Abstract
Kelp forests provide habitat for many species, but it remains uncertain whether specific kelp forest types support distinct biodiversity. Surface canopy-forming giant kelp (Macrocystis pyrifera) forests in Tasmania, Australia, have declined significantly due to climate change, and have been widely replaced by forests dominated by smaller stipitate kelps like Ecklonia radiata. However, there is limited knowledge of the community composition of Macrocystis forests and how this may differ from the stipitate kelp forest community. Underwater visual census surveys were conducted of the fishes and macroinvertebrates (> 2.5 cm length) in remnant Macrocystis forests in south-eastern Tasmania, and in adjacent stipitate kelp forests. 18 sites (9 of each forest type) were surveyed across two regions during the period of peak growth and canopy cover (i.e. winter-spring). Faunal community composition varied little between forest types, although Macrocystis forests supported more than double the abundance and biomass of mobile fishes, while cryptic fishes differed by forest type depending on region. Macroinvertebrate assemblages did not differ between forest types nor regions. Thus, for the taxa and time period examined, Tasmanian Macrocystis and stipitate kelp forests supported mostly similar faunal communities. Kelp forest communities showed spatial variation and were also likely influenced by a variety of other habitat characteristics, such as the relatively small patch sizes and/or ephemeral state of the remnant Macrocystis forests. Quantifying the community structure of these endangered communities informs the ecological changes that have occurred and will serve as an important reference for ongoing conservation and restoration activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat complexity is crucial factor that influences biodiversity, but its effects on community structure can be complex and multifaceted and are poorly understood (Beck 2000; Kovalenko et al. 2012; Tokeshi and Arakaki 2012). More complex habitats can support higher species richness and abundance, which at small-spatial scales is attributed to greater niche availability and/or increased surface area (e.g. Taniguchi et al. 2003; Willis et al. 2005; Stein et al. 2014). In some ecosystems, such as kelp forests and coral reefs, this complexity is provided by habitat-forming organisms or ecosystem engineers. As a result, the physical structure and morphology of these organisms is expected to play a key role in structuring associated communities (Teagle et al. 2017; Shelamoff et al. 2019).
Kelp forests are a conspicuous and valuable feature of temperate and subpolar coastal environments (Bennett et al. 2016; Eger et al. 2023), characterised by dense stands of large, canopy-forming, brown macroalgae (here adopting the broad functional definition of ‘kelp’ [sensu Steneck and Johnson 2014; Layton et al. 2020; discussed by Fraser 2012]). These forests are dominated by a variety of kelp species, with three broad morphological groups of kelp that create distinct forest structures (Dayton 1985; Steneck et al. 2002; Steneck and Johnson 2014). ‘Surface canopy’ kelps produce floating canopies that can extend 5–45 m above the substrate, whereas ‘stipitate’ kelps have rigid stalks or ‘stipes’ that can extend the canopy up to 2 m above the substrate, and ‘prostrate’ kelps lie on or just above the substratum. These kelp morphologies often occur sympatrically and alongside a wide variety of understorey macroalgae, turfing algae, and encrusting coralline algae, to produce highly complex and layered habitats (Dayton 1985; Steneck et al. 2002).
Kelps create habitat primarily through the provision of physical structure, which adds complexity and directly modifies local abiotic conditions (Teagle et al. 2017; Miller et al. 2018; Layton et al. 2019). Kelp structure provides shelter, spawning, and attachment sites for other organisms (Steneck et al. 2002; Teagle et al. 2017), while kelp canopies shade the seafloor (Reed and Foster 1984), modify local hydrodynamics (Gaylord et al. 2007), and also affect local biogeochemical conditions via their high productivity (Bustamante et al. 1995; Pessarrodona et al. 2022). These biophysical changes result in complex ecological interactions and feedbacks that influence associated organisms and further affect the structure, stability, and resilience of the entire ecosystem (e.g. ecosystem-engineer feedbacks [Dayton et al. 1984; Jones et al. 2010; Layton et al. 2019]).
In this way, kelp forests also play a vital role in creating unique biotic conditions that support productive and biodiverse communities (Schiel and Foster 2015; Teagle et al. 2017). These communities are the product of a complex and dynamic balance between positive and negative effects, with unique habitat conditions and biological interactions facilitating and inhibiting the colonising organisms in multifaceted ways (e.g. Connell 2003; Bennett et al. 2015). For example, certain fishes show increased recruitment within kelp forests, likely due to larval retention coupled with the forests’ physical structure and productivity (Carr 1994; O’Connor and Anderson 2010). Similarly, kelp-removal experiments demonstrate that many fishes are dependent on this forest habitat (e.g. Bodkin 1988; Carr 1989; Vanella et al. 2007). Invertebrates can likewise benefit from greater habitat complexity (Bué et al. 2020), substrate availability (Arkema et al. 2009), larval retention (Almanza et al. 2012), and productivity (Shelamoff et al. 2020a) within kelp forests, but may be negatively impacted by other processes like scour (Connell 2003).
Not only are these ecological interactions inherently complex but they also play out in a variable environment, driving significant spatial and temporal variability in kelp forests and their associated communities (e.g. Ebeling et al. 1980; Torres-Moye et al. 2013; Lamy et al. 2018). Some of this variability may arise because different forest growth forms (i.e. surface canopy-forming, stipitate, and prostrate) create distinct habitats that some species prefer due to differences in habitat quantity and complexity, or the provision of particular functional niches (Graham et al. 2007; Villegas et al. 2008). However, faunal communities in different forest types have rarely been compared explicitly or comprehensively (but see Graham et al. 2008; Villegas et al. 2008; Marzinelli et al. 2014). Current evidence suggests that while community structure can vary between kelp forest growth forms, these differences are typically minor, with only a few species showing habitat preferences (Graham et al. 2007; Villegas et al. 2008). In particular, midwater fishes appear to be closely associated with kelp surface canopies (e.g. Bodkin 1988; Moreno and Jara 1984; Graham 2004; Vanella et al. 2007), so these may prefer this type of forest. However, the drivers of variation in community structure between kelp forest types remain unclear, as does the ecological relevance of different kelp forest morphologies.
This is particularly relevant on the Great Southern Reef in Tasmania, Australia, a global hotspot of ocean warming, marine biodiversity, and endemism (Johnson et al. 2011; Hobday and Pecl 2014; Bennett et al. 2016). Here, more than 95% of surface canopy-forming giant kelp (Macrocystis pyrifera, hereafter Macrocystis) forests have been lost since the 1970s (Johnson et al. 2011; Butler et al. 2020). This ongoing decline has been driven primarily by the southward extension of the warm and nutrient-poor East Australian Current and its effects on regional oceanography (Johnson et al. 2011; Butler et al. 2020; Ridgway and Ling 2023). Here, Macrocystis forests have been widely replaced by forests dominated by the smaller, more thermally tolerant stipitate kelp Ecklonia radiata (hereafter Ecklonia; Wernberg et al. 2019), or by sea urchin barrens (Steneck and Johnson 2014; Ling and Keane 2024; Layton et al. 2020).
