxygen. Chemosphere 2(3):281–290
Ostrom N, Gandhi H, Murray A, Murray T (2015) The enigmatic nitrogen biogeochemistry of the cryoecosystem of Lake Vida, Victoria Valley, Antarctica. Geobiology. In review
Peters B, Casciotti KL, Samarkin VA, Madigan MT, Schutte CA, Joye SB (2014) Stable isotope analyses of NO −2 , NO −3 and N2O in the hypersaline ponds and soils of the McMurdo Dry Valleys, Antarctica. Geochem Cosmochim Acta 135:87–101
Picardal F (2012) Abiotic and microbial interactions during anaerobic transformations of Fe(II) and NO −x . Front Microbiol 3(112):1–7. doi:10.3389/fmicb.2012.00112
Porter LK (1969) Gaseous products produced by anaerobic reaction of sodium nitrite with oxime compounds and oximes synthesized from organic matter. Sol Sci Soc Am J 33(5):696–702
Priscu J, Christner B, Dore J, Popp B, Casciotti K, Lyons B (2008) Supersaturated N2O in a perennially ice-covered Antarctic lake: molecular and stable isotopic evidence for a biogeochemical relict. Limnol Oceanogr 53(6):2439–2450
Ravishankara A, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326(5949):123–125
Robertson G (1987) Nitrous oxide sources in aerobic soils: nitrification, denitrification and other biological processes. Soil Biol Biochem 19(2):187–193
Rubasinghege G, Spak SN, Stanier CO, Carmichael GR, Grassian VH (2011) Abiotic mechanism for the formation of atmospheric nitrous oxide from ammonium nitrate. Environ Sci Technol 45(7):2691–2697
Samarkin VA, Madigan MT, Bowles MW, Casciotti KL, Priscu JC, McKay CP, Joye SB (2010) Abiotic nitrous oxide emission from the hypersaline Don Juan Pond in Antarctica. Nat Geosci 3(5):341–344
Santoro AE, Buchwald C, McIlvin MR, Casciotti KL (2011) Isotopic signature of N2O produced by marine ammonia-oxidizing archaea. Science 333(6047):1282–1285
Santoro AE, Dupont CL, Richter RA, Craig MT, Carini P, McIlvin MR, Yang Y, Orsi WD, Moran DM, Saito MA (2015) Genomic and proteomic characterization of “Candidatus Nitrosopelagicus brevis”: an ammonia-oxidizing archaeon from the open ocean. Proc Natl Acad Sci USA 112(4):1173–1178
Schlesinger W (2009) On the fate of anthropogenic nitrogen. Proc Natl Acad Sci USA 106(1):203–208
Schreiber F, Polerecky L, de Beer D (2008) Nitric oxide microsensor for high spatial resolution measurements in biofilms and sediments. Anal Chem 80(4):1152–1158
Schreiber F, Loeffler B, Polerecky L, Kuypers MM, De Beer D (2009) Mechanisms of transient nitric oxide and nitrous oxide production in a complex biofilm. ISME J 3(11):1301–1313
Schreiber F, Wunderlin P, Udert K, Wells G (2012) Nitric oxide and nitrous oxide turnover in natural and engineered microbial communities: biological pathways, chemical reactions, and novel technologies. Front Microbiol 3:372
Schreiber F, Stief P, Kuypers MM, de Beer D (2014) Nitric oxide turnover in permeable river sediment. Limnol Oceanogr 59(4):1310–1320
Schweiger B, Hansen H, Bange H (2007) A time series of hydroxylamine (NH2OH) in the southwestern Baltic Sea. Geophys Res Lett 34:L24608. doi:10.1029/2007GL031086
Seitzinger S (1988) Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnol Oceanogr 33:702–724
Shacklette HT, Boerngen JG (1984) Element concentrations in soils and other surficial materials of the conterminous United States. Technical report, US Geological Survey Professional Paper 1270, Washington, DC
Shen Q, Ran W, Cao Z (2003) Mechanisms of nitrite accumulation occurring in soil nitrification. Chemosphere 50(6):747–753
Shrestha J, Rich JJ, Ehrenfeld JG, Jaffe PR (2009) Oxidation of ammonium to nitrite under iron-reducing conditions in wetland soils: laboratory, field demonstrations, and push-pull rate determination. Soil Sci 174(3):156–164
Skiba U, Ball B (2002) The effect of soil texture and soil drainage on emissions of nitric oxide and nitrous oxide. Soil Use Manag 18(1):56–60
Skipper H, Westermann D (1973) Comparative effects of propylene oxide, sodium azide, and autoclaving on selected soil properties. Soil Biol Biochem 5(4):409–414
