Abstract
The introduction of exotic herbivores to islands is one of the most harmful challenges to the conservation of insular biodiversity, causing the extinction or geographical reduction of many plant species worldwide and motivating conservation actions from environmental managers. Here, we evaluated the recovery of plant communities, specifically the endangered Medicago citrina, on a small islet close to Ibiza (Western Mediterranean Basin) after the complete eradication of introduced rabbits (fully eradicated in 2016). To evaluate the variation of plant richness and coverage, five permanent plots were installed in 2015 to record over five years. Additionally, an exhaustive census geolocating all M. citrina individuals was carried out in 2019. Total vegetation cover, plant richness and Shannon’s diversity index significantly increased throughout the study period. We counted 2,322 M. citrina individuals over 15 cm tall and a large number of seedlings. Therefore, this population not only successfully recovered, but also established the largest population in the entire geographic distribution of this species. The demographic structure is dominated by young individuals and a few large individuals, and the distribution across the island is heterogeneous since the largest spots of individuals were located in ravines where air currents probably provided seeds from plants located in inaccessible cliffs. However, the appearance of the invasive insect Icerya purchasi now threats the population of M. citrina as it feeds on the tissues of adult plants and compromises its development and survival. This study proves the eradication of herbivores is the most efficient way to conserve vulnerable species.
Similar content being viewed by others
Introduction
Biological invasions are the second leading cause of extinction worldwide (Gurevitch and Padilla 2004; Bellard et al. 2016b) and disturb the function and stability of ecosystems (Capizzi 2020). Insular plants and vertebrates have been particularly affected by alien species (Diamond 1989; Hänel and Chown 1998; Bellard et al. 2016b). Introduced herbivores such as rats, rabbits and goats interact with the native vegetation via direct impacts such as browsing and indirect impacts such as alteration of the soil composition or destruction of habitats (Coblentz 1978; Desender et al. 1998; Campbell and Donlan 2005). Moreover, introduced insects can also damage plant species by acting as plant disease vectors, phloem and leaf feeders, or florivores (Causton et al. 2006).
Goats are one of the most harmful introduced species and have severely damaged the flora of many islands, which has motivated eradication initiatives (Campbell and Donlan 2005). As examples, a 30-year eradication program was performed on La Pinta Island, Galápagos, Ecuador (Campbell et al. 2004), and a 12-year eradication program was carried out on Dirk Hartog Island, Australia (Heriot et al. 2019) among others, including 107 successful attempts in the Mediterranean islands (Capizzi 2020).
Rats are also very commonly introduced onto islands and islets and generally reach very high population densities (Cheylan 1999; Meyer and Butaud 2009). The impact of rats on flora is not negligible, for example the presence of rats altered the communities of plant species on islets in the Cabrera Archipelago National Park (Palmer and Pons 2001). Furthermore, rabbits can have an extremely deleterious impact on small islands, both directly by predation and indirectly by soil erosion and ecosystem transformation (Taylor and Lovegrove 1997; Eijzenga 2011; Schweizer et al. 2016).
The damage caused by invertebrate species has also severely affected plant populations on many islands, such as the example of predation of vegetation by Limnophyes minimus larvae on Marion Island (Hänel and Chown 1998), the severe defoliation of Buxus species by the alien moth Cydalima perspectalis (Baur et al. 2019) or the predation of the xylem vessels of many plant species by Icerya purchasi (Watson and Malumphy 2004; Alvarez et al. 2012). In particular, I. purchasi has invaded many islets in the western Mediterranean Basin, and threatens the conservation of narrowly distributed species like Medicago citrina on islands such as Columbretes and Cabrera (Laguna and Jiménez 1995; Laguna et al. 2017).
Monitoring of the ecosystem is essential after completion of an eradication program. Vegetation can significantly recover after eradication, as observed on Santa Cruz Island, California (Beltran et al. 2014), and Maui, Hawaii (Scowcroft and Hobdy 1987) or Es Vedrà, Balearic Islands (Capó et al. 2022). However, the chances of successful recovery of plant species may be lower when eradications are performed in areas where herbivores invaded the island many decades ago (Zavaleta et al. 2001; Eijzenga 2011). For example, other invasive alien plant species may benefit from eradications and colonize the ecosystem more rapidly than native species, as occurred for Casuarina equisetifolia on Nishi-jima Island, Ogasawara, after eradication of black rats (Rattus rattus) and feral goats (Capra hircus) (Abe et al. 2011). In this context, monitoring vegetation after eradication is crucial to elucidate how species recolonize their habitat and to evaluate if new threats have appeared requiring more interventions (IUCN/SSC 2013).
