Abstract
Lake Skadar with its surrounding springs, wetlands and larger affluents is among the most diverse freshwater ecosystems in the Mediterranean region and a key biodiversity/endemism hotspot in Europe. It is also highly endangered due to climate change and rapid tourism development in the area. Being abundant, diverse and mostly predatory, true aquatic bugs play an important role in the functioning of freshwater ecosystems and are used as indicators of aquatic habitat quality. Nevertheless, this taxonomic group has been scarcely studied in the area. Our survey provides the first comprehensive DNA barcode library for 24 out of 25 species of aquatic Heteroptera collected in the Skadar Lake basin and adjacent regions. By this, we extend the list of species known from the area by 60%. In the case of three species, Notonecta maculata, Hydrometra stagnorum and Nepa cinerea, we detected multiple highly divergent, and also new BINs indicating possible taxonomic inconsistencies, the potential for (pseudo)cryptic diversity and intricate phylogeographic patterns. We show that presumably well-known hotspots, such as Lake Skadar region, are heavily understudied regarding even the prominent insect taxa and, thus, particularly vulnerable to undocumented biodiversity loss. Finally, we underline the value of simple DNA-barcoding-based surveys for providing reference barcode libraries for effective biomonitoring and signalling taxonomic and biogeographic issues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Two decades ago, DNA barcoding has been proposed as a fast and objective way of identifying species, free from problems such as the intraspecific phenotypic diversity related to sex and developmental stage, poor preservation of identified organisms or imperfect taxonomic skills of people who identify the collected material (Hebert et al. 2003, 2004). DNA barcoding has been proven as an efficient and handy method for fast and preliminary biodiversity surveys, ecological forensics and, importantly, for quantifying species richness and evolutionary diversity within and among communities (e.g., Kress et al. 2015). Moreover, through the years, the idea evolved into a set of advanced tools, such as DNA metabarcoding or environmental DNA detection, that greatly enhance the assessment and biomonitoring of biotic communities, and ecosystem health as well as provide an invaluable insight into interactions between organisms (Lacoursière-Roussel et al. 2018; Gold et al. 2021). DNA-based biomonitoring is being developed and aimed to become the golden standard for the bioassessment of aquatic ecosystems within the European Union Water Framework Directive (Bruce et al. 2021; Thalinger et al. 2021). Public access to reliable, DNA-barcode reference libraries for all taxa in interest, generated by faunistic studies and well-curated by experts in their taxonomy, is a prerequisite for achieving such an aim (Kvist 2013). However, so far, apart from some emblematic taxa such as, e.g. Odonata, reference libraries are far from being completed even for fresh waters of the EU member states, and are just nascent for less emblematic and for European biodiversity hotspots, such as the Mediterranean Basin (Weigand et al. 2019).
The Mediterranean Basin, including the Balkan Peninsula, is considered among the World’s top important biodiversity hotspots, with at least 60% of the Palearctic and 6% of the World’s freshwater species present in the area (Tierno de Figueroa et al. 2013). Several recent molecular studies revealed the complex phylogeographical structures and cryptic diversity patterns within numerous morphologically defined species of freshwater organisms inhabiting the Mediterranean Basin (e.g. Previšić et al. 2014; Bisconti et al. 2016). Such patterns, driven by the dynamic geological history of the area and alterations of its hydrological network, are particularly prominent in the case of obligatorily aquatic organisms with no airborne dispersal stages, such as fishes and malacostracan crustaceans (Geiger et al. 2014; Sworobowicz et al. 2015; Mamos et al. 2016; Grabowski et al. 2017; Buj et al. 2019). Nevertheless, they were also observed in the amphibiotic aquatic insects, including skilled flyers, such as odonates (Galimberti et al. 2021). In the case of heteropterans, the variable dispersal skills combined with the patchiness and isolation of local freshwater ecosystems may promote genetic diversification, at least in some species. The very few studies that have tackled this issue involving molecular markers, also DNA barcodes, revealed already some taxonomic inconsistencies as well as cryptic diversity within some genera of true water bugs in Europe, pointing out the need for more detailed biogeographical and taxonomic studies using integrative approach (Berchi et al. 2018; Havemann et al. 2018). Nevertheless, the genetic diversity of heteropterans in the Mediterranean, particularly in the Balkan Peninsula, remains scarcely studied and there are no DNA barcode libraries for the local species.
Heteroptera is the most diverse hemimetabolous insect order represented in inland waters, including both aquatic and semi-aquatic species. The water bugs include 20 families, 326 genera and ca. 4700 species of the infraorders Nepomorpha, Gerromorpha and Leptopodomorpha inhabiting all types of inland waters on all continents, excluding Antarctica (Polhemus and Polhemus 2008). Among them, the Gerromorpha and Nepomorpha are considered primarily aquatic (Polhemus and Polhemus 2008; Lancaster and Downes 2013; Gullan and Cranston 2014; Henry 2017). Most of them are able to fly at the imago stage, however, their airborne dispersal skills vary depending on species (Fernando and Galbraith 1973; Savage 1989; Boda and Csabai 2013). Although they are most diverse in the tropics, some 340 species are known to occur in the Palearctic. The vast majority of species are predatory, with an exception of Corixidae, which are omnivorous. On the other hand, they are important prey items for a variety of other animals, predominantly fish and birds (Peckarsky 1982; McCafferty 1983; Zimmermann and Spence 1989; Hutchinson 1993; Klecka 2014; Boda et al. 2015). The diversity and distribution of Heteroptera in Europe have been the subject of numerous studies and, generally, the taxonomy of the group is considered well-established (Jansson 1986; Aukema and Rieger 1995; Aukema et al. 2013). Still, the particular number of species occurring in Europe is hard to confirm, according to the inability to verify older records. Summarizing available literature (Aukema and Rieger 1995; Fent et al. 2011; Aukema et al. 2013; Protić 2016), at least 147 species of aquatic and semi-aquatic Heteroptera were reported from Europe. Given their diversity, abundance and ecology, presence in various types of water bodies, habitat specialisation and sensitivity to pollutants, aquatic true bugs can be used in biomonitoring as important indicators of aquatic habitat quality and pollution impact (reviewed by Bakonyi et al. 2022).
Aquatic heteropterans of the Balkan Peninsula have remained largely understudied. Most information on Gerromorpha and Nepomorpha comes from the general works on Heteroptera by Josifov (1986); Aukema and Rieger (1995); Protić (1998); Protić (2001); Aukema et al. (2013), where they listed 83 species from the Balkan Peninsula, which makes 56% of European fauna. Only most recently the ecology of true aquatic bugs of Montenegro was studied by Gligorović et al. (2016) and the checklist was provided by Protić (2016), who reported the presence of 13 species of Gerromorpha and 21 species of Nepomorpha in the country (23% of European fauna, 41% of Balkan Peninsula). According to the Catalogue of the Heteroptera of the Palearctic Region, there are reports about 23 species of Nepomorpha and 16 species of Gerromorpha from Albania. Data about species lists occurring in the Skadar Lake can be found only in the two original papers of Kment et al. (2005) and Gligorović et al. (2016) and in the summary of the book about Skadar Lake zoobenthos by Pešić et al. (2018a). So far, 15 species of water bugs are known to occur in the Lake Skadar basin, however, considering the diversity of this group in the Balkans and the local habitat mosaic, we can expect much higher number of species to be present in the area.
Aims
Our study aims to initiate a comprehensive DNA-barcode library for aquatic Heteroptera (Gerromorpha, Nepomorpha) of the Balkan biodiversity hotspot, with a focus on the fauna of the ancient basin of Lake Skadar. Such DNA-barcode library for the local biota is an indispensable basis for setting up reference conditions and future biomonitoring/conservation efforts in the basin and is a response to challenges of the anthropogenic threat posed to Lake Skadar. Pešić et al. (2018b) underlined the need for employment of novel and efficient, DNA-based methods, in surveying the threatened biodiversity of Lake Skadar. Our study, performed within the project “DNA-Eco: DNA barcode reference library as a tool for sustainable management of freshwater ecosystems in the highly threatened Lake Skadar Basin” funded by the Ministry of Science, Montenegro, is among the first to address this need. Additionally, we verify the genetic identity of the local populations of widespread species in reference to those from other parts of Europe, available in the publicly accessible Barcode of Life Datasystems (BOLD). By this, we verify whether a simple survey of the collected material, employing DNA barcodes is efficient at taxonomic assignment using the currently available reference library and if such a survey hints at pronounced phylogeographic structure or hidden diversity within any species. We assume the latter may be expected in species of wide geographic distribution but limited dispersal abilities, i.e. due to wingless or poorly flying imagines. Finally, we provide the first thorough molecular study of the true water bugs of Skadar Lake and adjacent regions.