Smith R, Burns L, Doyle R, Lennox S, Kelso B, Foy R, Stevens R (1997) Free ammonia inhibition of nitrification in river sediments leading to nitrite accumulation. J Environ Qual 26(4):1049–1055
Sørensen J, Thorling L (1991) Stimulation by lepidocrocite (7-FeOOH) of Fe(II)-dependent nitrite reduction. Geochim Cosmochim Acta 55(5):1289–1294
Stark J, Firestone M (1995) Mechanisms for soil-moisture effects on activity of nitrifying bacteria. Appl Environ Microbiol 61(1):218–221
Stein LY (2011) Surveying N2O-producing pathways in bacteria. Methods Enzymol 486:131–151
Stein LY, Yung YL (2003) Production, isotopic composition, and atmospheric fate of biologically produced nitrous oxide. Ann Rev Earth Planet Sci 31(1):329–356
Stevens R, Laughlin R, Malone J (1998) Soil pH affects the processes reducing nitrate to nitrous oxide and di-nitrogen. Soil Biol Biochem 30(8–9):1119–1126
Stevenson F (1994) Humus chemistry: genesis, composition, reactions. Wiley, New York
Stevenson F, Swaby R (1964) Nitrosation of soil organic matter: I. Nature of gases evolved during nitrous acid treatment of lignins and humic substances. Soil Sci Soc Am J 28(6):773–778
Stevenson F, Harrison R, Wetselaar R, Leeper R (1970) Nitrosation of soil organic matter: III. Nature of gases produced by reaction of nitrite with lignins, humic substances, and phenolic constituents under neutral and slightly acidic conditions. Soil Sci Soc Am J 34(3):430–435
Stieglmeier M, Mooshammer M, Kitzler B, Wanek W, Zechmeister-Boltenstern S, Richter A, Schleper C (2014) Aerobic nitrous oxide production through N-nitrosating hybrid formation in ammonia-oxidizing archaea. ISME J 8(5):1135–1146
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural waters. Wiley, New York
Sutka R, Ostrom N, Ostrom P, Gandhi H, Breznak J (2003) Nitrogen isotopomer site preference of N2O produced by Nitrosomonas europaea and Methylococcus capsulatus Bath. Rapid Commun Mass Sp 17(7):738–745
Sutka R, Ostrom N, Ostrom P, Gandhi H, Breznak J (2004) Erratum: nitrogen isotopomer site preference of N2O produced by Nitrosomonas europaea and Methylococcus capsulatus Bath. Rapid Commun Mass Sp 18(12):1411–1412
Sutka RL, Ostrom N, Ostrom P, Breznak J, Gandhi H, Pitt A, Li F (2006) Distinguishing nitrous oxide production from nitrification and denitrification on the basis of isotopomer abundances. Appl Environ Microbiol 72(1):638–644
Tebo BM, Johnson HA, McCarthy JK, Templeton AS (2005) Geomicrobiology of manganese (II) oxidation. Trends Microbiol 13(9):421–428
Thamdrup B, Dalsgaard T (2000) The fate of ammonium in anoxic manganese oxide-rich sediment. Geochim Cosmochim Acta 64(24):4157–4164
Thomson A, Giannopoulos G, Pretty J, Baggs E, Richardson D (2012) Biological sources and sinks of nitrous oxide and strategies to mitigate emissions. Philos Trans R Soc B 367(1593):1157–1168
Thorn K, Mikita M (2000) Nitrite fixation by humic substances nitrogen-15 nuclear magnetic resonance evidence for potential intermediates in chemodenitrification. Soil Sci Soc Am J 64(2):568–582
Toyoda S, Mutobe H, Yamagishi H, Yoshida N, Tanji Y (2005) Fractionation of N2O isotopomers during production by denitrifier. Soil Biol Biochem 37(8):1535–1545
Vajrala N, Martens-Habbena W, Sayavedra-Soto LA, Schauer A, Bottomley PJ, Stahl DA, Arp DJ (2013) Hydroxylamine as an intermediate in ammonia oxidation by globally abundant marine archaea. Proc Natl Acad Sci 110(3):1006–1011
Van Cleemput O (1998) Subsoils: chemo- and biological denitrification, N2O and N2 emissions. Nutr Cycles Agroecosys 52(2–3):187–194
Van Cleemput O, Baert L (1984) Nitrite-a key compound in N-loss processes under acid conditions. Plant Soil 76(1–3):233–241
Van Cleemput O, Samater A (1996) Nitrite in soils: accumulation and role in the formation of gasseous N compounds. Fertil Res 45(1):81–89