Though herbivory by alien interferes with the whole ecosystem, range-restricted species are considered to be the most vulnerable species, especially if they lack defenses against herbivory or have high palatability (Bowen and Van Vuren 1997; Cubas et al. 2019; Capó et al. 2021). Therefore, range-restricted species may become extinct or constrain their distribution if alien herbivores are uncontrolled (Pisanu et al. 2012; Doherty et al. 2016; Fenu et al. 2016; Bellard et al. 2016a; Oliveira et al. 2021). Many species have been threatened by alien herbivores on archipelagos such as the Hawaiian Islands (Eijzenga 2011; Barton and Hanley 2013; Barton 2016), Galápagos Archipelago (Campbell et al. 2004; Carrion et al. 2011), Santa Cruz Island in California (Beltran et al. 2014), Canary Islands (Nogales et al. 2006; Garzón-Machado et al. 2010; Cubas et al. 2019) and Balearic Islands (Latorre et al. 2013; Capó et al. 2021).
The Balearic Islands are affected by alien herbivores, so mammal (goats, rats and rabbits) eradications have been performed on some islets of the Archipelago (Mayol et al. 2012; Capizzi 2020; Capó et al. 2022). Particularly, rabbits from s’Espartar, an islet of 20 ha close to Ibiza, were eradicated by the environmental authority in three steps using different methods: (i) first, most individuals were removed by hunting, (ii) then, combining hunting and poisonous traps and (iii) poisonous traps for remnant individuals (Colomar and Picorelli pers. com.). Major part of rabbits was eliminated from 2011 to 2013, and only few remnant individuals were removed in 2014 and 2015 (one individual in 2016). Since then, eradication was considered successful since any trace of rabbits was not recorded on the islet anymore. From the first attempt until the complete eradiation, ca. 300 rabbits were eliminated (Colomar and Picorelli, pers. com). This islet is home to the most abundant population of the threatened plant species Lamottea dianae and a remarkable population of the endangered species M. citrina, which was restricted to coastal cliffs areas where plants are inaccessible to rabbits (Mateu 2011).
Medicago citrina is exclusively located on twelve small and disjunct islets (see below). This species did not colonize larger islands or mainland, except for a few cases (Laguna et al. 2017), even when the islet is close to the mainland or other islands. The hypotheses proposed to explain this bizarre distribution are an extreme specialization to this particular microhabitat and seabirds are responsible for the colonization of these small islets or, more likely, there has been a contraction of the plants’ original range reduced to those islets where never have been herbivores. So, the main hypothesis, almost for Balearic populations, is that these islets acted as the last refuge for this species (Rita 2019). In fact, it is well known that this species is very vulnerable to mammal herbivores, especially goats, rats and rabbits (Palmer and Pons 2001; Latorre et al. 2013). Therefore, herbivores could have been the main reason of this so constricted distribution. Indeed, a recent study reported that M. citrina has lower tolerance to herbivory than the closely related and widespread species Medicago arborea (Capó et al. 2021). Like many other Mediterranean range-restricted species, M. citrina is well adapted to drought conditions and exposure to high salinity (Juan and Crespo 1999; Lefi et al. 2004), which has allowed it to effectively compete against other plants under the hard environmental conditions of the small islets (Juan and Crespo 1999). However, coinciding with eradication of the rabbits, the presence of I. purchasi was reported for the first time on s’Espartar islet and found to affect M. citrina individuals, probably due to the presence of this pest in surrounding islets (pers. observ.). This record implied that the spread of I. purchasi may interfere with the recovery of the M. citrina population after the eradication of rabbits.
In this context, the objectives of the present work were (i) to assess how plant communities responded to the eradication of rabbits from s’Espartar, (ii) to evaluate the whole population structure of M. citrina after the eradication of rabbits from the islet, and (iii) to assess the threat of I. purchasi to the recolonization of M. citrina on the islet. We hypothesized that (i) the coverage of the whole vegetation would increase in absence of rabbits, (ii) the population of M. citrina would increase and the recruitment of juveniles would be high once the herbivores were absent, and (iii) the stabilization of the population of M. citrina would increase the probability of the I. purchasii arrival from near infested islets.