Study area
Lake Skadar is located in the northern Mediterranean Region, western Balkans, and extends in an NW–SE direction, parallel to the coastline of the Adriatic Sea, and separated from it by the Rumija Massif. The northern part of the basin is situated on the Niksić karst field at an altitude of 600–630 m a.s.l. The karst field is intersected by the Zeta River and its major tributaries. The altitudes of the karst polje generally decrease from north to south and the southernmost part forms cryptodepression, which is flooded by the lake waters. Skadar Lake is the biggest lake on the Balkan Peninsula, approximately 44 km long and up to 18–20 km wide, with a surface area fluctuating seasonally from ca. 350 to ca. 600 km2, depending on the seasonal water level. The lake is generally shallow, with an average depth of about 5 m. Skadar Lake is fed by numerous sublacustrine karst springs deep down to 60 m, as well as by a few rivers, such as Morača, Crnojevića and Crmnica. Bojana River constitutes the lake's water outlet and connects Skadar Lake to the Adriatic Sea. The climate of Lake Skadar basin is Mediterranean, with hot summers and winter temperatures rarely going down below 0 °C. Thus, Lake Skadar is referred to as a subtropical water body (Lasca et al. 1981). The shallowness of the lake, together with the thermal conditions provides an opportunity for lush submerged vegetation, while the periodically flooded areas at the margin of the lakes are an extensive wetland system. The highly heterogeneous ecosystem encompassing a combination of warm lacustrine and wetland as well as cold spring and stream habitats is known to support very rich, yet still largely understudied fauna with more than 20 endemic species described so far and, most probably, many more awaiting discovery (Crnobrnja-Isailović et al. 2018; Pešić et al. 2018a; Gadawski et al. 2022b). Another peculiarity of the Skadar basin is related to its quite mysterious geological history; the basin with its karst spring systems and wetlands is most probably very old (of Pliocene, ca. 3 mya, or even earlier origin), while the present lake formed only about 1200 years ago (for review see Grabowski et al. 2018).
The unique ecosystem, high biodiversity (at least 1900 species) with number of endemics as well as the ecological importance of Lake Skadar have been recognized and appreciated by establishing a National Park (IUCN Management Category II) on the Montenegrin side, and a “Managed Natural Reserve” (IUCN Category IV) covering the Albanian part of the lake, while at the international level, the lake is recognized as a Ramsar site (a wetland area of international significance) and one of the Key Biodiversity Areas across the Mediterranean biodiversity hotspot (Darwall et al. 2014). Despite its peculiar value, international recognition and protection, Lake Skadar basin is highly endangered by degradation. As summarised by Pešić et al. (2018b), the main threats to the environment of Lake Skadar and its basin are: (1) pollution (including industrial, municipal, solid, and liquid waste), (2) poaching, (3) lakeshore development, and (4) water management measures, with additional challenges posed by: (a) increasing tourism pressure, (b) intensification of the agricultural sector, (c) introduction of the invasive species, (d) using the sublacustrine springs of Lake Skadar for the regional water supply of the Montenegrin coastal area, (e) hydropower development of the Drin River in Albania and the Morača Valley in Montenegro, and (f) dredging of the Bojana River to reduce flooding problems. One of the initial countermeasures to these threats requires a definition of “reference conditions” leading to the establishment of protocols for use in biomonitoring programs (Pešić et al. 2018b).
Material and methods
Field collections and taxonomic identification
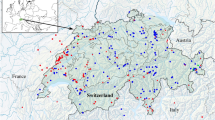
The material for the study was collected during several field expeditions to Lake Skadar in April/May and September/October 2014, July/August 2015 and May/June 2018 organised jointly by the Department of Invertebrate Zoology & Hydrobiology, University of Lodz, the Department of Invertebrate Zoology and Limnology, University of Szczecin, and the Department of Biology, University of Montenegro. The material was collected with qualitative methods from a variety of aquatic habitats (Fig. 1) in and around the lake, including kick-sampling in shallow inshore habitats, dredging of underwater meadows, submerged light traps in the open lake, kick-sampling in karst springs, streams and wetlands. This material was supplemented by water bugs attracted to light in the open areas, ensuring that the light was visible over a long distance above the lake's surface. For the latter purpose, we used a 500W white-light bulb connected to a power generator, placed in front of a white screen (2 m × 3 m). The expositions started ca. 45 min before sunset and continued for three hours. All the collected individuals were preserved in 96% ethanol for further examination. The distribution of the collection sites is presented on the map (Fig. 2) and the collection details are summarised in Table S1, S2.
The material was identified by GT, using a stereomicroscope Nikon SMZ800, to the lowest possible taxonomic level according to the available taxonomic literature on the European aquatic Heteroptera (Jansson 1986; Strauss and Niedringhaus 2014). Vouchers were deposited in the invertebrate collection of the Department of Invertebrate Zoology and Hydrobiology, University of Lodz.
DNA extraction, amplification, sequencing, data depository
Molecular studies were done either in the Canadian Center for DNA Barcoding (CCDB), Guelph, Canada, or in the Department of Invertebrate Zoology and Hydrobiology (UniLodz), University of Lodz (see Table S1 for details).
Two DNA extraction protocols were implemented for samples analysed in the CCDB. For larger specimens, the standard procedure involved sampling a leg for DNA extraction at a later stage (Ivanova et al. 2006). Conversely, for smaller individuals measuring less than 3 mm, the entire specimen was placed in a 96-well microplate and used for DNA extraction. An additional step was incorporated into the procedure to recover exoskeletal remains following non-destructive lysis (Porco et al. 2010).
For samples analysed at UniLodz, total DNA was extracted using either the Chelex procedure (Casquet et al. 2012) or the phenol–chloroform method following the procedure by Grabowski et al. (2012). Amplification of COI region and enzymatic purification was done according to primers and the procedure described by Rewicz et al. (2021). Sequencing was done in Macrogen Europe either one-way or bi-directional according to the obtained quality. DNA extracts were deposited either in UniLodz, or in CCDB (See Table S1 for particular individuals).
The obtained sequences were first identified using BLAST (Altschul et al. 1990) searches on the NCBI nucleotide database (https://www.ncbi.nlm.nih.gov/). Then, sequences were manually edited, aligned, and trimmed as well as checked for the absence of frameshifts, double peaks, and stop codons in the Geneious 10.2.6 (Kearse et al. 2012).
Dataset assemblage
Relevant voucher information, photos, and newly generated DNA barcodes are publicly accessible through the dataset DS-MOHET (https://doi.org/10.5883/DS-MOHET) in BOLD (Ratnasingham and Hebert 2007). Additional publicly available COI sequences from European Gerromorpha and Nepomorpha were obtained from BOLD (public dataset DS-HETEUR). The dataset DS-HETEUR consists of 1168 sequences that are publicly available (accessed 16-06-2023) with 220 sequences (18.8%) developed during this study (DS-MOHET). Sequences stored in BOLD as private records are not included in the analysis and are not present on BIN distribution maps. Analysis are based only on published records with GPS coordinates. The data were used for constructing the distance-based NJ tree and the haplotype networks (see below). Newly obtained sequences were deposited in GenBank under accession numbers ON406644-ON406861.
DNA barcode analysis
We calculated the sequence divergences (mean and maximum intraspecific variation and minimum genetic distance to the nearest-neighbour species) using ‘Barcode Gap Analysis’ and ‘Distance Summary’ tools in BOLD (boldsystems.org), employing the Kimura-2-Parameter (K2P), and p-distance metrics. Unique BIN (Barcode Index Number) identifiers, which group DNA sequences based on the genetic distance (entative equivalents of species) were assigned for sequences deposited in BOLD (Ratnasingham and Hebert 2013).
We provided a Neighbour-Joining cluster analysis (NJ; (Saitou and Nei 1987)) for all the studied COI barcode sequences, based on the K2P distance (Kimura 1980), with a bootstrap test (1000 replicates) (Felsenstein 1985), using MEGA X software (Kumar et al. 2018). Separate trees were provided for Nepomorpha and Gerromorpha.,
Species delimitation
In order to explore the molecular threshold between species and verify clustering of species having more than one BIN, we performed species delimitation analyses. The delimitation methods were based on genetic distances and phylogeny reconstructions. Firstly, we downloaded COI sequences from DS-HETEUR BOLD dataset, only in case of sequences > 500 bp long, excluding contaminants and sequences containing stop codons. The downloaded sequences were realigned using MAFFT v. 7 with an automatic search for the best algorithm (Katoh et al. 2013). Sequences that would shorten the alignment below 500 bp, but had longer representation for particular species, were removed. The final dataset of Heteroptera from Europe consisted of 1091 sequences, each 513 bp long. We used the distance-based species delimitation method based on the barcode gap detection, i.e. ASAP: assemble species by automatic partitioning software (Puillandre et al. 2021). ASAP was run with the Kimura (K80) distance method. For the phylogeny based delimitation method, we used Bayesian implementation of the multi-rate PTP (mPTP, Kapli et al. 2017). The method implements MCMC sampling that provides a fast and comprehensive evaluation of the inferred delimitation. We decided to use this method as other methods like bPTP or GMYC have a tendency to over-split species (e.g. Wattier et al. 2020; Mamos et al. 2021). Two runs of 500 M MCMC generations-long chain with burn-in of 10% were performed on the local server. As an input tree for mPTP, we reconstructed the maximum-likelihood phylogeny using W-IQ-Tree (Trifinopoulos et al. 2016), with 1000 bootstrap replicates and automatic substitution model selection (Fig S1).