Representative examples of surface canopy-forming Macrocystis pyrifera forests (left) and stipitate Ecklonia radiata-dominated forests (right) in Tasmania, Australia. Note differences in the canopy (top panels) and understorey (bottom panels) structure of each forest type (photo credits, clockwise from top-left: Stefan Andrews courtesy Great Southern Reef Foundation; Matthew Doggett; Hunter Forbes; Stefan Andrews courtesy Great Southern Reef Foundation)
The dramatic losses of Tasmanian Macrocystis forests led to their national listing as an ‘endangered ecological community’ in 2012 (Department of the Environment 2022). However, the identity and uniqueness of these giant kelp forest communities is yet to be clearly defined, especially relative to the lower-lying stipitate kelp forests that have replaced them (Fig. 1). The ongoing decline of this endangered ecosystem coupled with growing interest in their conservation and restoration (e.g. Layton et al. 2020; Layton and Johnson 2021; Eger et al. 2022), has created an urgency to characterise these communities and evaluate whether they are functionally distinct (see Walker 1992; Lawton and Brown 1994; Rosenfeld 2002).
The aims of this study were to: (1) characterise the forest structure of kelp canopies across remnant Tasmanian Macrocystis and stipitate kelp forests; (2) characterise the fish and macroinvertebrate communities of the remnant Macrocystis forests (in the dominant period of canopy cover); and (3) compare them to the communities associated with sympatric stipitate kelp forests (dominated by Ecklonia). It was hypothesised that these two forest types would support a mostly similar faunal species pool, but that the broader community-level composition would differ with kelp forest type.
Methods
Study area
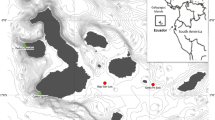
Surveys were conducted in two regions of south-eastern Tasmania, Australia, within Macrocystis forests and stipitate kelp forests (mostly dominated by Ecklonia) at similar depths. Site selection was based on availability of remnant Macrocystis forests of sufficient patch size (i.e. able to fit a 50 m by 10 m transect), height (i.e. kelp reaching the surface), and density (i.e. >75% surface canopy cover). Nearby stipitate kelp forest sites were then chosen accordingly. In total, surveys were conducted at 18 sites (n = 9 = Macrocystis forest; n = 9 = stipitate kelp forest), with 6 (3 of each forest type) in the Tinderbox region and 12 (6 of each forest type) in the Southport region (Fig. 2). Survey sites were typically separated by > 250 m (but two Macrocystis sites were separated by ∼65 m; see Fig. 2a), and surveys were conducted at depths of 6–11 m (mean = 7.8 m ± 0.3 SE). Tinderbox and Southport sites spanned the Bruny and Davey marine bioregions (Edgar et al. 1997). All surveys were conducted throughout May-September of 2022, which coincides with the period of peak Macrocystis surface-canopy growth and cover in Tasmania (Cribb 1954; Butler et al. 2020).
Survey methods
SCUBA divers conducted surveys using modified Reef Life Survey (RLS) underwater visual census methods (Reef Life Survey Foundation 2019; Edgar et al. 2020). Along with standard RLS methods, additional techniques were incorporated to better characterise the kelp forest habitat and align with methods used by the Australian Temperate Reef Collaboration (ATRC, https://atrc.au/). At each site, one 50 m transect was deployed along a constant depth contour. Divers first swam slowly along the transect and counted and sized any fishes and other large swimming animals (e.g. cephalopods, marine mammals, etc.) sighted within 5 m either side of the transect line, and within 5 m of the seafloor. Given the depth of the study sites (6–11 m) this captured most of the water column, and we observed very few, if any, animals above this 5 m band. Divers then swam back along the transect, searching through crevices and understorey macroalgae to count and size all mobile macroinvertebrates and cryptic fishes (i.e. inconspicuous species closely associated with the seabed; see Reef Life Survey Foundation 2019) within 1 m either side of the transect line. All fauna were sized using bins from 2.5 to > 62.5 cm based on their estimated total length (Reef Life Survey Foundation 2019), except for four invertebrate taxa, which were sized based on arm radius (Asteroidea and Crinoidea), carapace length (Decapoda), or test diameter (Echinoidea).
Macroalgae density and species richness were also measured in order to relate forest habitat structure to faunal community structure. The number of Macrocystis individuals and stipes was measured within 1 m either side of the transect, while stipe counts of other canopy or subcanopy-forming brown macroalgae (e.g. Ecklonia, Phyllospora comosa, Carpoglossum confluens, Cystophora spp., Sargassum spp.; hereafter referred to as ‘subcanopy macroalgae’) were conducted within 1 × 1 m quadrats every 10 m along the transect (i.e. 5 in total per survey; except at one site where subcanopy macroalgae density was not measured [Actaeon Island, at Southport]). We define these species as ‘subcanopy macroalgae’ for clarity and simplicity, to differentiate them from the larger surface canopy-forming Macrocystis as well as smaller understorey macroalgae, but acknowledge that these species do form canopies of their own in the absence of larger, surface canopy-forming macroalgae.
Statistical analyses
Multivariate generalised linear modelling (GLM) with a negative binomial distribution was used to test the effect of forest type (fixed factor) and region (fixed factor) on the abundance of fauna. Univariate GLMs were then conducted to explore key sources of variation in three functional groups (i.e. large and actively-swimming “mobile fishes”, cryptic fishes, and macroinvertebrates). Non-Metric Multidimensional Scaling (nMDS) ordination was used to compare community structure between kelp forest types and regions. For this, a Bray-Curtis dissimilarity matrix was used, following square-root transformation and the exclusion of all data from one anomalous site that obscured differences between the remaining sites in the nMDS (Fossil Cliffs, at Tinderbox). Key species (with a Pearson’s correlation coefficient of ≥ 0.6 and that were observed on more than one survey) were depicted on the nMDS with vector overlays.
Univariate linear or generalised linear models were undertaken to test the effects of forest type and region and their interaction on the species richness, diversity (Shannon-Weiner diversity index; hereafter ‘SW diversity’), abundance, and estimated biomass (hereafter ‘biomass’) of faunal groups (i.e. all fauna, all fishes, mobile fishes, cryptic fishes, and macroinvertebrates), as well as on the density of Macrocystis and on the density and species richness of subcanopy macroalgae. Different error distributions were used to better meet assumptions for these models (i.e. Gaussian for diversity and density data; Poisson for count data; negative binomial for over-dispersed count data; quasi-Poisson for over-dispersed non-count data). Diagnostic plots of model fit and a test statistic of overdispersion were used to examine test assumptions and data were transformed to better satisfy model assumptions where necessary.
Community biomass was estimated using published length-weight relationships to estimate body mass (fishes [Edgar and Stuart-Smith 2009]; octopus [Macroctopus maorum; Lalas 2009]; urchins [Centrostephanus rodgersii, Heliocidaris erythrogramma; Ling et al. 2015]; all other invertebrates [Heather et al. 2021]; excluding the anemone Phlyctenactis tuberculosa which did not have a published length-weight relationship]).
All statistical analyses were undertaken in R 4.2.1 (R Core Team 2022), except for the nMDS which was conducted in Primer 6. Multivariate GLMs were undertaken using the package mvabund (Wang et al. 2012) and figures were produced using the package ggplot2 (Wickham 2016). Plots are presented with untransformed data for clarity, and in-text values are provided as medians with interquartile range (IQR) rather than means due to non-normal distribution.
Results
Overall, we recorded a total of 51 species across both kelp forest types (Appendix Table A.1), inclusive of fishes (n = 24 [12 large “mobile fishes”; 12 cryptic fishes]) and mobile macroinvertebrates (n = 27). These fishes belonged to 10 orders and 16 families and the macroinvertebrates to 4 phyla and 8 classes. Of these, 37 species were observed in Macrocystis forests and 36 in stipitate kelp forests, with 24 species (47%) found in both forest types. In both habitats, communities were dominated by reef-associated fishes and invertebrates (Fig. 3). The most abundant species by far was the crinoid Cenolia spp. (median = 498 individuals/transect [447.3 IQR]), while the most abundant fish was the labrid Notolabrus tetricus (median = 8 individuals/transect [5.8 IQR]). The abalone Haliotis rubra and urchin Heliocidaris erythrogramma were typically the next most abundant species at Southport and Tinderbox, respectively. Most other species were rare, occurring in low numbers on few surveys (Fig. 3; Appendix Table A.1).