Materials and methods
Study system
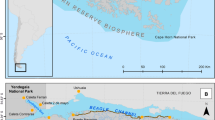
S’Espartar is an islet (20 ha) located 1,300 m away from the western coast of Eivissa (38° 57′ N, 1° 11′ E) with a maximum length and width of 890 m and 360 m respectively, and an altitude of 70 m asl (Fig. 1). The most extensive vegetation communities on s’Espartar are grasslands of Stipa tenacissima, which occupies the warmest and driest slops; shrublands of Olea europaea var. sylvestris, Pinus halepensis and Pistacia lentiscus, located in the higher areas; and coastal slopes and ravines of Atriplex halimus, among others (Fig. 2). Some areas of the islet have a high density of range-restricted or threatened species such as Lamottea dianae, Diplotaxis ibicensis and Silene hifacensis (Mateu 2011).
Vegetation map of the islet of s’Espartar in 2010, the yellow squares with numbers correspond to the permanent plots installed in 2015. Data from Mateu (2011), with modifications
Medicago citrina (Fabaceae) is a shrub that reaches up to 3 m tall and wide, with yellow flowers and spiral-shaped pods (Sales and Hedge 2000). The species does not exhibit asexual reproduction. Flowers are self-compatible but depend on large floral visitors (mainly hymenopterans, hoverflies and blowflies) for tripping and pollination (Pérez-Bañón et al. 2003). It produces a large number of seeds, although seed set in small-islet populations may be lowered by scarcity of appropriate pollinators (Juan 2002; Pérez-Bañón et al. 2003). The species forms a long-term soil seed bank that ensures the population regeneration, and germination rate seems to increase when seeds pass through the digestive tract of animals (Laguna et al. 2017). Besides seabird dispersal, fruits can be easily dispersed by wind as they consist of flat and very light legumes with wide wings. The distribution of this species is restricted to 12 small islets in the Balearic and Columbretes Archipelagos, and a small islet close to the Iberian Peninsula (Fig. 3). The overall population of the species was estimated at 2,500 individuals (Laguna et al. 2017), thus M. citrina is in the IUCN Endangered category (Rita 2019) and is one of the 50 most threatened plant species of the Mediterranean Islands (Pasta et al. 2017).
Geographic distribution of Medicago citrina populations. Each point can cover more than one island. Original populations are labeled in red; introduced populations are labeled in blue. The population on Grossa de Columbretes Island was reintroduced after it became extinct. The population on Dragonera Island was probably introduced. Isolated individuals appear sporadically in the larger islands of the Balearic Islands and also on the coast of the Iberian Peninsula (these individuals have not been indicated)
Medicago citrina is severely affected by I. purchasi, which feeds on the tissues of the species and compromises its development and survival. The first records of I. purchasi on the Columbretes Islands were made in 1996 (Juan 2002) and at almost the same time on the Cabrera Islets, and the species expanded to all islets in the archipelago between 2004 and 2006. The first records of I. purchasii in the populations of Eivissa were made on na Bosc Islet (ses Bledes) in 2018 and on s’Espartar in 2019 (pers. observ.). This pest has caused severe damage on the Columbretes Islets, reducing the M. citrina population by close to 85% (Laguna et al. 2017), and severe damage of the population on na Bosc Islet close to Ibiza has also been observed (pers. observ.).
Monitoring recovery of plant communities after the eradication of rabbits
Five 10 × 10 m permanent plots were installed in different areas of s’Espartar in 2015 to include the main plant communities of the islet. The habitats selected for the installation of each plot were: grasslands of Stipa tenacissima, ravines with Atriplex halimus, communities of Lamottea dianae, and shrublands of Pistacia lentiscus and Olea europaea var. sylvestris. Each plot was visited annually from 2015 to 2019, and the monitoring was performed always in middle of May to account for the therophyte species and to accomplish data homogenization among years. We compiled an inventory of the vascular flora by direct observations or sampling difficult-to-identify species for identification in the laboratory using determination manuals (Gil and Llorens 2017). The Braun-Blanquet index was used to evaluate the abundance-dominance of each species (Braun-Blanquet 1979). Additionally, 20-m transects were used to evaluate species coverage by reporting each individual found every 10 cm (200 sampling points per plot and year). Transects were placed from one vertex of the plot to the opposite diagonal vertex. From this data, we calculated indexes related to diversity: species richness (S), as the number of total species, Shannon’s diversity index (H’) (Shannon 1948) and Pielou’s evenness (J), calculated as J = H’/ln(S) (Pielou 1975). Diversity indexes were calculated using the ‘diversity’ and ‘specnumber’ functions of ‘vegan’ package (Oksanen et al. 2008).