Additionally, for species in which more than one BIN was detected, we displayed the haplotype relationship through a Minimum Spanning Network using PopART (Leigh and Bryant 2015).
Results
We present 220 DNA barcode sequences of 24 species of aquatic bugs (see Table S1 for details). We failed to obtain the sequence only from Lethocerus patruelis (Stål, 1854) as this specimen was found in the field, it was already dead and dry. In Montenegro, we collected 191 specimens from 24 species (25 BINs), while in Albania we caught 30 specimens, from 11 species (12 BINs). Our findings added the first records of 4 species for Montenegro (Micronecta poweri (Douglas & Scott, 1869), Hesperocorixa sahlbergi (Fieber, 1848), Notonecta viridis Delcourt, 1909 and Gerris lateralis Schummel, 1832). In Albania, Micronecta pusilla (Horvát, 1895) was recorded for the first time. Even, though we focused mainly on the Skadar Lake catchment area, we covered 63% of Montenegrin (24 from 38, 4 species new) and 27% of Albanian (11 from 40, 1 species new) aquatic Heteroptera fauna. According to the list provided by Pešić et al. (2018a, b), our findings added the first records for Micronecta pusilla, Hesperocorixa sahlbergi, Aquarius paludum (Fabricius, 1794) and Velia affinis Kolenati, 1857 to the 15 previously known species from the transboundary Skadar Lake area itself.
Lengths of the analyzed DNA barcode fragment ranged from 402 to 658 base pairs (bp). In two sequences of Ilyocoris cimicoides (Linnaeus, 1758), we detected putative pseudogenes (NUMT) and removed them from further analysis as such NUMTs often co-amplify with the targeted COI region but lack the proper phylogenetic information (Jordal and Kambestad 2014; Hawlitschek et al. 2017; Zhao et al. 2022). All the sequences were characterized by high AT content, with mean values of G = 16%, C = 17.5%, A = 32.2%, and T = 34.4%. The number of BINs per species ranged from one (21 species, 87.5%) to two, for three species: Hydrometra stagnorum (Linnaeus, 1758), Nepa cinerea Linnaeus, 1758, and Notonecta maculata Fabricius, 1794. We did not detect BIN sharing between species, and seven BINs from the detected 27 were unique for BOLD (sequences deposited for the first time to BOLD and clustered to the new BIN). Intraspecific K2P distance varied from null to a maximum of 6.08%, 3.45%, and 1.25% for the aforementioned species for which double BINs were detected. Interspecific distances varied from 5.61% (Notonecta viridis; Notonecta glauca, 1758) to 20.15% (Micronecta pusilla; Ilyocoris cimicoides) (Table 1). We found non-overlapping clusters in the case of all analyzed species. In all cases, the distance at which barcode gap (i.e. distance to the nearest neighbor) could be observed exceeded the maximum intraspecific distance. For a better presentation of the phylogenetic relationships between species, we showed them on two figures (Nepomorpha: Fig. 3; Gerromorpha: Fig. 4). Wider overview of molecular identification showed that our data provided the first COI sequences for Sigara scripta (Rambur, 1840) BOLD:AEI1642, Micronecta pusilla BOLD:AEG9578, Velia affinis BOLD:AEG0715, and Velia sp. BOLD:AEF9274 (Fig. S1). Moreover, sequences from the Skadar Lake region added a formerly unknown BINs to Nepa cinerea BOLD:AEH5788, Hesperocorixa sahlbergi BOLD:AEF2957, Notonecta maculata BOLD:AEH5379, BOLD:AEF8405 and Micronecta poweri BOLD:AET2410 (Fig. S1). Minimum Spanning Networks shown for the species with multiple BINs (Fig. 5) revealed presence of geographical patterns. In Hydrometra stagnorum, BIN AEC2693 contained most of the sequences (6 haplotypes) from Montenegro, plus one sequence from Portugal. Single indvidual from Norway was assigned to BIN ACX 7898. BIN AAK5632 consists of sequences from Montenegro and Albania and also sequences from Central and Western Europe. The most common haplotype is shared between France, Montenegro, and Germany. A complex of five BINs can be observed within Nepa cinerea, four of which are restricted to Norway (ADP8747), Portugal (AEC3215), China (ADL0058), and Montenegro (AEH5788), respectively. Sequences from Finland, Germany, Poland, and Montenegro share BIN AAK8359. Notonecta maculata sequences were assigned to three BINs. Individuals from the Lake Skadar area are within BIN AEH5379 (singleton from Albania), and BIN AEF8405, together with individuals from Albania and Montenegro. The last BIN (AAN1703) consists of individuals from Western Europe (Fig. 5).
Neighbour-joining topology of the analysed Nepomorpha species based on Kimura 2-parameter distances. Triangles indicate the relative number of individuals sampled (height) and sequence divergence (width). Dashed rectangles encircled species, where double BINs were detected. Numbers next to nodes represent bootstrap values > 50% (1000 replicates)
Neighbour-joining topology of the analysed Gerromorpha species based on Kimura 2-parameter distances. Triangles indicate the relative number of individuals sampled (height) and sequence divergence (width). Dashed rectangle encircles species, where double BINs were detected. Numbers next to nodes represent bootstrap values > 50% (1000 replicates)
Minimum Spanning Network showing phylogenetic relationships within three species: Hydrometra stagnorum, Nepa cinerea, Notonecta maculata, for which multiple BINs were detected. Each bar represents a single mutational change The diameter of the circles is proportional to the number of individuals in each haplotype sampled (see open circles with numbers). The lines illustrate the results of the species delimitation analysis: solid line—mPTP, dotted line—ASAP, dashed line—BINs
The ASAP delimitation method provides similar results as the BIN clustering, except for two BINs being fused (AEH5788 and ADP8747) in the case of N. cinerea, and merging all BINs of N. maculata. (Fig. 5, Table S3). The mPTP results for the three BIN discordant species (H. stagnorum, N. cinerea, N. maculata) show an unambiguous overlap of the molecular units and morphological species (Fig. 5). Using the barcode gap analysis, we estimated the threshold distance separating species at 2.2% K2-p (Fig. 6, Table S3).
Rivers and springs were the richest in terms of species number. We noticed 19 and 20 species of aquatic true bugs in such water bodies, respectively. Sigara dorsalis dominated in both. Fewer species (11) were found in Lake Skadar itself. However, species composition varied between the sites. Sublacustrine springs showed the greatest diversity (8 species), with the dominance of larvae of the genus Micronecta (only the imagines of M. pusilla were found in the Skadar Lake), followed by Sigara dorsalis and Ilyocoris cimicoides. The littoral zone of Lake Skadar was less diverse. Heteropteran communities in this habitat consisted of five species, and the dominant was Sigara dorsalis. The benthic zone was least diverse in a number of species. Only Corixinae larvae (the same species as detected in the other sampling sites) and specimens of Micronecta pusilla (larvae and imagines) were found there.
Discussion
The true aquatic bugs have neither been frequently targeted by studies involving DNA barcoding (i.e. Havemann et al. 2018; Meyin A Ebong et al. 2016) nor included in wider true-bugs DNA-oriented papers (i.e. Park et al. 2011; Raupach et al. 2014; Sousa et al. 2021). In our study focusing on the Lake Skadar basin and adjacent areas, we found 20 species (83% of our dataset) of which each was associated with only a single BIN, congruent with our morphological identification. An identity of one species of Velia remains unknown and needs further morphological evaluation. Three species were represented by multiple BINs (two in each case). Surprisingly, we did not detect BIN sharing between species, as reported in other studies regarding heteropterans (Havemann et al. 2018; Raupach et al. 2014) and other insect orders (i.e. Raupach et al. 2020a, b; Rewicz et al. 2021). Interestingly, the estimated 2.2% K2-p threshold distance separating species in our data (Fig. 6), being identical with the initial distance threshold used in the BIN-defining algoritm (Ratnasingham and Hebert 2013), provides some suggestion for existence of species complexes in the species characterised by presence of multiple BINs.
Species with high intraspecific variability
Three species in which we found multiple BINs were characterised by relatively high intraspecific genetic distances: Notonecta maculata (1.25%), Hydrometra stagnorum (6.08%), Nepa cinerea (3.45%). In the case of of three other species with high intraspecific distances (Aquarius paludum, Gerris lacustris, Ilyocoris cimicoides), a continuum of differentiation without any clear barcoding gap could be observed (Table 1). All delimitation methods (BINs, ASAP, mPTP) also clearly pointed out that each represents a single species/lineage (Fig S1, Table S3).