Ranked abundance (% of total count) of the 8 most abundant species of fishes (left panels) and invertebrates (right panels), with all remaining species pooled, in Macrocystis and stipitate kelp forests at Southport (n = 6 for each; light grey) and Tinderbox (n = 3 for each; dark grey), Tasmania. * Denotes where species were pooled into ‘all others’ when they had equally low abundances as species not in the top 8. † Only 8 species were recorded in this habitat
Univariate analyses of forest structure
Measurements of macroalgal density confirmed that Macrocystis and stipitate kelp forests had distinct habitat structures (Fig. 4; Appendix Table B.1). Median Macrocystis density within Macrocystis forests was 0.2 individuals/m2 (0.4 IQR; Fig. 4a) and 1.0 stipes/m2 (2.9 IQR; Fig. 4b), significantly higher than stipitate forests (p < 0.001; Appendix Table B.1). Macrocystis densities did not significantly differ between regions (Appendix Table B.1). However, there was a significant difference in the density of subcanopy macroalgae between regions (p = 0.008; Fig. 4c; Appendix Table B.1; Fig. 10), with higher densities of subcanopy macroalgae at Tinderbox (18 stipes/m2 [6 IQR]) than Southport (10 stipes/m2 [5 IQR]). The interaction between forest type and region also had a significant effect on the species richness of understorey macroalgae (p = 0.004; Appendix Table B.1; Fig. 10), which differed between stipitate kelp forests in Southport and Tinderbox but was similar in Macrocystis forests (median = 4 species/transect [1.3 IQR]; Fig. 4d).
Macrocystis density (a [entire plants], b [stipes]) and subcanopy macroalgae (i.e. other habitat-forming brown macroalgae in the subcanopy) density (c) and species richness (d) in Macrocystis and stipitate kelp forests at Southport (n = 6 for each) and Tinderbox (n = 3 for each), Tasmania (Appendix Table B.1)
Multivariate analyses of community composition
Multivariate GLM indicated that community composition differed significantly across regions but not forest types (Table 1). Univariate GLMs showed that the composition of mobile fishes and cryptic fishes (but not invertebrates) differed by region, while forest type did not have a significant effect on the composition of any of these faunal groups.
nMDS ordination reflected this, showing clear separation in community structure between regions and some separation between forest types within the same region (Fig. 5). All Tinderbox sites were closely grouped, while Southport sites were widely dispersed but still separated from the Tinderbox sites. Stipitate kelp forest communities were generally more closely grouped than Macrocystis forests. The vector overlay of Pearson’s correlation coefficients suggested that Southport sites had higher abundances of Haliotis rubra (Gastropoda, Mollusca) and Tinderbox sites had higher abundances of Notolabrus tetricus (Labridae, Osteichthyes). Cenolia spp. (Crinoidea, Echinodermata) and Ranella australasia (Gastropoda, Mollusca) were also key drivers of site separation, primarily due to site level differences in these very abundant and very rare species, respectively.
Non-Metric Multidimensional Scaling (nMDS) ordination of kelp forest faunal communities in Macrocystis (circles) and stipitate kelp (triangles) forests at Southport (green) and Tinderbox (blue), Tasmania. Vectors correspond to the strength and direction of species with a Pearson’s correlation coefficient of ≥ 0.6 that occurred on more than one survey
Univariate analyses of community composition
Total faunal (i.e. fish and macroinvertebrate) SW diversity, species richness, abundance, or biomass did not differ by forest types or region (Fig. 6; Table 2; Appendix Table C.1). Median faunal SW diversity was 0.4/transect (0.3 IQR), median species richness was 11 species/transect (3 IQR), median abundance was 542 individuals/transect (487.5 IQR), and median biomass was 98.0 kg/transect (132.4 IQR). There was also no difference in the SW diversity, species richness, abundance, or biomass of all fishes (i.e. mobile and cryptic fish together) between forest types (Appendix Table C.2; Fig. 11).
Total fauna (i.e. fish and macroinvertebrate) SW diversity (a), species richness (b), abundance (c), and biomass (d) per transect in Macrocystis and stipitate kelp forests at Southport (n = 6 in each) and Tinderbox (n = 3 in each), Tasmania (Appendix Table C.1)
For the mobile fish assemblage, there were no differences in diversity or species richness between forest types or regions (Fig. 7; Table 2; Appendix Table C.3). Median mobile fish SW diversity was 0.7/transect (0.3 IQR) and median species richness was 3 species/transect (2 IQR). However, the abundance of mobile fishes did differ between both forest types (p < 0.001) and regions (p = 0.002; Fig. 7c), with more mobile fishes in Macrocystis forests and at Tinderbox. In Macrocystis forests, median mobile fish abundance was 22 individuals/transect (22 IQR), more than double that of stipitate kelp forests (median = 8 individuals/transect [7 IQR]). There were also differences in mobile fish biomass between forest types (p = 0.046; Fig. 7d), with higher biomass in Macrocystis forests (median = 1.3 kg/transect [0.6 IQR]) than in stipitate kelp forests (median = 0.8 kg/transect [0.4 IQR]), but with no regional differences.
Mobile fish SW diversity (a), species richness (b), abundance (c), and biomass (d) per transect in Macrocystis and stipitate kelp forests at Southport (n = 6 of each) and Tinderbox (n = 3 of each), Tasmania (Appendix Table C.3)
Cryptic fishes generally made only a small contribution to surveys, with a median SW diversity of 0.4/transect (0.7 IQR), a median species richness of 2 species/transect (2.8 IQR), a median abundance of 2 individuals/transect (4 IQR), and a median biomass of 0.02 kg/transect (0.1 IQR). Cryptic fish assemblages were also mostly similar between forest types but did differ between regions (Fig. 8; Table 2; Appendix Table C.4). Cryptic fish SW diversity and richness were both higher (p = 0.017 and p = 0.005, respectively) at Tinderbox (median diversity = 0.7 [0.6 IQR]; median richness = 3 species/transect [2 IQR]) than at Southport (median SW diversity = 0.0 [0.7 IQR]; median richness = 1 species/transect [2 IQR]). There was also a significant interaction between forest type and region for cryptic fish abundance (p = 0.042; Fig. 8c), which was highest in stipitate kelp forests at Tinderbox and lowest in stipitate kelp forests at Southport. There was no difference in cryptic fish biomass between forest types or regions (Fig. 8d).
Cryptic fish SW diversity (a), species richness (b), abundance (c), and biomass (d) per transect in Macrocystis and stipitate kelp forests at Southport (n = 6 in each) and Tinderbox (n = 3 in each), Tasmania (Appendix Table C.4)
Macroinvertebrates generally made the greatest contribution to surveys, with a median species richness of 7 species/transect (3.8 IQR), a median abundance of 535 individuals/transect (457.5 IQR), and a median biomass of 96.2 kg/transect (125.8 IQR). This pattern was driven by large numbers of Cenolia spp. on almost all surveys, resulting in a relatively low median SW diversity of 0.3/transect (0.1 IQR). However, macroinvertebrate assemblages did not differ between forest types or regions in any of these parameters (Fig. 9; Table 2; Appendix Table C.5).