All statistical analyses were performed in R 3.6.1 (R Core Team 2021). Recovery of vegetation was assessed using a Generalised Additive Mixed Model (GAMM), considering plant coverage or diversity indexes as explained variables, years as a smooth variable and plots as random factor. Moreover, the number of individuals of M. citrina observed inside the plot were recorded to evaluate the species recovery; the temporal variation was assessed using a Generalised Linear Model (GLM) with a Poisson distribution for the number of M. citrina individuals. Subsequently, analysis of variance was performed using the ‘Anova’ function of ‘car’ package (Fox and Weisberg 2019). To evaluate ad-hoc groups, the Tukey test was performed using the ‘lsmeans’ function of the ‘lsmeans’ package (Lenth 2016).
Analysis of the population structure of Medicago citrina
During the final year of monitoring the permanent plots, we georeferenced and measured the total population of M. citrina, except for seedlings (< 15 cm high) and individuals located on inaccessible cliffs. Each individual was georeferenced using a differential GPS corrected by the Servei d’Informació Territorial de les Illes Balears (SITIBSA) network. All GPS points were obtained using a Leica System RTK RX 1200 and Leica TPS800, which provide a maximum deviation of 0.05 m that allows individuals to be accurately located (Leica Geosystems 2013). Data on the plant height (cm), canopy diameter (cm), trunk diameter (mm) at 5 cm above the terrain, and presence of reproductive structures were collected for each individual. We also calculated the size index using (plant height + canopy diameter)/2, as described by De Cáceres et al. (2013). Seedlings that measured less than 15 cm were not sampled, but considered as recruitment of the surrounding adults. Individuals of M. citrina infested by I. purchasi were also recorded to assess the impact of the pest on the population. Biometric parameters were calculated for the general population and for patches of high-density of individuals and also differentiating infested from non-affected individuals. Statistical analysis was conducted in R 4.1.1 (R Core Team 2021) by implementing a linear model including biometric parameters described above as fixed factors and patches of high density or infestation as response variables. Analysis of the variance was performed by ‘Anova’ function from the ‘car’ package (Fox and Weisberg 2019).
The distribution of the M. citrina population was represented with QGIS 3.10 (QGIS Development Team 2020). The results were compared with data from previous studies (Bibiloni et al. (2003); Mateu (2011)) on the same islet.
Results
Plant community coverage and richness increased after the eradication of rabbits
Analysis of the five permanent plots between 2015 and 2019 (rabbits were fully eradicated in 2016) revealed a significant increase in the total vegetation cover (Fig. 4). Moreover, the diversity indexes indicated significant increases in plant richness and Shannon’s diversity over the study period but non-significant changes for Pielou evenness index (Fig. 4).
Seedlings of M. citrina were only observed in one of the five plots at the beginning of the experiment (2015), in three plots in the following year (2016), and in four plots in 2017 (Fig. 5). Globally, there was a significant increase both in the number of individual plants and coverage of M. citrina. These increases were especially more rapid for plots 2 and 3, those closest to cliffs with rupicolous populations of M. citrina. Some seedlings survived in the four plots and then died the following year, though a general increasing trend was still observed (Fig. 5). Plot 1, which was not colonized by M. citrina throughout the study period, was the furthest from the putative source area and located inside a dense grassland of Stipa tenacissima (Fig. 3).
Number of Medicago citrina individuals reported each year of the study period (2015–2019). The overall trend is indicated in the dotplot (left) using error bars to indicate standard error. Letters indicate significant differences determined using the Tukey test. The recovery process of each plot is detailed in the diagram (right); each color represents a permanent plot, and the size of the plant is classified as seedling, juvenile or adult. Plot 1 was not included as no individuals were reported in any year
The population of M. citrina recovered after to rabbits’ eradication
A total of 2,322 individuals taller than 15 cm were recorded, which represented a density of 116 individuals/ha. The geographic distribution of the population was heterogeneous, with some spots containing a high density of individuals (Fig. 6A). Grasslands with predominance of the perennial graminoid Stipa tenacissima had the lowest density of M. citrina individuals. On the contrary, areas close to cliffs, where population inaccessible to rabbits exist, showed the highest density of M. citrina. In particular, M. citrina was dominant in two spots on the islet. One spot is located on the western slope of the central ravine and the other is on the western part of the islet; both of these spots are close to the cliffs on the north coast. Interestingly, the eastern part of the islet has not been colonized by M. citrina yet, with only few individuals found along the south-eastern zone.