Venkiteswaran J, Rosamond M, Schiff S (2014) Nonlinear response of riverine N2O fluxes to oxygen and temperature. Environ Sci Technol 48(3):1566–1573
Venterea RT (2007) Nitrite-driven nitrous oxide production under aerobic soil conditions: kinetics and biochemical controls. Global Change Biol 13(8):1798–1809
Venterea R, Rolston D (2000) Nitric and nitrous oxide emissions following fertilizer application to agricultural soil: biotic and abiotic mechanisms and kinetics. Global Change Biol 105(D12):15117–15129
Von Breymann MT, De Angelis MA, Gordon LI (1982) Gas chromatography with electron capture detection for determination of hydroxylamine in seawater. Anal Chem 54(7):1209–1210
Walker C, de La Torre J, Klotz M, Urakawa H, Pinel N, Arp D, Brochier-Armanet C, Chain P, Chan P, Gollabgir A (2010) Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci USA 107(19):8818–8823
Ward B, Zafiriou O (1988) Nitrification and nitric oxide in the oxygen minimum of the eastern tropical North Pacific. Deep Sea Res 35(7):1127–1142
Weber KA, Achenbach LA, Coates JD (2006) Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4(10):752–764
Williams E, Hutchinson G, Fehsenfeld F (1992) NOx and N2O emissions from soil. Global Biogeochem Cycles 6(4):351–388
Wolf D, Dao T, Scott H, Lavy T (1989) Influence of sterilization methods on selected soil microbiological, physical, and chemical properties. J Environ Qual 18(1):39–44
Wrage N, Velthof G, van Beusichem M, Oenema O (2001) Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem 33(12–13):1723–1732
Wunderlin P, Lehmann M, Siegrist H, Tuzson B, Joss A, Emmenegger L, Mohn J (2013) Isotope signatures of N2O in a mixed microbial population system: constraints on N2O producing pathways in wastewater treatment. Environ Sci Technol 47(3):1339–1348
Yang W, Weber K, Silver W (2012) Nitrogen loss from soil through anaerobic ammonium oxidation coupled to iron reduction. Nat Geosci 5(8):538–541
Zafiriou OC, McFarland M (1980) Determination of trace levels of nitric oxide in aqueous solution. Anal Chem 52(11): 1662–1667
Zafiriou O, McFarland M, Bromund R (1980) Nitric oxide in seawater. Science 207(4431):637–639
Zamora L, Oschlies A (2014) Surface nitrification: a major uncertainty in marine N2O emissions. Geophys Res Lett 41(12):4247–4253
Zhu X, Burger M, Doane T, Horwath W (2013a) Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc Natl Acad Sci USA 110(16):6328–6333
Zhu X, Silva LC, Doane TA, Horwath WR (2013b) Iron: the forgotten driver of nitrous oxide production in agricultural soil. PLoS ONE 8(3):e60146
Acknowledgments
We thank Martin Klotz, Lisa Stein, Nicolas Brüggemann, Bess Ward, and Fourth International Conference on Nitrification (ICoN4) participants for helpful discussions. We also thank Timothy A. Doane, G. Philip Robertson, associate editor R. Kelman Wieder, and four anonymous reviewers for thoughtful comments on earlier versions of this manuscript. WRH and XZB acknowledge support provided by the J. G. Boswell Endowed Chair in Soil Science and USDA National Institute of Food and Agriculture (NIFA; Grant Number: 2011-67003-30371). ARC acknowledges support from the NSF Graduate Research Fellowship Program and the Georgia Institute of Technology Goizueta Foundation Fellowship. NEO acknowledges support from the NSF Geobiology and Low Temperature Geochemistry program (Grants 1053432 and 1348935). JBG acknowledges support from NASA Exobiology Grant NNX14AJ87G and a Center for Dark Energy Biosphere Investigations (NSF-CDEBI OCE-0939564) Small Research Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: R. Kelman Wieder.
Rights and permissions
About this article
Cite this article
Zhu-Barker, X., Cavazos, A.R., Ostrom, N.E. et al. The importance of abiotic reactions for nitrous oxide production. Biogeochemistry 126, 251–267 (2015). https://doi.org/10.1007/s10533-015-0166-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-015-0166-4