Notonecta maculata is a medium size (ca. 16 mm) predatory water bug and one of the nine congeneric species occurring in Europe. It inhabits various habitats with an emphasis on small lotic water bodies with well-developed aquatic vegetation (Strauss and Niedringhaus 2014). In the analysed material, it was represented by 19 barcoded individuals assigned to two BINs, BOLD:AEF8405 (18 individuals, 11 in Montenegro, 7 in Albania) and BOLD:AEH5379 (1 individual, Albania). The latter BIN is unique and a singleton, while BIN AEF8405 also contains private sequences from the Mediterranean region (3 from Malta, 1 from Cyprus) deposited in BOLD. Both BINs differ from the BIN BOLD:AAN1703, already known from Western and Central Europe (Germany, Austria, Italy, Portugal) (Fig. 5, Fig. S1, Fig. 7), by 1.38% K2P distance. Such spatial segregation and split into ‘southern-Mediterranean’ haplogroup (including two BINs revealed in this study) and Central-Western ‘continental haplogroup’ may reflect the complex evolutionary history of N. maculata in Europe. Regarding BIN AEH5379, we cannot exclude that it may be an artifact generated by sampling bias. Broader sampling in the southern part (Albania, Greece), and north (Croatia, Slovenia, Bosnia and Hercegovina) of the Balkan Peninsula may provide more individuals to fill this gap. A further morphological examination is necessary, as this group (Hebsgaard et al. 2004) is known for complicated taxonomy, and it is possible that potential cryptic (or pseudo-cryptic) species can be revealed.
Nepa cinerea is a widespread and ubiquitous water bug, one of three species within the genus, occurring in Europe. It may reach a total length of 15–23 mm, plus an elongated breathing tube which may be as long as the body. This sneaky predator feeds on various aquatic invertebrates and fish fry. It inhabits various flowing and stagnant waters, often among the submerged vegetation and detritus (Strauss and Niedringhaus 2014). We barcoded 5 individuals, which were assigned to two BINs: AEH5788, a new unique BIN with 3 individuals from Montenegro, and AAK8359 (2 individuals from Montenegro), which is definitely the most widespread in Europe and recorded from Germany, Poland, Finland, Netherlands (BOLD private data, accessed on 16-06-2023), Austria (BOLD private data, accessed on 16-06-2023), Romania (BOLD private data, accessed on 16-06-2023), and the United Kingdom (BOLD private data, accessed on 16–06-2023) (Fig. 8). The phylogeographic structure within this species seems to be intricate, with at least 5 haplogroups, of which 4 are restricted to limited areas (countries) like China, Norway, Montenegro, and Portugal, and one is widespread in Europe (Fig. 5). The occurrence of two lineages in sympatry within the Skadar Lake area serves as a definitive confirmation of the area's uniqueness and emphasizes the pressing need for its protection. An integrative approach involving morphology, ultrastructure, and nuclear markers is needed to solve this putative species complex.
Hydrometra stagnorum is one of two Hydrometra species widely distributed in Europe. Associated with the water surface, it occurs in the coastal zone of various types of stagnant and flowing waters. Its length reaches 7–9 mm, and the species predates and scavenges on organisms falling on the water surface (Strauss and Niedringhaus 2014). We revealed the presence of three haplogroups equivalent to BINs BOLD:AEC2693 (6 individuals from Montenegro), BOLD ACX7898 (1 individual from Norway), and BOLD:AAK5632 (5 individuals from Montenegro, 1 from Albania). Two BINs were sympatric, even in the same exact localities, in the Skadar Lake region. Nevertheless, there are differences in their distribution at the scale of Europe. BIN AEC2693 is limited to its southern part, recorded so far from Montenegro, France (BOLD private data), and Portugal, ACX7898 is a single individual from Norway, while AAK5632 is much more widespread and found in Montenegro, Albania, Germany, Austria, Poland, France, Romania (BOLD private data, accessed on 16-06-2023), and Netherlands (BOLD private data, accessed on 16-06-2023) (Fig. 9). The high 6.08% K2P intraspecific distance may indicate the presence of cryptic species and requires further attention. All three lineages seem to be separate species according to ASAP and BIN delimitation. mPTP merges them into one, but this method is known to provide the most conservative/lumping approach combining even well-recognized different species into single MOTU (Silva et al. 2023; Parslow et al. 2021). Taking into account limited dispersal abilities and geographic distribution of Hydrometra, we can assume the existence of some local (pseudo)cryptic species. An integrative approach involving morphology and more molecular markers is needed to elucidate the taxonomy of this species.
Cryptic diversity within the Skadar Lake area
Due to the turbulent geological history of the Balkan Peninsula and Skadar Lake Lake itself (details by (Grabowski et al. 2018)), the aquatic fauna of the basin is rich in endemics (Pešić and Glöer 2013, 2018; Jabłońska et al. 2018, 2020). Most of the endemicity was detected in obligatory aquatic invertebrates (the whole life cycle in water), such as amphipods (Jabłońska et al. 2020), shrimps (Jabłońska et al. 2018), snails (Pešić and Glöer 2013, 2018; Pešić et al. 2019), leeches (Grosser et al. 2015; Marinković et al. 2019; Jovanović et al. 2021), and water mites (Pešić 2022; Pešić et al. 2021a, 2021b, 2020; Pešić and Smit 2020). Recent morpho-molecular studies on Lake Skadar Chironomidae done by Gadawski et al. (2022a) revealed the presence of new BINs, which could be identified only at the genus level. Thus, we cannot exclude the presence of endemic or non-described species there, yet it needs further work integrating morphological and molecular data. Our findings revealed new BINs within Nepa cinerea and Hesperocorixa sahlbergi. First DNA barcodes obtained for Velia affinis, Micronecta pusilla, and Sigara scripta need urgent comparison with specimens from other parts of their broad occurrence range (Eastern-Mediterranean, Pontic and Caucasus), to determine their taxonomic and phylogenetic position (Aukema and Rieger 1995). The finding of Velia sp. and Notonecta maculata representing the same BINs in the Lake Skadar area as in Cyprus, and Malta, respectively (BOLD private data) provides a perspective for the description of new species with not strictly Balkan, but the broader Southern European-Mediterranean range. This area and the Mediterranean islands, in particular, are known for their high level of diversity and endemicity (Bisconti et al. 2016; Grković et al. 2015; Hupało et al. 2018, 2021; Ivković et al. 2021). On the other hand, Mediterranean fresh waters and wetlands are scattered, and heavily vulnerable to both, anthropogenic pressure and habitat loss (Cuttelod et al. 2009; Darwall et al. 2014; Hermoso and Clavero 2011; Leberger et al. 2020; Milano et al. 2013; Tachos et al. 2022), as well as threatened by invasive species (Deidun et al. 2018; Hermoso et al. 2011; Naselli-Flores and Marrone 2019). As mentioned in the study area's description, various anthropogenic threats also apply to Lake Skadar, and the need for documenting its biodiversity's 'reference condition' becomes apparent (Pešić et al. 2018b).
Heteroptera of Lake Skadar—perspective for further findings
Our data confirmed the dependence of the obtained species composition on the sampling technique. Light trapping, which we conducted on the Skadar Lake shores, and spring systems, revealed only one species—Sigara dorsalis. Surprisingly, we did not detect any other representatives of Corixinae among more than 5000 invertebrates lured to light and barcoded (Grabowski et al. unpublished data). Such low effectiveness of collecting water boatmen at light is surprising, given that they are reported to be highly phototactic (Weigelhofer et al. 1993). Light traps set at the bottom of the lake bring us only representatives of Corixinae and Micronectinae, mostly juvenile specimens. Using classic hydrobiological equipment (kick net, dredge) was the most effective and resulted in collecting 24 species.
The fauna of true water bugs in the Skadar Lake area is definitely understudied. So far, including our findings, 24 species are known from the area and, in a broader view, only 38 and 40 species are known from Montenegro and Albania, respectively, at the country scale (Aukema and Rieger 1995; Aukema et al. 2013; Protić 2016). The majority of our records represent species that have already been reported from the region. Still, we were able to add nine new species and increase the number of species known from Lake Skadar area by 60%. An obvious reason for that is scarce literature data with only two original papers published recently on aquatic heteropterans in the Skadar Lake area (Kment et al. 2005; Gligorović et al. 2016) and one review paper including this group (Pešić et al. 2018a). The general diversity of water bugs fauna of the Balkans (83 species) and Europe (147 species) together with a mosaicity of various aquatic habitats present in the Lake Skadar basin suggest that many more true water bugs can still be found in this area. However, there is no data available on the diversity of this group from other big Balkan lakes, such as Ohrid, Prespa or Doiran, that would allow any prediction or comparison regarding that matter. Interestingly, only one species generally considered rare in Europe, i.e. Sigara scripta, was discovered in Lake Skadar. However, it should be emphasized that our study is a preliminary survey. Even as such, being also the first insight into the molecular diversity of the local aquatic bugs, it has already pinpointed several taxonomic issues that need to be tackled in future research and provided valuable contribution to documentation of the “reference condition” of local biodiversity. Further studies, including a more systematic approach to various habitats and elevation gradients, are needed to complete the DNA barcode reference library. Having such a library will enable eDNA-based surveys on larger geographic scales that may be a very effective way of estimating biodiversity patterns and studying biogeographical affiliation of species, particularly in standing waters (Carraro et al. 2020).