Macroinvertebrate SW diversity (a), species richness (b), abundance (c), and biomass (d) per transect in Macrocystis and stipitate kelp forests at Southport (n = 6 in each) and Tinderbox (n = 3 in each), Tasmania (Appendix Table C.5)
Discussion
Overall, Tasmanian Macrocystis forests and Ecklonia-dominated stipitate kelp forests appeared to support similar faunal communities during the period of peak canopy cover, but more than double the abundance and biomass of mobile fishes occurred in Macrocystis forests. Cryptic fishes showed a more complex pattern, with particularly high abundance within stipitate kelp forests in one region. Despite different habitat structure in Macrocystis and stipitate kelp forests, there were few community-level differences in fish and macroinvertebrate assemblages, and differences were mostly between regions rather than forest types. The overall composition (i.e. multivariate community structure) of mobile and cryptic fish assemblages differed between regions, as did mobile fish abundance and cryptic fish diversity, richness, and abundance. In contrast, surveys did not detect any difference in macroinvertebrate assemblages between forest types or regions. Overall, these results suggest that these distinct kelp forest types support mostly similar faunal communities but that some taxa do respond to differences between these habitats.
Regional variation was generally found to be important, and this is consistent with prior research that emphasises kelp forest communities do display high spatial variation between regions and sites (Moreno and Jara 1984; Torres-Moye et al. 2013; Lamy et al. 2018). Evidence suggests that spatial variation in kelp forest communities can be driven by differences in local substrate complexity (Torres-Moye et al. 2013) or by broad-scale environmental conditions like sea surface temperature or oceanographic connectivity (Graham et al. 2008; Lamy et al. 2018). Indeed, Southport and Tinderbox sites spanned a border between bioregions (Edgar et al. 1997), so regional differences in community composition were not unexpected. In addition to bioregional differences in fauna, the Tinderbox sites were mostly located inside a no-take marine reserve, which could have contributed to regional variation in fish assemblages. The positive effects of marine reserves on fish communities are well documented (e.g. Willis et al. 2003; Edgar and Stuart-Smith 2009; Sala and Giakoumi 2018), and likely contributed to higher mobile fish abundance at Tinderbox compared to Southport (Fig. 7c). Interestingly however, even non-target cryptic fishes were more abundant, diverse, and species rich within the marine reserve (Fig. 8), suggesting potentially complex interactive effects and trophic cascades (Shears and Babcock 2003; Edgar et al. 2017).
Faunal responses to differences in kelp forest habitat structure
Differences in faunal assemblages were detected between kelp forest types, but only for fishes, and for mobile and cryptic fishes in different ways. Macrocystis forests supported∼2.5 times more mobile fishes than stipitate kelp forests, and close to double the biomass. This suggests that surface canopy-forming and stipitate kelp forests can be distinct habitats that are preferred by some species due to differences in habitat quality or quantity (Graham et al. 2007; Villegas et al. 2008). Similar differences in fish abundance and biomass between different kelp forest types have been previously recorded (Bodkin 1986), and macroalgal species identity and density have been found to better predict fish biomass than fish composition, diversity, or density (Srednick and Steele 2022). Relative to stipitate kelp forests, Macrocystis forests may offer more algal surface area throughout more of the water column along with greater biomass, and thus provide more physical structure coupled with much greater primary productivity (Sanderson 1990; Pessarrodona et al. 2021, 2022) that in turn might support a greater abundance and biomass of fish. Specifically, this increased macroalgal biomass occurs as midwater and surface canopy structure that is primarily available to mobile fishes that more readily vary their behaviour and vertical distribution to exploit this habitat (Carr 1991; Srednick and Steele 2019, 2022).
Habitat complexity generally enhances fish richness in kelp forest ecosystems (e.g. Angel and Ojeda 2001; Efird and Konar 2014; Parsons et al. 2016) and certain fish species and functional groups often show a close or obligate association with kelp surface canopies (Moreno and Jara 1984; Graham 2004; Graham et al. 2007; Vanella et al. 2007). Macroalgal size has also been found to be a good predictor of fish assemblage composition, richness, and diversity (Srednick and Steele 2019, 2022). So, while some fish assemblages did seem to respond in terms of abundance and biomass to the larger and more complex canopy structure provided by Macrocystis, it was unexpected that overall species composition did not also differ with kelp forest type. One explanation for this is that no specialised surface canopy-associated species were identified in this study. These specialists seem to be the primary driver of differences in species composition between surface canopy-forming and other kelp forest types (Graham et al. 2007), yet there appear to be relatively few surface canopy specialist species worldwide (Limbaugh 1955; Ebeling et al. 1980; Moreno and Jara 1984). In Australia, kelps arrived relatively recently (ca. 3 mya; Durrant et al. 2015; Starko et al. 2019), compared to deep evolutionary ties of many reef fish and invertebrate taxa (Edgar et al. 2023), perhaps limiting the need for species to adapt to surface-canopy environments. Fishes may also respond to variation in kelp forest habitat complexity on different scales, for instance with different fish species or life stages responding more strongly to microhabitats or landscape-scale complexity created by kelp forests. Indeed, kelp density and number of stipes can influence fish taxonomic and functional diversity, abundance, and biomass on patch and within-patch scales (Shelamoff et al. 2020b; Sgarlatta et al. 2022), and significantly different fish assemblages can also be found at patch edges relative to patch interiors (Efird and Konar 2014). It seems that fish communities respond in complex ways to different characteristics of foundational macroalgae, with a combination of factors including macroalgal identity, density, surface area, and height variably influencing different aspects of fish assemblages (Srednick and Steele 2019, 2022). Consequently, generalisations of kelp forest structure may not entirely capture the subtleties and diversity in macroalgae form and ecology to which fishes are responding.
Cryptic fishes showed a distinct response from mobile fishes, differing in abundance with a significant interaction between forest type and region, where Tinderbox stipitate kelp forests supported the highest abundance of cryptic fishes (Fig. 8c). In part, this may be driven by stochastic observations and fish schooling behaviour, as large schools of small cryptic fishes (e.g. Pempheridae) were observed on a few occasions at Tinderbox. However, forest type and region were also found to affect subcanopy macroalgae species richness, with the lowest richness in Tinderbox stipitate kelp forests (Fig. 4d; Appendix Table B.1). In contrast, subcanopy macroalgae density was higher at Tinderbox than at Southport (Fig. 4c). Since cryptic fishes were primarily bottom-associated, benthic complexity and subcanopy structure is likely more important to these species than canopy structure, which can even indirectly inhibit bottom-associated fishes (Holbrook et al. 1990; Shelamoff et al. 2020b; Srednick and Steele 2022). Therefore, the less species-rich but denser subcanopy found in Tinderbox stipitate kelp forests could add benthic complexity and improve habitat quality for cryptic fishes without providing additional niches that alter species composition.