A Distribution map of Medicago citrina after the eradication of rabbits in 2016 (data from 2020); B distribution of M. citrina individuals with a trunk diameter greater than 40 mm; C potential routes of M. citrina recolonization according to the slope and orientation of ravines in the islet (solid arrows) and other putative sources of seeds (dotted arrows). Individuals detected before the eradication are marked in red, both those found in cliff areas (polygons) and isolated individuals in areas accessible to rabbits across the islet (dots)
The western part of the islet had the densest population of M. citrina, representing 37.2% of the whole population. In this area there were the 65.5% of total individuals with a trunk diameter greater than 40 mm, and the 76.6% of those with trunk wider than 50 mm. The average trunk diameter of this subpopulation differed significantly to that of the overall population (df = 1, F = 19.33, p < 0.001); therefore, the largest—and potentially oldest—M. citrina individuals were mainly located at that area (Fig. 6A, B).
Overall, the M. citrina individuals were mainly young (28.1% had a stem diameter less than 1 cm, and up to 65.7% had a stem diameter less than 2 cm), with only a small number of older individuals (2.0% had a diameter greater than 5 cm and only six had a diameter larger than 8 cm). M. citrina plants were generally small, as only 4.8% were taller than 100 cm (the tallest plant was 163 cm). Also, the trunk diameter significantly correlated with the height (r = 0.66) and the width of the plant (r = 0.82). Therefore, the trunk diameter and size index exhibited similar demographic trends (Fig. 7). Overall, 56% of plants were sexually mature, although this data may be underestimated since part of the census was carried out in summer.
Infestation by Icerya purchasi affected M. citrina individuals during recolonization
Recolonization of the islet by M. citrina after the eradication of rabbits coincided with the first records of I. purchasi infestation on the islet. Of the overall M. citrina population, 3.5% of plants (81 individuals) showed signs of the pest. The infested plants were mainly located in the western area of the islet and some individuals exhibited considerable damage that could affect their survival (Fig. 8). Damaged individuals had higher trunk diameters and higher size indexes than the average values. Therefore, I. purchasi preferentially attacks larger, adult individuals than smaller plants (df = 1, F = 19.33, p < 0.001). During our study, the pest did not lead to the death of any plants, though the damage may become more severe in the future.
Discussion
Plant communities increased in coverage and richness after eradication of rabbits
The entire plant community of the islet exhibited higher plant coverage, species richness and Shannon’s diversity index after the eradication of rabbits. Plant coverage notably increased after the eradication, indicating that plants have the ability to respond rapidly. This event is not rare, as many plant communities in other areas increased after eradication of herbivores (Bullock et al. 2002; Beltran et al. 2014; Chapuis et al. 2004). The impact of herbivores is more severe under insular conditions, especially for range-restricted species without defences (Bowen and Van Vuren 1997; Cubas et al. 2019; Capó et al. 2021), and can also negatively affect other plant-animal interactions (Traveset and Richardson 2006). Moreover, species richness in the permanent plots had doubled four years after the total eradication of rabbits, indicating a clear expansion of less-abundant species across habitats, as observed in other areas (Kessler 2001). However, further monitoring programs are necessary to guarantee species recovery and avoid the appearance of alien species (Abe et al. 2011), which are abundant along the Eivissa coastline. Our temporal monitoring of the permanent plots showed a remarkable increase in M. citrina from 2015 to 2019, indicating a good and fast response for this species after to the eradication of rabbits, as previously reported for other insular endemic species, such as on the Mauritius Archipelago (North et al. 1994).
Eradication of rabbits was essential to preserve endangered species
The population of M. citrina on the islet of s'Espartar consisted almost 2,322 individuals in 2019 (4 years after the total eradication of rabbits), not counting the individuals on the cliffs nor seedlings. Therefore, this is an example of a rapid colonization process from less 10 plants detected in 2003 (Bibiloni et al. 2003) and 2010 (Mateu 2011) to the actual size of the population once herbivores are removed. Hence, the new population after the eradication of rabbits is of a similar size to the total estimated population (2,500 individuals) for the entire distribution area of this species in 2017 (Laguna et al. 2017), which means that eradication of rabbits in s’Espartar allowed to double the global population of the target species. The severe damage of rabbits on this plant species has been well documented as this animal was one of the main factors which drive to its extinction in illa Grossa of Columbretes Archipelago (Laguna et al., 2017) where it was later reintroduced. Moreover, the demographic structure of the population of M. citrina reveals a predominance of young individuals with a few larger plants, which further supports the short-time period of recolonization enhanced by the high seed production of individuals and the long-term soil seed bank (Pérez-Bañón et al. 2003), which predicts that the colonization would be still in progress. Indeed, numerous recently germinated seedlings were observed during the exhaustive census of the population, which reveals that M. citrina has a great capacity to regenerate in the absence of herbivores. In addition, these observations are compatible with the hypothesis that M. citrina may have had a wider distribution in the past and that an ancient introduction of herbivores constrained its distribution to smallest islets where rats, goats or other animals never were introduced. Moreover, other species endemic to the Balearic Islands has been ecologically restricted to rupicolous habitats or islets without mammal herbivores (Capó 2021). Indeed, some plants endemic to the Balearic Islands recolonized shrublands on sa Dragonera islet after eradication of rats such as Hippocrepis balearica, Cheirolophus intybaceus, Helichrysum fontanesii or Lomelosia cretica (pers. observ.), and could probably colonize other areas if herbivores were eradicated.