Conclusions
Answering the need for employment of novel and efficient, DNA-based methods, in surveying the threatened biodiversity of Lake Skadar, we provided the first comprehensive DNA barcode library for aquatic Heteroptera from its basin and adjacent regions, a unique and diverse part of the Balkan Peninsula. At the same time, we extended the list of species known from the area by 60%. Out of the 24 barcoded species, we detected multiple highly divergent, and some new, BINs for Notonecta maculata, Hesperocorixa sahlbergi, Hydrometra stagnorum and Nepa cinerea, indicating possible taxonomic inconsistencies, the potential for (pseudo)cryptic diversity and intricate phylogeographic patterns. Our study showed that the presumably well-known and threatened diversity hotspots, such as the Lake Skadar region are heavily understudied regarding even the usually large and common insect taxa, such as true water bugs. We also pinpointed the value of simple DNA-barcoding-based surveys, not only for providing reference barcode libraries for effective biomonitoring but also for signaling taxonomic and biogeographic issues.
Data availability
All new COI DNA sequences used in the present study are deposited in GenBank under Accession Numbers ON406644-ON406861.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Aukema B, Rieger C (1995) Catalogue of the Heteroptera of the Palaearctic Region. Vol. 1. Enicocephalomorpha, Dipsocoromorpha, Nepomorpha, Gerromorpha and Leptopodomorpha. Netherlands Entomological Society, Amsterdam, Wageningen
Aukema B, Rieger C, Rabitsch W (2013) Catalogue of the Heteroptera of the Palaearctic Region, vol 6. The Netherlands Entomological Society, Amsterdam
Bakonyi G, Vásárhelyi T, Szabó B (2022) Pollution impacts on water bugs (Nepomorpha, Gerromorpha): state of the art and their biomonitoring potential. Environ Monit Assess 194:301. https://doi.org/10.1007/s10661-022-09961-2
Berchi GM, Copilas-Ciocianu D, Kment P, Buzzetti FM, Petrusek A, Rakosy L, Cianferoni F, Damgaard J (2018) Molecular phylogeny and biogeography of the West-Palaearctic Velia (Heteroptera: Gerromorpha: Veliidae). Syst Entomol 43(2):262–276. https://doi.org/10.1111/syen.12273
Bisconti R, Canestrelli D, Tenchini R, Belfiore C, Buffagni A, Nascetti G (2016) Cryptic diversity and multiple origins of the widespread mayfly species group Baetis rhodani (Ephemeroptera: Baetidae) on northwestern Mediterranean islands. Ecol Evol 6(21):7901–7910. https://doi.org/10.1002/ece3.2465
Boda P, Csabai Z (2013) When do beetles and bugs fly? A unified scheme for describing seasonal flight behaviour of highly dispersing primary aquatic insects. Hydrobiologia 703(1):133–147. https://doi.org/10.1007/s10750-012-1350-3
Boda P, Bozóki T, Vásárhelyi T, Bakonyi G, Várbíró G (2015) Revised and annotated checklist of aquatic and semi-aquatic Heteroptera of Hungary with comments on biodiversity patterns. ZooKeys 501:89. https://doi.org/10.3897/zookeys.501.8964
Bruce K, Blackman R, Bourlat SJ, Hellström AM, Bakker J, Bista I, Bohmann K, Bouchez A, Brys R, Clark K, Elbrecht V, Fazi S, Fonseca V, Hänfling B, Leese F, Mächler E, Mahon AR, Meissner K, Panksep K, Pawlowski J, Schmidt Yáñez P, Seymour M, Thalinger B, Valentini A, Woodcock P, Traugott M, Vasselon V, Deiner K (2021) A practical guide to DNA-based methods for biodiversity assessment. Adv Books. https://doi.org/10.3897/ab.e68634
Buj I, Šanda R, Zogaris S, Freyhof J, Geiger MF, Vukić J (2019) Cryptic diversity in Telestes pleurobipunctatus (Actinopterygii; Leuciscidae) as a consequence of historical biogeography in the Ionian Freshwater Ecoregion (Greece, Albania). Hydrobiologia 835(1):147–163. https://doi.org/10.1007/s10750-019-3935-6
Carraro L, Mächler E, Wüthrich R, Altermat F (2020) Environmental DNA allows upscaling spatial patterns of biodiversity in freshwater ecosystems. Nat Commun 11(3585):1–12. https://doi.org/10.1038/s41467-020-17337-8
Casquet J, Thebaud C, Gillespie RG (2012) Chelex without boiling, a rapid and easy technique to obtain stable amplifiable DNA from small amounts of ethanol-stored spiders. Mol Ecol Resour 12(1):136–141. https://doi.org/10.1111/j.1755-0998.2011.03073.x
Crnobrnja-Isailović J, Polović L, Ljubisavljević K, Čađenović N, Čubrić T, Haxhiu I (2018) Diversity and Conservation Status of Batrachofauna and Herpetofauna in the Lake Skadar Region. In: Pešić V, Karaman G, Kostianoy AG (eds) The Skadar/Shkodra Lake Environment. Springer International Publishing, Cham, pp 383–414
Cuttelod A, García N, Malak DA, Temple HJ, Katariya V (2009) The Mediterranean: a biodiversity hotspot under threat. Wildlife in a Changing World–an analysis of the 2008 IUCN Red List of Threatened Species 89(2019):9
Darwall W, Carrizo S, Numa C, Barrios V, Freyhof J, Smith K (2014) Freshwater key biodiversity areas in the Mediterranean Basin Hotspot: informing species conservation and development planning in freshwater ecosystems. IUCN, Cambridge
Deidun A, Sciberras A, Formosa J, Zava B, Insacco G, Corsini-Foka M, Crandall KA (2018) Invasion by non-indigenous freshwater decapods of Malta and Sicily, central Mediterranean Sea. J Crustac Biol 38(6):748–753. https://doi.org/10.1093/jcbiol/ruy076
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evol 39(4):783–791. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
Fent M, Kment P, ÇamurEli̇pek B, Kirgiz T (2011) Annotated catalogue of Enicocephalomorpha, Dipsocoromorpha, Nepomorpha, Gerromorpha, and Leptopodomorpha (Hemiptera: Heteroptera) of Turkey, with new records. Zootaxa 2856(1):1. https://doi.org/10.11646/zootaxa.2856.1.1
Fernando CH, Galbraith D (1973) Seasonality and dynamics of aquatic insects colonizing small habitats. SIL Proc 1922–2010 18(3):1564–1575. https://doi.org/10.1080/03680770.1973.11899644
Gadawski P, Montagna M, Rossaro B, Giłka W, Pešić V, Grabowski M, Magoga G (2022a) DNA barcoding of Chironomidae from the Lake Skadar region: reference library and a comparative analysis of the European fauna. Divers Distrib 28(12):2838–2857. https://doi.org/10.1111/ddi.13504
Gadawski P, Rossaro B, Giłka W, Montagna M, Zawal A, Grabowski M (2022b) First insights into the diversity and ecology of non-biting midges (Diptera: Chironomidae) of the unique ancient Skadar Lake basin (Montenegro/Albania). J Gt Lakes Res 48(2):538–550. https://doi.org/10.1016/j.jglr.2021.02.003
Galimberti A, Assandri G, Maggioni D, Ramazzotti F, Baroni D, Bazzi G, Chiandetti I, Corso A, Ferri V, Galuppi M, Ilahiane L, La Porta G, Laddaga L, Landi F, Mastropasqua F, Ramellini S, Santinelli R, Soldato G, Surdo S, Casiraghi M (2021) Italian odonates in the Pandora’s box: a comprehensive DNA barcoding inventory shows taxonomic warnings at the Holarctic scale. Mol Ecol Resour 21(1):183–200. https://doi.org/10.1111/1755-0998.13235
Geiger MF, Herder F, Monaghan MT, Almada V, Barbieri R, Bariche M, Berrebi P, Bohlen J, Casal-Lopez M, Delmastro GB, Denys GPJ, Dettai A, Doadrio I, Kalogianni E, Kärst H, Kottelat M, Kovačić M, Laporte M, Lorenzoni M, Marčić Z, Özuluğ M, Perdices A, Perea S, Persat H, Porcelotti S, Puzzi C, Robalo J, Šanda R, Schneider M, Šlechtová V, Stoumboudi M, Walter S, Freyhof J (2014) Spatial heterogeneity in the Mediterranean Biodiversity Hotspot affects barcoding accuracy of its freshwater fishes. Mol Ecol Resour 14(6):1210–1221. https://doi.org/10.1111/1755-0998.12257