In contrast, there were no differences in macroinvertebrate assemblages between forest types. While they might be expected to respond in similar ways as fishes, macroinvertebrates may not respond as quickly or at all if the Macrocystis forests are not stable enough for larvae to settle and allow distinct assemblages to develop. Indeed, there is likely a spectrum of mobility and response speed that determines the effect of remnant Macrocystis forests on different organisms. Fishes, which are more mobile, and even fast-growing and prolific macroalgae, can likely respond much more quickly than macroinvertebrates to exploit or avoid the ephemeral habitat created by relatively unstable remnant Macrocystis forests. Therefore, persistent differences in kelp forest habitat structure may be needed to produce distinct macroinvertebrate communities. The comparatively stable subcanopy macroalgae assemblages may also impart some resilience to Macrocystis loss, since like cryptic fishes, these lower-lying macroalgae are likely more important for macroinvertebrates (as they are for epibiotic and holdfast invertebrates [see Parker et al. 2001; Goodsell et al. 2004]). Indeed, there seem to be few large canopy-associated invertebrates in Tasmania (the gastropods Phasianotrochus spp. and the urchins Holopneustes spp. are exceptions but were rare or absent on these surveys) or elsewhere (Limbaugh 1955). Thus, as for fishes, there may not be strong differences in invertebrate species composition between forest types if they simply do not occupy those surface canopy niches.
Ecosystem dynamics and management considerations
When interpreting the findings of this study, a number of other important ecological considerations need to be considered. Firstly, this study examined a subset of kelp forest biodiversity (i.e. fishes and mobile macroinvertebrates) over a limited temporal window (i.e. winter-spring, in one year). It is possible that differences exist between forest types for taxa not assessed in this study. For instance, previous studies have found differences between kelp forest types in epiphytic or holdfast-associated invertebrates that might respond to subtle differences in kelp morphology (e.g. Arnold et al. 2016; Marzinelli et al. 2016; Fraser et al. 2021). These invertebrates are important and highly abundant secondary producers in kelp forests (Taylor 1998; Davenport and Anderson 2007), and would likely benefit from the greater algal surface area and productivity in Macrocystis forests relative to stipitate kelp forests. Differences in forest structure also likely affect the abundance and composition of smaller, understorey macroalgae that were not quantified in this study, such as foliose red, green, and brown macroalgae, and turfing or encrusting species (Kimura and Foster 1984; Santelices and Ojeda 1984; Flukes et al. 2014), which contribute both directly and indirectly to forest biodiversity (Fraser et al. 2021). Similarly, while megafauna were not observed on-transect, they might also utilise these habitats in unique ways (e.g. Schrope 2007; Bullen et al. 2021). Different species (and even different life stages) likely respond differently to the complexity that kelps add on scales from the individual to the patch and landscape (see Tokeshi and Arakaki 2012). In fact, the landscape-scale complexity created by a mosaic of different kelp forest habitats could have the greatest benefit to biodiversity (Goodsell et al. 2004; Villegas et al. 2008). Understanding how different taxa respond to kelp forest structure on different spatial scales should be a priority for future research, especially in the context of the broader coastal systems they occupy.
A second consideration when interpreting the results presented here is that Tasmanian Macrocystis forests are already a severely altered ecosystem, having been in continuing decline since at least the 1970s (Johnson et al. 2011; Butler et al. 2020) and also likely altered by human activity since long before then (Jackson et al. 2001; Edgar et al. 2005; Barrett et al. 2009), coupled with ongoing Australia-wide declines in shallow temperate reef life (Edgar et al. 2023). In Tasmania, remnant patches of Macrocystis forest are much reduced in size and density from their historical state (see Cribb 1954), and many have become more transient (Butler et al. 2020; Layton and Johnson 2021). Indeed, over the course of this study, some adjacent patches of Macrocystis forest developed or disappeared entirely, and most remnant patches surveyed were well under 500 m in diameter. Consequently, the home range of many species observed likely extended beyond the edge of these remnant forests, particularly for mobile fishes (Barrett 1995). But this could also be true for less wide-ranging species given that many of these forest patches appear to be periodically reduced in size and habitat quality over summer (Butler et al. 2020), and community structure may be affected by this instability (Lamy et al. 2020) and habitat fragmentation (Efird and Konar 2014). Indeed, many of the biophysical effects of kelps scale with forest size and density (Gaylord et al. 2007; Layton et al. 2019; Shelamoff et al. 2020a), so the notion that larger, denser, older, or more stable Macrocystis forests may have historically created a distinct habitat is plausible. It is important to consider this issue of changing social memory or ‘sliding baselines’ in the management of these ecosystems (Dayton et al. 1998; Jackson 2001), especially when setting targets for kelp forest restoration and management (Hobbs 2007; Layton et al. 2020; Eger et al. 2022). It is unclear whether the apparent similarity of Macrocystis and stipitate kelp forests reflects functional redundancy between these kelp forest types in terms of habitat provision, or if it reflects a shifted baseline and state of already-diminished biodiversity in remnant Macrocystis forests.
Conclusions
Surface canopy-forming Macrocystis forests and Ecklonia-dominated stipitate kelp forests supported mostly similar fish and macroinvertebrate communities during the period of peak surface canopy cover, with almost all of the variation found between sampling regions rather than between forest types. However, Macrocystis forests supported more than double the abundance and biomass of mobile fishes, and the abundance of cryptic fishes differed with forest type in some regions. This suggests that Ecklonia-dominated forests might provide some compensation for the severe losses of Macrocystis in Tasmania, in terms of the habitat that they provide and biodiversity they support, but that Macrocystis forests may provide some unique habitat benefits to particular faunal groups, namely mobile fishes.
The responses of fauna to differences in kelp forest habitat structure are multifaceted, with different faunal groups variably influenced or unaffected by different habitat features. While mobile fishes seem more readily able to exploit midwater and surface canopy structure, cryptic fishes may respond to subcanopy macroalgae, and invertebrates appear unaffected by differences in kelp habitat structure. Fauna clearly respond to habitat characteristics on different spatial and temporal scales, and better understanding these dynamics is essential. If there is functional redundancy between Macrocystis and stipitate kelp forests in terms of habitat provision, biodiversity losses from Macrocystis forest declines may be minimal, with stipitate kelp forests serving as an important safeguard for reef biodiversity. However, the nature and variability of the relatively degraded remnant forests may also have contributed to the patterns observed, with these kelp forest types possibly creating more similar habitats today than they did historically, with Macrocystis forests not persisting long enough for a distinct community to develop.
This research offers key insights for conservation, restoration, and management in an ocean warming and marine biodiversity hotspot, and also helps to continue developing our understanding of how fauna respond to differences in kelp forest structure, and habitat complexity more broadly. While more research is still needed to characterise Tasmanian Macrocystis forest communities on greater temporal scales and including other taxa, it is clear that these habitats support valuable faunal communities and that this fundamental ecological knowledge is required to inform species prioritisation and conservation decision-making in the face of climate change. Ultimately, we must continue to improve our understanding of kelp forest communities and processes before it is too late to appreciate and conserve the biodiversity that these unique and important ecosystems support.
Data availability
The datasets generated and/or analysed during the current study are available from the National Reef Monitoring Network via the Australian Ocean Data Network (AODN) repository (https://portal.aodn.org.au/), or from the corresponding author on request.