Medicago citrina has received intense attention from environmental authorities for conservation purposes due to its endangered status in many red lists such as the IUCN (Laguna et al. 2017; Rita 2019). Both in situ and ex situ actions have been performed to guarantee its conservation (Santamaría et al. 2007; Fenu et al. 2020). Also, translocations of M. citrina to new islands were performed after eliminating herbivores, as recommended by the IUCN (IUCN/SSC 2013). Therefore, the conservation of this endangered species strongly depends on the absence of herbivores, similarly to other threatened species (Gómez-Aparicio et al. 2005). Furthermore, the population that was sheltered in the inaccessible cliffs of the islet enabled rapid recolonization and encouraged conservation of the genetic structure of the M. citrina population during the period rabbits were present on the islet. However, these small isolated populations could also represent a genetic bottleneck that may reduce the genetic variability of the new population, as has occurred in other species (Jiménez et al. 2017).
Recolonization may be mediated by abiotic factors
The heterogeneous distribution of the new M. citrina population on the islet may possibly be explained by two reasons. On one hand, inaccessible individuals on cliffs could have acted as a seed source for dispersion of the population to other areas. From there, the population may have first colonized the areas closest to cliffs and then spread to other areas of the islet by wind action or slope mediation (Fig. 6C), so the colonisation is still in process. This might explain why some areas remain uninhabited at present but may be colonised in future. On the other hand, not all of the habitats on the islet are suitable for the target species. For example, the grasslands with a predominance of Stipa tenacissima located on the south slopes of the islet are generally not colonized by M. citrina. For this reason, the heterogeneous distribution of M. citrina may be determined by habitat suitability and could depend on the coexisting plant community.
The high-density spot on the western site of the islet contained a higher proportion of old individuals than other areas. This area is likely to have been the initial site of M. citrina during its colonization of the islet and from which the population subsequently spread. Rabbits were concentrated in the central part of the islet (pers. observ.), and therefore marginal areas could have been the first to be released from herbivore pressure during the eradication process. As another possibility, the western side of the islet is closer to another islet where M. citrina occurs, and the possible arrival of M. citrina mediated by seabirds cannot be neglected. Further studies are required to evaluate the interinsular connection and explore the effect of abiotic features on the spread and distribution of M. citrina.
Infestation by Icerya purchasi affected M. citrina individuals during recolonization
Icerya purchasi was previously reported to be present on islets where M. citrina occurs. In 1996–1997 and 2013–2015, I. purchasi damaged and caused 40% and 85% reductions, respectively, in the populations of M. citrina on the Columbretes Islands (Laguna et al. 2017). This pest was also detected in 2018 on an islet near Ibiza (na Bosc), where it caused the death of many large M. citrina individuals (pers. obs.). In the present study, we recorded the presence of I. purchasi on s’Espartar in 2019. Though the infestation only affected 3.5% of the census population, the pest has a high capacity to spread and could affect the entire population in a few years, as reported in other studies (Laguna et al. 2017). At present, biological control using Rodalia cardinalis (Quezada and DeBach 1973) has been very effective for controlling I. purchasi on M. citrina on the Columbretes Islands (Laguna et al. 2017) and for other species in the Galápagos Archipelago (Alvarez et al. 2012). However, the use of exotic species as biological control remains controversial, especially when such management is applied in protected natural areas. All in all, based on previous experience (Laguna et al. 2017), we consider using the beetle (R. cardinalis) represents a suitable solution to promote complete recovery of the M. citrina population on the islet.
Conclusions
Introduced mammal herbivores in islands and islets represent a serious threat to the conservation of threatened flora and insular ecosystems in general. Herbivory can force defenceless species to reduce their distribution or even become extinct. We report a clear case of how a threatened plant species can rapidly recover its population size in the short-term and recolonize a whole islet once alien rabbits have been eradicated. Medicago citrina has long been a target for conservation and the focus of in situ and ex situ conservation actions. Eradication of alien herbivores has been the most successful action to preserve its population, as its overall population size on s’Espartar doubled in only a few years. Inaccessible populations that remained undamaged while rabbits were present on the islet acted as a seed reservoir and enhanced the recovery of the population after the eradication of rabbits, illustrating the importance of these residual populations to the recovery of the species as a whole. The damage caused by rabbits, combined with the current presence of I. purchasi on the islet, are examples of how invasive alien species—not only mammals, but also arthropods—strongly threaten narrow and threatened plant species. Further monitoring and management actions must be performed in the future to guarantee the eradication of the scale pest and ensure the islet free remains free of alien herbivores.