Gligorović B, Savić A, Protić L, Pešić V (2016) Ecological patterns of water bug (Hemiptera: Heteroptera) assemblages in karst springs: a case study from central Montenegro. Oceanol Hydrobiol Stud 45(4):554–563. https://doi.org/10.1515/ohs-2016-0046
Gold Z, Sprague J, Kushner DJ, Zerecero Marin E, Barber PH (2021) eDNA metabarcoding as a biomonitoring tool for marine protected areas. PLoS ONE 16(2):e023855. https://doi.org/10.1371/journal.pone.0238557
Grabowski M, Rewicz T, Bacela-Spychalska K, Konopacka A, Mamos T, Jazdzewski K (2012) Cryptic invasion of Baltic lowlands by freshwater amphipod of Pontic origin. Aquat Invasions 7(3):337–346. https://doi.org/10.3391/ai.2012.7.3.005
Grabowski M, Mamos T, Bącela-Spychalska K, Rewicz T, Wattier RA (2017) Neogene paleogeography provides context for understanding the origin and spatial distribution of cryptic diversity in a widespread Balkan freshwater amphipod. PeerJ 5:e3016. https://doi.org/10.7717/peerj.3016
Grabowski M, Jabłońska A, Wysocka A, Pešić V (2018) The Obscure History of the Lake Skadar and Its Biota: a perspective for future research. In: Pešić V, Karaman G, Kostianoy AG (eds) The Skadar/Shkodra Lake environment. Springer International Publishing, Cham, pp 47–61
Grković A, Vujić A, Radenković S, Chroni A, Petanidou T (2015) Diversity of the genus Eumerus Meigen (Diptera, Syrphidae) on the eastern Mediterranean islands with description of three new species. Ann Soc Entomol Fr (NS) 51(4):361–373. https://doi.org/10.1080/00379271.2016.1144483
Grosser C, Pešić V, Gligorović B (2015) A checklist of the leeches (Annelida: Hirudinea) of Montenegro. Ecol Montenegrina 2(1):20–28
Gullan PJ, Cranston PS (2014) The insects: an outline of entomology, 5th edn. John Wiley & Sons, New York
Havemann N, Gossner MM, Hendrich L, Morinière J, Niedringhaus R, Schäfer P, Raupach MJ (2018) From water striders to water bugs: the molecular diversity of aquatic Heteroptera (Gerromorpha, Nepomorpha) of Germany based on DNA barcodes. PeerJ 6:e4577. https://doi.org/10.7717/peerj.4577
Hawlitschek O, Morinière J, Lehmann GUC, Lehmann AW, Kropf M, Dunz A, Glaw F, Detcharoen M, Schmidt S, Hausmann A, Szucsich NU, Caetano-Wyler SA, Haszprunar G (2017) DNA barcoding of crickets, katydids and grasshoppers (Orthoptera) from Central Europe with focus on Austria, Germany and Switzerland. Mol Ecol Resour 17(5):1037–1053. https://doi.org/10.1111/1755-0998.12638
Hebert PDN, Cywinska A, Ball SL, deWaard JR (2003) Biological identifications through DNA barcodes. Proc R Soc b Biol 270(1512):313–321. https://doi.org/10.1098/rspb.2002.2218
Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W (2004) Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc Natl Acad Sci 101(41):14812–14817. https://doi.org/10.1073/pnas.0406166101
Hebert PDN, Braukmann TWA, Prosser SWJ, Ratnasingham S, deWaard JR, Ivanova NV, Janzen DH, Hallwachs W, Naik S, Sones JE, Zakharov EV (2018) A Sequel to Sanger: amplicon sequencing that scales. BMC Genom 19(1):219. https://doi.org/10.1186/s12864-018-4611-3
Hebsgaard MB, Andersen NM, Damgaard J (2004) Phylogeny of the true water bugs (Nepomorpha: Hemiptera–Heteroptera) based on 16S and 28S rDNA and morphology. Syst Entomol 29(4):488–508. https://doi.org/10.1111/j.0307-6970.2004.00254.x
Henry TJ (2017) Biodiversity of Heteroptera Insect Biodiversity. John Wiley & Sons, New York
Hermoso V, Clavero M (2011) Threatening processes and conservation management of endemic freshwater fish in the Mediterranean basin: a review. Mar Freshw Res 62(3):244–254. https://doi.org/10.1071/MF09300
Hermoso V, Clavero M, Blanco-Garrido F, Prenda J (2011) Invasive species and habitat degradation in Iberian streams: an analysis of their role in freshwater fish diversity loss. Ecol Appl 21(1):175–188. https://doi.org/10.1890/09-2011.1
Hupało K, Mamos T, Wrzesińska W, Grabowski M (2018) First endemic freshwater Gammarus from Crete and its evolutionary history—an integrative taxonomy approach. PeerJ 6:e4457. https://doi.org/10.7717/peerj.4457
Hupało K, Stoch F, Karaouzas I, Wysocka A, Rewicz T, Mamos T, Grabowski M (2021) Freshwater Malacostraca of the Mediterranean Islands – Diversity, Origin, and Conservation Perspectives. In: Wehrtmann IS (ed) Advances in Crustacean Research 22 CRC Press, 139–220.
Hutchinson GE (1993) A treatise on limnology. Wiley, New York, Chichester, Brisbane, Toronto, London
Ivanova NV, DeWaard JR, Hebert PDH (2006) An inexpensive, automation-friendly protocol for recovering high-quality DNA. Mol Ecol Notes 6(4):998–100. https://doi.org/10.1111/j.1471-8286.2006.01428.x
Ivković M, Perović M, Grootaert P, Pollet M (2021) High endemicity in aquatic dance flies of Corsica, France (Diptera, Empididae, Clinocerinae and Hemerodromiinae), with the description of a new species of Chelipoda. ZooKeys. https://doi.org/10.3897/zookeys.1039.66493
Jabłońska A, Mamos T, Zawal A, Grabowski M (2018) Morphological and molecular evidence for a new shrimp species, Atyaephyra vladoi sp. Nov. (Decapoda, Atyidae) in the ancient Skadar Lake system, Balkan Peninsula—its evolutionary relationships and demographic history. Zool Anz 275:66–79. https://doi.org/10.1016/j.jcz.2018.05.004
Jabłońska A, Wrzesińska W, Zawal A, Pešić V, Grabowski M (2020) Long-term within-basin isolation patterns, different conservation units, and interspecific mitochondrial DNA introgression in an amphipod endemic to the ancient Lake Skadar system. Balkan Peninsula Freshw Biol 65(2):209–225. https://doi.org/10.1111/fwb.13414
Jansson A (1986) The Corixidae (Heteroptera) of Europe and some adjacent regions. Entomol Fenn 47:1–94
Jordal BH, Kambestad M (2014) DNA barcoding of bark and ambrosia beetles reveals excessive NUMTs and consistent east-west divergence across Palearctic forests. Mol Ecol Resour 14(1):7–17. https://doi.org/10.1111/1755-0998.12150
Josifov M (1986) Catalogue of the Heteroptera species (Insecta) known from the Balkan Peninsula. Faunistische Abhandlungen Staatliches Museum Für Tierkunde in Dresden 14(6):61–93
Jovanović M, Haring E, Sattmann H, Grosser C, Pesic V (2021) DNA barcoding for species delimitation of the freshwater leech genus Glossiphonia from the Western Balkan (Hirudinea, Glossiphoniidae). Biodivers Data J 9:e66347
Kapli P, Lutteropp S, Zhang J, Kobert K, Pavlidis P, Stamatakis A, Flouri T (2017) Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics 33(11):1630–1638. https://doi.org/10.1093/bioinformatics/btx025
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software Version 7: improvements in performance and usability. Mol Biol Evol 30(4):772–780. https://doi.org/10.1093/molbev/mst010
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28(12):1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16(2):111–120. https://doi.org/10.1007/BF01731581
Klecka J (2014) The role of a water bug, Sigara striata, in freshwater food webs. PeerJ 2:389. https://doi.org/10.7717/peerj.389
Kment P, Bryja J, Jindra Z (2005) New records of true bugs (Heteroptera) of the Balkan peninsula. Acta Entomol Serbica 13(1):9–20
Kress JW, García-Robledo C, Uriarte M, Erickson DL (2015) DNA barcodes for ecology, evolution, and conservation. Trends Ecol Evol 30(1):25–35. https://doi.org/10.1016/j.tree.2014.10.008
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549. https://doi.org/10.1093/molbev/msy096
Kvist S (2013) Barcoding in the dark?: a critical view of the sufficiency of zoological DNA barcoding databases and a plea for broader integration of taxonomic knowledge. Mol Phylogenet Evol 69(1):39–45. https://doi.org/10.1016/j.ympev.2013.05.012