References
Almanza V, Buschmann AH, Hernández-González MC, Henríquez LA (2012) Can giant kelp (Macrocystis pyrifera) forests enhance invertebrate recruitment in southern Chile? Mar Biol Res 8:855–864. https://doi.org/10.1080/17451000.2012.692159
Angel A, Ojeda FP (2001) Structure and trophic organization of subtidal fish assemblages on the northern Chilean coast: the effect of habitat complexity. Mar Ecol Prog Ser 217:81–91. https://doi.org/10.3354/meps217081
Arkema KK, Reed DC, Schroeter SC (2009) Direct and indirect effects of giant kelp determine benthic community structure and dynamics. Ecology 90:3126–3137. https://doi.org/10.1890/08-1213.1
Arnold M, Teagle H, Brown MP, Smale DA (2016) The structure of biogenic habitat and epibiotic assemblages associated with the global invasive kelp Undaria pinnatifida in comparison to native macroalgae. Biol Invasions 18:661–676. https://doi.org/10.1007/s10530-015-1037-6
Barrett NS (1995) Short- and long-term movement patterns of six temperate reef fishes (families Labridae and Monacanthidae). Mar Freshw Res 46:853–860. https://doi.org/10.1071/mf9950853
Barrett NS, Buxton CD, Edgar GJ (2009) Changes in invertebrate and macroalgal populations in tasmanian marine reserves in the decade following protection. J Exp Mar Biol Ecol 370:104–119. https://doi.org/10.1016/j.jembe.2008.12.005
Beck MW (2000) Separating the elements of habitat structure: independent effects of habitat complexity and structural components on rocky intertidal gastropods. J Exp Mar Biol Ecol 249:29–49. https://doi.org/10.1016/S0022-0981(00)00171-4
Bennett S, Wernberg T, de Bettignies T et al (2015) Canopy interactions and physical stress gradients in subtidal communities. Ecol Lett 18:677–686. https://doi.org/10.1111/ele.12446
Bennett S, Wernberg T, Connell SD et al (2016) The ‘Great southern reef’: social, ecological and economic value of Australia’s neglected kelp forests. Mar Freshw Res 67:47–56. https://doi.org/10.1071/MF15232
Bodkin JL (1986) Fish assemblages in Macrocystis and Nereocystis kelp forests off central California. Fish Bull 84:799–808
Bodkin JL (1988) Effects of kelp forest removal on associated fish assemblages in central California. J Exp Mar Biol Ecol 117:227–238. https://doi.org/10.1016/0022-0981(88)90059-7
Bué M, Smale DA, Natanni G et al (2020) Multiple-scale interactions structure macroinvertebrate assemblages associated with kelp understory algae. Divers Distrib 26:1551–1565. https://doi.org/10.1111/ddi.13140
Bullen CD, Campos AA, Gregr EJ et al (2021) The ghost of a giant – six hypotheses for how an extinct megaherbivore structured kelp forests across the North Pacific Rim. Glob Ecol Biogeogr 30:2101–2118. https://doi.org/10.1111/geb.13370
Bustamante RH, Branch GM, Eekhout S (1995) Maintenance of an exceptional intertidal Grazer Biomass in South Africa: Subsidy by Subtidal Kelps. Ecology 76:2314–2329. https://doi.org/10.2307/1941704
Butler CL, Lucieer VL, Wotherspoon SJ, Johnson CR (2020) Multi-decadal decline in cover of giant kelp Macrocystis pyrifera at the southern limit of its Australian range. Mar Ecol Prog Ser 653:1–18. https://doi.org/10.3354/meps13510
Carr MH (1989) Effects of macroalgal assemblages on the recruitment of temperate zone reef fishes. J Exp Mar Biol Ecol 126:59–76. https://doi.org/10.1016/0022-0981(89)90124-X
Carr MH (1991) Habitat selection and recruitment of an assemblage of temperate zone reef fishes. J Exp Mar Biol Ecol 146:113–137. https://doi.org/10.1016/0022-0981(91)90257-W
Carr MH (1994) Effects of Macroalgal Dynamics on Recruitment of a temperate reef fish. Ecology 75:1320–1333. https://doi.org/10.2307/1937457
Connell SD (2003) Negative effects overpower the Positive of Kelp to Exclude invertebrates from the Understorey Community. Oecologia 137:97–103
Cribb AB (1954) Macrocystis pyrifera (L.) Ag. In Tasmanian Waters. Mar Freshw Res 5:1–34. https://doi.org/10.1071/mf9540001
Davenport AC, Anderson TW (2007) Positive indirect effects of reef fishes on kelp performance: the importance of mesograzers. Ecology 88:1548–1561. https://doi.org/10.1890/06-0880
Dayton PK (1985) Ecology of Kelp communities. Annu Rev Ecol Syst 16:215–245
Dayton PK, Currie V, Gerrodette T et al (1984) Patch Dynamics and Stability of some California Kelp Communities. Ecol Monogr 54:254–289. https://doi.org/10.2307/1942498
Dayton P, Tegner M, Edwards P, Riser K (1998) Sliding baselines, ghosts, and reduced expectations in Kelp Forest communities. ECOL APPL 8:309–322. https://doi.org/10.2307/2641070
Department of the Environment (2022) Giant Kelp Marine Forests of South East Australia in Community and Species Profile and Threats Database. http://www.environment.gov.au/sprat. Accessed 3 Mar 2022
Durrant HMS, Barrett NS, Edgar GJ et al (2015) Shallow phylogeographic histories of key species in a biodiversity hotspot. Phycologia 54:556–565. https://doi.org/10.2216/15-24.1
Ebeling AW, Larson RJ, Alevizon WS, Bray RN (1980) Annual variability of reef-fish assemblages in kelp forests off Santa Barbara, California. Fish Bull 78:361–377
Edgar GJ, Stuart-Smith RD (2009) Ecological effects of marine protected areas on rocky reef communities—a continental-scale analysis. Mar Ecol Prog Ser 388:51–62. https://doi.org/10.3354/meps08149
Edgar GJ, Moverley J, Barrett NS et al (1997) The conservation-related benefits of a systematic marine biological sampling programme: the tasmanian reef bioregionalisation as a case study. Biol Conserv 79:227–240. https://doi.org/10.1016/S0006-3207(96)00095-X
Edgar GJ, Samson CR, Barrett NS (2005) Species extinction in the Marine Environment: Tasmania as a Regional Example of overlooked losses in Biodiversity. Conserv Biol 19:1294–1300. https://doi.org/10.1111/j.1523-1739.2005.00159.x
Edgar GJ, Stuart-Smith RD, Thomson RJ, Freeman DJ (2017) Consistent multi-level trophic effects of marine reserve protection across northern New Zealand. PLoS ONE 12:e0177216. https://doi.org/10.1371/journal.pone.0177216
Edgar GJ, Cooper A, Baker SC et al (2020) Establishing the ecological basis for conservation of shallow marine life using reef life survey. Biol Conserv 252:108855. https://doi.org/10.1016/j.biocon.2020.108855
Edgar GJ, Stuart-Smith RD, Heather FJ et al (2023) Continent-wide declines in shallow reef life over a decade of ocean warming. Nature 615:858–865. https://doi.org/10.1038/s41586-023-05833-y
Efird TP, Konar B (2014) Habitat characteristics can influence fish assemblages in high latitude kelp forests. Environ Biol Fish 97:1253–1263. https://doi.org/10.1007/s10641-013-0211-x
Eger AM, Marzinelli EM, Christie H et al (2022) Global kelp forest restoration: past lessons, present status, and future directions. Biological Reviews n/a: https://doi.org/10.1111/brv.12850
Eger AM, Marzinelli EM, Beas-Luna R et al (2023) The value of ecosystem services in global marine kelp forests. Nat Commun 14:1894. https://doi.org/10.1038/s41467-023-37385-0
Flukes EB, Johnson CR, Wright JT (2014) Thinning of kelp canopy modifies understory assemblages: the importance of canopy density. Mar Ecol Prog Ser 514:57–70. https://doi.org/10.3354/meps10964