Data availability
Authors will share databases with anyone who will be interested to use it for scientific purposes.
Code availability
Not applicable.
References
Abe T, Yasui T, Makino S (2011) Vegetation status on Nishi-jima Island (Ogasawara) before eradication of alien herbivore mammals: Rapid expansion of an invasive alien tree, Casuarina equisetifolia (Casuarinaceae). J Forest Res 16:484–491. https://doi.org/10.1007/s10310-010-0239-0
Alvarez CC, Causton CE, Hoddle MS, Hoddle CD, van Driesche R, Stanek EJ (2012) Monitoring the effects of Rodolia cardinalis on Icerya purchasi populations on the Galápagos Islands. Biocontrol 57:167–179. https://doi.org/10.1007/s10526-011-9429-8
Barton KE (2016) Low tolerance to simulated herbivory in Hawaiian seedlings despite induced changes in photosynthesis and biomass allocation. Ann Bot 117:1053–1062. https://doi.org/10.1093/aob/mcw021
Barton KE, Hanley ME (2013) Seedling-herbivore interactions: insights into plant defence and regeneration patterns. Ann Bot 112:643–650. https://doi.org/10.1093/aob/mcw021
Baur B, Jung J, Rusterholz HP (2019) Defoliation of wild native box trees (Buxus sempervirens): does box rust (Puccinia buxi) infection influence herbivory, survival and growth of the invasive Cydalima perspectalis? J Appl Entomol 143:766–775. https://doi.org/10.1111/jen.12640
Bellard C, Cassey P, Blackburn TM (2016) Alien species as a driver of recent extinctions. Biol Lett. https://doi.org/10.1098/rsbl.2015.0623
Bellard C, Genovesi P, Jeschke JM (2016) Global patterns in threats to vertebrates by biological invasions. Proc Roy Soc B-Biol Sci. https://doi.org/10.1098/rspb.2015.2454
Beltran RS, Kreidler N, Van Vuren DH, Morrison SA, Zavaleta ES, Newton K, Tershy BR, Croll DA (2014) Passive recovery of vegetation after herbivore eradication on Santa Cruz Island, California. Restor Ecol 22:790–797. https://doi.org/10.1111/rec.12144
Bibiloni G, Rita J, Moragues E, Conesa MA, Fontcuberta C (2003) Estudi corològic de la flora vascular endémica dels illots del Parc Natural de Cala d’Hort. Conselleria de Medi Ambient, Govern de les Illes Balears. Report
Bowen L, Van Vuren D (1997) Insular endemic plants lack defenses against herbivores. Conserv Biol 11:1249–1254. https://doi.org/10.1046/j.1523-1739.1997.96368.x
Braun-Blanquet J (1979) Fitosociología. Bases para el estudio de las comunidades vegetales. H. Blume Ediciones, Madrid
Bullock D, North S, Dulloo M, Thorsen M (2002) The impact of rabbit and goat eradication on the ecology of Round Island, Mauritius. In: Veitch CR, Clout MN (eds). Turning the tide: the eradication of invasive species: Proceedings of the International Conference on eradication of island invasives. IUCN-The World Conservation Union, Gland, pp 53–63
Campbell K, Donlan CJ (2005) Feral goat eradications on islands. Conserv Biol 19:1362–1374. https://doi.org/10.1111/j.1523-1739.2005.00228.x
Campbell K, Donlan CJ, Cruz F, Carrion V (2004) Eradication of feral goats Capra hircus from Pinta Island, Galápagos, Ecuador. Oryx 38:328–333. https://doi.org/10.1017/S0030605304000572
Capizzi D (2020) A review of mammal eradications on Mediterranean islands. Mammal Rev 50:124–135. https://doi.org/10.1111/mam.12190
Capó A, Roig-Oliver M, Cardona C, Cursach J, Bartolomé J, Rita J, Baraza E (2021) Historic exposure to herbivores, not constitutive traits, explains plant tolerance to herbivory in the case of two Medicago species (Fabaceae). Plant Sci 307:110890
Capó M, Cursach J, Picorelli V, Baraza E, Rita J (2022) Eradication of feral goats not population control as a strategy to conserve plant communities on Mediterranean islets. J Nat Conserv 65:126108. https://doi.org/10.1016/j.jnc.2021.126108