Lacoursière-Roussel A, Howland K, Normandeau E, Grey EK, Archambault P, Deiner K, Lodge DM, Hernandez C, Leduc N, Bernatchez L (2018) eDNA metabarcoding as a new surveillance approach for coastal Arctic biodiversity. Ecol Evol 8(16):7763–7777. https://doi.org/10.1002/ece3.4213
Lancaster J, Downes BJ (2013) Aquatic entomology. OUP Oxford, Oxford
Lasca N Radulović V, Ristić R, Cherkauer D (1981) Geology, hydrology, climate and bathymetry of Lake Skadar The biota and limnology of Lake Skadar. University Veljko Vlahović, Institute of Biological and Medicine Research, Titograd, Montenegro, Yugoslavia
Leberger R, Geijzendorffer IR, Gaget E, Gwelmami A, Galewski T, Pereira HM, Guerra CA (2020) Mediterranean wetland conservation in the context of climate and land cover change. Reg Environ Change 20(2):67. https://doi.org/10.1007/s10113-020-01655-0
Leigh JW, Bryant D (2015) POPART: full-feature software for haplotype network construction. Methods Ecol Evol 6(9):1110–1116. https://doi.org/10.1111/2041-210X.12410
Mamos T, Wattier R, Burzyński A, Grabowski M (2016) The legacy of a vanished sea: a high level of diversification within a European freshwater amphipod species complex driven by 15 My of Paratethys regression. Mol Ecol 25(3):795–810. https://doi.org/10.1111/mec.13499
Mamos T, Jażdżewski K, Čiamporová-Zaťovičová Z, Fedor Č Jr, Grabowski M (2021) Fuzzy species borders of glacial survivalists in the Carpathian biodiversity hotspot revealed using a multimarker approach. Sci Rep 11:21629. https://doi.org/10.1038/s41598-021-00320-8
Marinković N, Karadžić B, Pešić V, Gligorović B, Grosser C, Paunović M, Nikolić V, Raković M (2019) Faunistic patterns and diversity components of leech assemblages in karst springs of Montenegro. Knowl Manag Aquat Ecosyst 420:26
McCafferty WP (1983) Aquatic entomology: the fishermen’s and ecologists’ illustrated guide to insects and their relatives. Jones & Bartlett Learning, Boston
Meyin A, Ebong S, Petit E, Le Gall P, Chen PP, Nieser N, Guilbert E, Njiokou F, Marsollier L, Guégan JF, Pluot-Sigwalt D, Eyangoh S, Harry M (2016) Molecular species delimitation and morphology of aquatic and sub-aquatic bugs (Heteroptera) in Cameroon. PLoS ONE 11(5):e0154905. https://doi.org/10.1371/journal.pone.0154905
Milano M, Ruelland D, Fernandez S, Dezetter AJ, Fabre J, Servat E, Fritsch JM, Ardoin-Bardin S, Thivet G (2013) Current state of Mediterranean water resources and future trends under climatic and anthropogenic changes. Hydrol Sci J 58(3):498–518. https://doi.org/10.1080/02626667.2013.774458
Naselli-Flores L, Marrone LF (2019) Different invasibility of permanent and temporary waterbodies in a semiarid Mediterranean Island. Inland Waters 9(4):411–421. https://doi.org/10.1080/20442041.2019.1653110
Park DS, Foottit R, Maw E, Hebert PDN (2011) Barcoding bugs: DNA-based identification of the true bugs (Insecta: Hemiptera: Heteroptera). PLoS ONE 6(4):18749. https://doi.org/10.1371/journal.pone.0018749
Parslow BA, Schwarz MP, Stevens MI (2021) Molecular diversity and species delimitation in the family Gasteruptiidae (Hymenoptera: Evanioidea). Genome 64:253–264
Peckarsky BL (1982) Aquatic insect predator-prey relations. Bioscience 32(4):261–266. https://doi.org/10.2307/1308532
Pešić V (2022) Sperchon milisai nov. sp., an overlooked new species of water mites (Acari, Hydrachnidia, Sperchontidae) from Montenegro and Croatia, based on morphological and DNA barcode evidence. Ecol Montenegrina 51:81–92
Pešić V, Glöer P (2018) The Diversity and Conservation Status of the Molluscs of Lake Skadar/Shkodra. In: Pešić V, Karaman G, Kostianoy AG (eds) The Skadar/Shkodra lake environment. Springer International Publishing, Cham, pp 295–310
Pešić V, Smit H (2020) Mideopsis milankovici sp. nov. a new water mite from Montenegro based on morphological and molecular data (Acariformes, Hydrachnidia, Mideopsidae). Acarologia 60(3):566–575. https://doi.org/10.24349/acarologia/20204387
Pešić V, Gadawski P, Gligorović B, Glöer P, Grabowski M, Kovács T, Murányi D, Płóciennik M, Šundić D (2018a) The diversity of the Zoobenthos communities of the Lake Skadar/Shkodra Basin. In: Pešić V, Karaman G, Kostianoy AG (eds) The Skadar/Shkodra lake environment. The Handbook of Environmental Chemistry. Springer International Publishing, Cham, pp 255–293
Pešić V, Karaman GS, Kostianoy AG, Vukašinović-Pešić V (2018b) Conclusions recent advances and the future prospects of the Lake Skadar/Shkodra environment. In: Pešić V, Karaman G, Kostianoy AG (eds) The Skadar/Shkodra Lake environment. The Handbook of environmental chemistry. Springer International Publishing, Cham, pp 481–500. https://doi.org/10.1007/698_2018_274
Pešić V, Hofman S, Rysiewska A, Osikowski A, Falniowski A (2019) Species distinctness of Bithynia cettinensis Clessin, 1887 and B. zeta Glöer et Pešić, 2007 (Caenogastropoda: Truncatelloidea). Folia Malacol 27(2):111–118. https://doi.org/10.12657/folmal.027.013
Pešić V, Zawal A, Bańkowska A, Jovanović M, Dabert M (2020) A new crenobiontic water mite species of the genus Atractides Koch, 1837 from Montenegro and Bulgaria, based on morphological and molecular data (Acariformes, Hydrachnidia, Hygrobatidae). Syst Appl Acarol 25(10):1889–1900
Pešić V, Jovanović M, Manović A, Karaouzas I, Smit H (2021a) New records of water mites from the Balkans revealed by DNA barcoding (Acari, Hydrachnidia). Ecol Montenegrina 49:20–34. https://doi.org/10.37828/em.2021.49.2
Pešić V, Zawal A, Manović A, Bańkowska A, Jovanović M (2021b) A DNA barcode library for the water mites of Montenegro. Biodivers Data J 9:e78311. https://doi.org/10.3897/BDJ.9.e78311
Pesic V, Glöer P (2013) A new freshwater snail genus (Hydrobiidae, Gastropoda) from Montenegro, with a discussion on gastropod diversity and endemism in Skadar Lake. ZooKeys. https://doi.org/10.3897/zookeys.281.4409
Polhemus JT, Polhemus DA (2008) Global diversity of true bugs (Heteroptera; Insecta) in freshwater. Hydrobiologia 595(1):379–391. https://doi.org/10.1007/s10750-007-9033-1
Porco D, Rougerie R, DeHarveng L, Hebert PDH (2010) Coupling non-destructive DNA extraction and voucher retrieval for small soft-bodied Arthropods in a high-throughput context: the example of Collembola. Mol Ecol Resour 10(6):942–945. https://doi.org/10.1111/j.1755-0998.2010.2839.x
Previšić A, Graf W, Vitecek S, Kučinić M, Bálint M, Keresztes L, Pauls SU, Waringer J (2014) Cryptic diversity of caddisflies in the Balkans: the curious case of Ecclisopteryx species (Trichoptera: Limnephilidae). Arthropod Syst Phylogeny 72(3):309–329
Protić L (1998) Catalogue of the Heteroptera fauna of Yugoslav countries: part one. Nat Hist Mus Belgrade Special Issue 38:1–215
Protić L (2001) Catalogue of the Heteroptera fauna of Yugoslav countries. Part two. Nat Hist Mus Belgrade Special Issue 39:1–272
Protić L (2016) Checklist of Heteroptera of Montenegro. Ecol Montenegrina 7:350–393. https://doi.org/10.37828/em.2016.7.12
Puillandre N, Brouillet S, Achaz G (2021) ASAP: assemble species by automatic partitioning. Mol Ecol Resour 21:609–620. https://doi.org/10.1111/1755-0998.13281
Ratnasingham S, Hebert PDN (2007) BOLD: the barcode of life data system (http://www.barcodinglife.org). Mol Ecol Notes 7(3):355–364. https://doi.org/10.1111/j.1471-8286.2007.01678.x