Fraser C (2012) Is bull-kelp kelp? The role of common names in science. N Z J Mar Freshwat Res 46:279–284. https://doi.org/10.1080/00288330.2011.621130
Fraser KM, Stuart-Smith RD, Ling SD, Edgar GJ (2021) Small invertebrate consumers produce consistent size spectra across reef habitats and climatic zones. Oikos 130:156–170. https://doi.org/10.1111/oik.07652
Gaylord B, Rosman J, Reed D et al (2007) Spatial patterns of flow and their modification within and around a giant kelp forest. Limnol Oceanogr 52:1838–1852. https://doi.org/10.2307/4502339
Goodsell PJ, Fowler-Walker MJ, Gillanders BM, Connell SD (2004) Variations in the configuration of algae in subtidal forests: implications for invertebrate assemblages. Austral Ecol 29:350–357. https://doi.org/10.1111/j.1442-9993.2004.01372.x
Graham MH (2004) Effects of local Deforestation on the Diversity and Structure of Southern California Giant Kelp Forest Food Webs. Ecosystems 7:341–357. https://doi.org/10.1007/s10021-003-0245-6
Graham MH, Vásquez J, Buschmann AH (2007) Global ecology of the giant kelp Macrocystis: from ecotypes to ecosystems. CRC
Graham M, Halpern B, Carr M (2008) Diversity and Dynamics of Californian Subtidal Kelp Forests. In: Food Webs and the Dynamics of Marine Reefs. pp 103–134
Heather FJ, Blanchard JL, Edgar GJ et al (2021) Globally consistent reef size spectra integrating fishes and invertebrates. Ecol Lett 24:572–579. https://doi.org/10.1111/ele.13661
Hobbs RJ (2007) Setting effective and realistic restoration goals: key directions for Research. Restor Ecol 15:354–357. https://doi.org/10.1111/j.1526-100X.2007.00225.x
Hobday AJ, Pecl GT (2014) Identification of global marine hotspots: sentinels for change and vanguards for adaptation action. Rev Fish Biol Fisheries 24:415–425. https://doi.org/10.1007/s11160-013-9326-6
Holbrook S, Carr M, Schmitt R, Coyer J (1990) Effect of Giant Kelp on local abundance of reef fishes: the importance of Ontogenetic Resource requirements. Bull Mar Sci 47:104–114
Jackson JBC (2001) What was natural in the coastal oceans? Proceedings of the National Academy of Sciences 98:5411–5418. https://doi.org/10.1073/pnas.091092898
Johnson CR, Banks SC, Barrett NS et al (2011) Climate change cascades: shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. J Exp Mar Biol Ecol 400:17–32. https://doi.org/10.1016/j.jembe.2011.02.032
Jones CG, Gutiérrez JL, Byers JE et al (2010) A framework for understanding physical ecosystem engineering by organisms. Oikos 119:1862–1869. https://doi.org/10.1111/j.1600-0706.2010.18782.x
Kimura RS, Foster MS (1984) The effects of harvesting Macrocystis pyrifera on the algal assemblage in a giant kelp forest. Hydrobiologia 116:425–428. https://doi.org/10.1007/BF00027714
Kovalenko KE, Thomaz SM, Warfe DM (2012) Habitat complexity: approaches and future directions. Hydrobiologia 685:1–17. https://doi.org/10.1007/s10750-011-0974-z
Lalas C (2009) Estimates of size for the large octopus Macroctopus maorum from measures of beaks in prey remains. N Z J Mar Freshwat Res 43:635–642. https://doi.org/10.1080/00288330909510029
Lamy T, Reed DC, Rassweiler A et al (2018) Scale-specific drivers of kelp forest communities. Oecologia 186:217–233. https://doi.org/10.1007/s00442-017-3994-1
Lamy T, Koenigs C, Holbrook SJ et al (2020) Foundation species promote community stability by increasing diversity in a giant kelp forest. Ecology 101:e02987. https://doi.org/10.1002/ecy.2987
Lawton JH, Brown VK (1994) Redundancy in ecosystems. In: Schulze E-D, Mooney HA (eds) Biodiversity and ecosystem function. Springer, Berlin, Heidelberg, pp 255–270
Layton C, Johnson C (2021) Assessing the feasibility of restoring giant kelp forests in Tasmania. Marine Biodiversity Hub
Layton C, Shelamoff V, Cameron MJ et al (2019) Resilience and stability of kelp forests: the importance of patch dynamics and environment-engineer feedbacks. PLoS ONE 14:e0210220. https://doi.org/10.1371/journal.pone.0210220
Layton C, Coleman MA, Marzinelli EM et al (2020) Kelp Forest Restoration in Australia. Front Mar Sci 7. https://doi.org/10.3389/fmars.2020.00074
Limbaugh C (1955) Fish life in the kelp beds and the effects of kelp harvesting. Scripps Institute of Oceanography
Ling SD, Keane JP (2024) Climate-driven invasion and incipient warnings of kelp ecosystem collapse. Nat Commun 15:400. https://doi.org/10.1038/s41467-023-44543-x
Ling SD, Scheibling RE, Rassweiler A et al (2015) Global regime shift dynamics of catastrophic sea urchin overgrazing. Philosophical Trans Royal Soc B: Biol Sci 370:20130269. https://doi.org/10.1098/rstb.2013.0269
Marzinelli EM, Campbell AH, Vergés A et al (2014) Restoring seaweeds: does the declining fucoid Phyllospora comosa support different biodiversity than other habitats? J Appl Phycol 26:1089–1096. https://doi.org/10.1007/s10811-013-0158-5
Marzinelli EM, Leong MR, Campbell AH et al (2016) Does restoration of a habitat-forming seaweed restore associated faunal diversity? Restor Ecol 24:81–90. https://doi.org/10.1111/rec.12292
Miller RJ, Lafferty KD, Lamy T et al (2018) Giant kelp, Macrocystis pyrifera, increases faunal diversity through physical engineering. Proc Royal Soc B: Biol Sci 285:20172571. https://doi.org/10.1098/rspb.2017.2571
Moreno C, Jara H (1984) Ecological studies on fish fauna associated with Macrocystis pyrifera belts in the south of Fueguian Islands, Chile. https://doi.org/10.3354/MEPS015099. Marine Ecology Progress Series
O’Connor KC, Anderson TW (2010) Consequences of habitat disturbance and recovery to recruitment and the abundance of kelp forest fishes. J Exp Mar Biol Ecol 386:1–10. https://doi.org/10.1016/j.jembe.2010.01.016
Parker JD, Duffy JE, Orth RJ (2001) Plant species diversity and composition: experimental effects on marine epifaunal assemblages. Mar Ecol Prog Ser 224:55–67. https://doi.org/10.3354/meps224055
Parsons DF, Suthers IM, Cruz DO, Smith JA (2016) Effects of habitat on fish abundance and species composition on temperate rocky reefs. Mar Ecol Prog Ser 561:155–171. https://doi.org/10.3354/meps11927
Pessarrodona A, Filbee-Dexter K, Alcoverro T et al (2021) Homogenization and miniaturization of habitat structure in temperate marine forests. Glob Chang Biol 27:5262–5275. https://doi.org/10.1111/gcb.15759
Pessarrodona A, Assis J, Filbee-Dexter K et al (2022) Global seaweed productivity. Sci Adv 8:eabn2465. https://doi.org/10.1126/sciadv.abn2465
Reed DC, Foster MS (1984) The effects of Canopy shadings on Algal Recruitment and Growth in a Giant Kelp Forest. Ecology 65:937–948. https://doi.org/10.2307/1938066
Reef Life Survey Foundation (2019) Standardised Survey procedures for Monitoring Rocky & Coral reef Ecological communities. Reef Life Survey Foundation, Hobart
Ridgway KR, Ling SD (2023) Three decades of variability and warming of nearshore waters around Tasmania. Prog Oceanogr 215:103046. https://doi.org/10.1016/j.pocean.2023.103046
Rosenfeld JS (2002) Functional redundancy in ecology and conservation. Oikos 98:156–162. https://doi.org/10.1034/j.1600-0706.2002.980116.x
Sala E, Giakoumi S (2018) No-take marine reserves are the most effective protected areas in the ocean. ICES J Mar Sci 75:1166–1168. https://doi.org/10.1093/icesjms/fsx059
Sanderson JC (1990) Subtidal macroalgal studies in East and South Eastern Tasmanian coastal waters. Thesis, University of Tasmania
Santelices B, Ojeda F (1984) Effects of canopy removal on the understory algal community structure of coastal forests of Macrocystis pyrifera from southern South America. Mar Ecol Prog Ser 14:165–173. https://doi.org/10.3354/meps014165
Schiel DR, Foster MS (2015) The Biology and Ecology of Giant Kelp forests. University of California Press
Schrope M (2007) Killer in the kelp. Nature 445:703–705. https://doi.org/10.1038/445703a
Sgarlatta MP, Ramírez-Valdez A, Ladah LB, Calderón-Aguilera LE (2022) Fish functional diversity is modulated by small-scale habitat complexity in a temperate ecosystem. https://doi.org/10.1007/s10750-022-05061-x. Hydrobiologia
Shears NT, Babcock RC (2003) Continuing trophic cascade effects after 25 years of no-take marine reserve protection. Mar Ecol Prog Ser 246:1–16. https://doi.org/10.3354/meps246001
Shelamoff V, Layton C, Tatsumi M et al (2019) Patch size and density of canopy-forming kelp modify influences of ecosystem engineering on understorey algal and sessile invertebrate assemblages. Mar Ecol Prog Ser 632:59–79. https://doi.org/10.3354/meps13155
Shelamoff V, Layton C, Tatsumi M et al (2020a) Kelp patch size and density influence secondary productivity and diversity of epifauna. Oikos 129:331–345. https://doi.org/10.1111/oik.06585
Shelamoff V, Layton C, Tatsumi M et al (2020b) High kelp density attracts fishes except for recruiting cryptobenthic species. Mar Environ Res 161:105127. https://doi.org/10.1016/j.marenvres.2020.105127
Srednick GS, Steele MA (2019) Macroalgal height is more important than species identity in driving differences in the distribution and behavior of fishes. Mar Ecol Prog Ser 613:139–149. https://doi.org/10.3354/meps12898
Srednick GS, Steele MA (2022) Macroalgal physical structure predicts variation in some attributes of temperate fish assemblages better than macroalgal species composition. Mar Biol 169:147. https://doi.org/10.1007/s00227-022-04135-7
Starko S, Soto Gomez M, Darby H et al (2019) A comprehensive kelp phylogeny sheds light on the evolution of an ecosystem. Mol Phylogenet Evol 136:138–150. https://doi.org/10.1016/j.ympev.2019.04.012
Stein A, Gerstner K, Kreft H (2014) Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol Lett 17:866–880. https://doi.org/10.1111/ele.12277
Steneck RS, Johnson CR (2014) Kelp forests: dynamic patterns, processes, and feedbacks. In: Bertness MD, Bruno JF, Silliman BR, Stachowicz JJ (eds) Marine Community Ecology and Conservation. Sinauer Associates, Inc., Massachusetts, USA, pp 315–336
Steneck RS, Graham MH, Bourque BJ et al (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29:436–459. https://doi.org/10.1017/S0376892902000322
Taniguchi H, Nakano S, Tokeshi M (2003) Influences of habitat complexity on the diversity and abundance of epiphytic invertebrates on plants. Freshw Biol 48:718–728. https://doi.org/10.1046/j.1365-2427.2003.01047.x
Taylor R (1998) Density, biomass and productivity of animals in four subtidal rocky reef habitats: the importance of small mobile invertebrates. Mar Ecol Prog Ser 172:37–51. https://doi.org/10.3354/meps172037
Teagle H, Hawkins SJ, Moore PJ, Smale DA (2017) The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J Exp Mar Biol Ecol 492:81–98. https://doi.org/10.1016/j.jembe.2017.01.017
Tokeshi M, Arakaki S (2012) Habitat complexity in aquatic systems: fractals and beyond. Hydrobiologia 685:27–47. https://doi.org/10.1007/s10750-011-0832-z
Torres-Moye G, Edwards MS, Montaño-Moctezuma CG (2013) Benthic community structure in kelp forests from the Southern California Bight. Ciencias Marinas 39:239–252. https://doi.org/10.7773/cm.v39i3.2250
Vanella FA, Fernández DA, Carolina Romero M, Calvo J (2007) Changes in the fish fauna associated with a sub-antarctic Macrocystis pyrifera kelp forest in response to canopy removal. Polar Biol 30:449–457. https://doi.org/10.1007/s00300-006-0202-x
Villegas MJ, Laudien J, Sielfeld W, Arntz WE (2008) Macrocystis integrifolia and Lessonia trabeculata (Laminariales; Phaeophyceae) kelp habitat structures and associated macrobenthic community off northern Chile. Helgol Mar Res 62:33–43. https://doi.org/10.1007/s10152-007-0096-1
Walker BH (1992) Biodiversity and Ecological redundancy. Conserv Biol 6:18–23. https://doi.org/10.1046/j.1523-1739.1992.610018.x
Wang Y, Naumann U, Wright ST, Warton DI (2012) Mvabund– an R package for model-based analysis of multivariate abundance data. Methods Ecol Evol 3:471–474. https://doi.org/10.1111/j.2041-210X.2012.00190.x
Wernberg T, Coleman M, Babcock R et al (2019) Biology and Ecology of the Globally Significant Kelp Ecklonia radiata. In: Oceanography and Marine Biology. pp 265–323
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-, New York
Willis TJ, Millar RB, Babcock RC (2003) Protection of exploited fish in temperate regions: high density and biomass of snapper Pagrus auratus (Sparidae) in northern New Zealand marine reserves. J Appl Ecol 40:214–227. https://doi.org/10.1046/j.1365-2664.2003.00775.x
Willis SC, Winemiller KO, Lopez-Fernandez H (2005) Habitat structural complexity and morphological diversity of fish assemblages in a neotropical floodplain river. Oecologia 142:284–295. https://doi.org/10.1007/s00442-004-1723-z
Acknowledgements
We sincerely thank Claire Butler and Elizabeth Oh for their assistance conducting field surveys, and also thank the others who provided field support as field attendants. Thanks to Sylvie King for creating the site map. Thanks to Jeffrey Wright and Catriona Hurd for input and support on the project. This work was funded by, and in collaboration with, Eaglehawk Dive Centre, the Sea Forest Foundation, and the Tasmanian Smart Seafood Partnership.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was funded by, and in collaboration with, Eaglehawk Dive Centre, the Sea Forest Foundation, and the Tasmanian Smart Seafood Partnership between National Resource Management South and the Tasmanian Seafood Industry Council which is funded by the Australian Government’s National Landcare Program.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. H.F., C.L., S.B., and B.S. collected the data. H.F., B.S., and C.L. analysed the data. H.F. wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Cesar Cordeiro.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Forbes, H., Strain, E.M., Bennett, S. et al. Endangered giant kelp forests support similar fish and macroinvertebrate communities to sympatric stipitate kelp forests. Biodivers Conserv (2024). https://doi.org/10.1007/s10531-024-02867-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10531-024-02867-0