Ratnasingham S, Hebert PD (2013) A DNA-based registry for all animal species: the barcode index number (BIN) system. PLoS ONE 8:e66213
Raupach MJ, Hendrich L, Küchler SM, Deister F, Morinière J, Gossner MM (2014) Building-up of a DNA barcode library for true bugs (Insecta: Hemiptera: Heteroptera) of Germany reveals taxonomic uncertainties and surprises. PLoS ONE 9(9):e106940. https://doi.org/10.1371/journal.pone.0106940
Raupach MJ, Hannig K, Morinière J, Hendrich L (2020a) A DNA barcode library for ground beetles of Germany: the genus Agonum Bonelli, 1810 (Insecta, Coleoptera, Carabidae). Deutsche Entomologische Zeitschrift 67(2):197–207. https://doi.org/10.3897/dez.67.56163
Raupach MJ, Hannig K, Morinière J, Hendrich L (2020b) A DNA barcode library for ground beetles of Germany: the genus Pterostichus Bonelli, 1810 and allied taxa (Insecta, Coleoptera, Carabidae). ZooKeys 980:93–117. https://doi.org/10.3897/zookeys.980.55979
Rewicz T, Móra A, Tończyk G, Szymczak A, Grabowski M, Calleja EJ, Pernecker B, Csabai Z (2021) First records raise questions: DNA barcoding of Odonata in the middle of the Mediterranean. Genome 64(3):196–206. https://doi.org/10.1139/gen-2019-0226%M32502367
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Savage AA, (1989) Adults of the British aquatic Hemiptera Heteroptera: a key with ecological notes. Freshwater biological association, Cumbria
Silva FL, Pinho L, Stur E, Nihei S, Ekrem T (2023) DNA barcodes provide insights into the diversity and biogeography of the non-biting midge Polypedilum (Diptera, Chironomidae) in South America. Authorea. preprint. https://doi.org/10.22541/au.167592622.27180121/v1
Sousa P, Grosso-Silva JM, Andrade A, Chaves C, Pinto J, Paupério J, Beja P, Ferreira S (2021) The InBIO barcoding initiative database: DNA barcodes of Portuguese Hemiptera 01. Biodivers Data J 8:e49985
Strauss G, Niedringhaus R (2014) Die Wasserwanzen Deutschlands: Bestimmungsschlüssel für alle Nepo-und Gerromorpha. WABV Fründ
Sworobowicz L, Grabowski M, Mamos T, Burzyński A, Kilikowska A, Sell J, Wysocka A (2015) Revisiting the phylogeography of Asellus aquaticus in Europe: insights into cryptic diversity and spatiotemporal diversification. Freshw Biol 60(9):1824–1840. https://doi.org/10.1111/fwb.12613
Tachos V, Dimitrakopoulos PG, Zogaris S (2022) Multiple anthropogenic pressures in Eastern Mediterranean rivers: insights from fish-based bioassessment in Greece. Ecohydrol Hydrobiol 22(1):40–54. https://doi.org/10.1016/j.ecohyd.2021.06.001
Thalinger B, Deiner K, Harper LR, Rees HC, Blackman RC, Sint D, Traugott M, Goldberg CS, Bruce K (2021) A validation scale to determine the readiness of environmental DNA assays for routine species monitoring. Environ DNA 3(4):823–836. https://doi.org/10.1002/edn3.189
Tierno de Figueroa JM, López-Rodríguez MJ, Fenoglio S, Sánchez-Castillo P, Fochetti R (2013) Freshwater biodiversity in the rivers of the Mediterranean Basin. Hydrobiologia 719(1):137–186. https://doi.org/10.1007/s10750-012-1281-z
Trifinopoulos J, Nguyen L-T, von Haeseler A, Minh BQ (2016) W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res 44:W232–W235
Wattier R, Mamos T, Copilaş-Ciocianu D, Jelić M, Ollivier A, Chaumot A, Danger M, Felten V, Piscart C, Žganec K, Rewicz T, Wysocka A, Rigaud T, Grabowski M (2020) Continental-scale patterns of hyper-cryptic diversity within the freshwater model taxon Gammarus fossarum (Crustacea, Amphipoda). Sci Rep 10:16536. https://doi.org/10.1038/s41598-020-73739-0
Weigand H, Beermann AJ, Čiampor F, Costa FO, Csabai Z, Duarte S, Geiger MF, Grabowski M, Rimet F, Rulik B, Strand M, Szucsich N, Weigand AM, Willassen E, Wyler SA, Bouchez A, Borja A, Čiamporová-Zaťovičová Z, Ferreira S, Dijkstra KDB, Eisendle U, Freyhof J, Gadawski P, Graf W, Haegerbaeumer A, van der Hoorn BB, Japoshvili B, Keresztes L, Keskin E, Leese F, Macher JN, Mamos T, Paz G, Pešić V, Pfannkuchen DM, Pfannkuchen MA, Price BW, Rinkevich B, Teixeira MAL, Várbíró G, Ekrem T (2019) DNA barcode reference libraries for the monitoring of aquatic biota in Europe: gap-analysis and recommendations for future work. Sci Total Environ 678:499–524. https://doi.org/10.1016/j.scitotenv.2019.04.247
Weigelhofer G, Weißmair W, Waringer J (1993) Night migration activity and the influence of meteorological parameters on light-trapping for aquatic Heteroptera. Zool Anz 229(5–6):209–218
Zhao Y, Wang H, Huang H, Zhou Z (2022) A DNA barcode library for katydids, cave crickets, and leaf-rolling crickets (Tettigoniidae, Rhaphidophoridae and Gryllacrididae) from Zhejiang Province, China. ZooKeys. https://doi.org/10.3897/zookeys.1123.86704
Zimmermann M, Spence JR (1989) Prey use of the fishing spider Dolomedes triton (Pisauridae, Araneae): an important predator of the neuston community. Oecologia 80(2):187–194. https://doi.org/10.1007/BF00380149
Acknowledgements
The authors wish to thank all the people contributing help and friendly atmosphere to the fieldwork during all the Lake Skadar expeditions, i.e. Aleksandra Bańkowska, Edyta Buczyńska, Karolina Bącela-Spychalska, Tomasz Czernicki, Katarzyna Gadawska, Joanna Grabowska, Janusz Hejduk, Aleksandra Jabłońska, Radomir Jaskuła, Magdalena Kłosowska, Grzegorz Michoński, Marek Michalski, Mateusz Puchalski, Ewa Sarnacka, Piotr Spychalski, Agnieszka Szlauer-Łukaszewska, Robert Sobczyk, Przemysław Śmietana, Przemysław Włodarczyk.
Funding
The research was financed by: (1) the Ministry of Science, Montenegro (grant “DNA-Eco: DNA barcode reference library as a tool for sustainable management of freshwater ecosystems in the highly threatened Lake Skadar Basin”); (2) the National Science Center, Poland (grants number 2014/15/B/NZ8/00266, 2016/23/NZ8/02123); (3) the statutory funds of the University of Lodz; (4) the statutory fund of the University of Szczecin. T.R. was supported by a Scholarship from the Polish National Agency for Academic Exchange (NAWA) through the Bekker Programme [PPN/BEK/2018/1/00162]. PG was supported by a scholarship from the Polish National Agency for Academic Exchange (NAWA) through the Iwanowska Programme [PPN/IWA/2018/1/00021/DEC/2].
Author information
Authors and Affiliations
Contributions
Conceptualization: MG; Methodology: TR, PG, TM, ŁT; Formal analysis and investigation: TR, MG, GT, PG, TM, ŁT, VP, AZ; Taxonomic expertise: GT; Writing—original draft preparation: TR, MG; Writing—review and editing: TR, MG, ŁT, PG, TM; Funding acquisition: VP, AZ, MG, PG, TR; Resources: TR, MG, GT, PG, TM, ŁT, VP, AZ; Supervision: MG.
Corresponding author
Ethics declarations
Declarations
None of the studied species is listed in national laws as protected or endangered. The material was collected partly from the Skadar Lake National Park according to the terms of permit number 02-UPI/1070/3, provided by the Environmental Agency of Montenegro for V. Pešić.
Conflict of interest
The authors have no conflict of interest to declare, relevant to the content of this article.
Additional information
Communicated by David Hawksworth.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rewicz, T., Tończyk, G., Trębicki, Ł. et al. DNA barcode-based survey documents underestimated diversity and intricate phylogeographic patterns of aquatic Heteroptera in an endangered Balkan biodiversity hotspot: ancient Lake Skadar basin. Biodivers Conserv 32, 4111–4138 (2023). https://doi.org/10.1007/s10531-023-02686-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-023-02686-9