Carrion V, Donlan CJ, Campbell KJ, Lavoie C, Cruz F (2011) Archipelago-wide island restoration in the galápagos islands: Reducing costs of invasive mammal eradication programs and reinvasion risk. PLoS ONE 6(5):e18835. https://doi.org/10.1371/journal.pone.0018835
Causton CE, Peck SB, Sinclair BJ, Roque-Albelo L, Hodgson CJ, Landry B (2006) Alien insects: threats and implications for conservation of Galápagos Islands. Ann Entomol Soc Am 99:121–143. https://doi.org/10.1603/0013-8746(2006)099[0121:AITAIF]2.0.CO;2
Chapuis JL, Frenot Y, Lebouvier M (2004) Recovery of native plant communities after eradication of rabbits from the subantarctic Kerguelen Islands, and influence of climate change. Biol Conserv 117:167–179. https://doi.org/10.1016/S0006-3207(03)00290-8
Cheylan G (1999) Evolution rapide de petites populations insulaires méditerranéennes de Rattus rattus. In: Alcover JA (ed) Ecologia de les illes. Monografies de la Societat d’Història Natural de les Illes Balears 6. Societat d’Història Natural de les Illes Balears: Palma, pp 88–103
Coblentz BE (1978) The effects of feral goats (Capra hircus) on island ecosystems. Biol Conserv 13:279–286. https://doi.org/10.1016/0006-3207(78)90038-1
Cubas J, Irl SDH, Villafuerte R, Bello-Rodríguez V, Rodríguez-Luengo JL, Del Arco M, Martín-Esquivel JL, González-Mancebo JM (2019) Endemic plant species are more palatable to introduced herbivores than non-endemics. Proc Roy Soc B-Biol Sci. https://doi.org/10.1098/rspb.2019.0136
De Cáceres M, Legendre P, He F (2013) Dissimilarity measurements and the size structure of ecological communities. Methods Ecol Evol 4:1167–1177. https://doi.org/10.1111/2041-210X.12116
Desender K, Baert L, Maelfait JP, Verdyck P (1998) Conservation on Volcan Alcedo (Galápagos): terrestrial invertebrates and the impact of introduced feral goats. Biol Conserv 87:303–310. https://doi.org/10.1016/S0006-3207(98)00078-0
Diamond JM (1989) Overview of recent extinctions. In: Western D, Pearl MC (eds) Conservation for the Twenty-first Century. Wildlife Conservation International, New York, pp 37–41
Doherty TS, Glen AS, Nimmo DG, Ritchie EG, Dickman CR (2016) Invasive predators and global biodiversity loss. PNAS USA 113:11261–11265. https://doi.org/10.1073/pnas.1602480113
Eijzenga H (2011) Vegetation change following rabbit eradication on Lehua Island, Hawaiian Islands. In: Veitch CR, Clout MN, Towns DR (eds) Island invasives: eradication and management. IUCN, Gland, Switzerland, pp 290–294
Fenu G, Cogoni D, Bacchetta G (2016) The role of fencing in the success of threatened plant species translocation. Plant Ecol 217(2):207–217. https://doi.org/10.1007/s11258-015-0517-1
Fenu G, Bacchetta G, Christodoulou C, Cogoni D, Fournaraki C, Giusso del Galdo G, Pietro G, Gotsiou P, Kyratzis A, Piazza C, Vicens M, Montmollin B (2020) A common approach to the conservation of threatened island vascular plants: first results in the Mediterranean Basin. Diversity 12(4):157. https://doi.org/10.3390/d12040157
Fox J, Weisberg S (2019) An R Companion to Applied Regression. Sage, Thousand Oaks CA
Garzón-Machado V, González-Mancebo JM, Palomares-Martínez A, Acevedo-Rodríguez A, Fernández-Palacios JM, Del-Arco-Aguilar M, Pérez-de-Paz PL (2010) Strong negative effect of alien herbivores on endemic legumes of the Canary pine forest. Biol Conserv 143:2685–2694. https://doi.org/10.1016/j.biocon.2010.07.012
Gil L, Llorens L (2017) Flora vascular de les Illes Balears. Clau analítica. Universitat de les Illes Balears, Palma
Gómez-Aparicio L, Zamora R, Gómez JM (2005) The regeneration status of the endangered Acer opalus subsp. granatense throughout its geographical distribution in the Iberian Peninsula. Biol Conserv 121:195–206. https://doi.org/10.1016/j.biocon.2004.04.019
Gurevitch J, Padilla DK (2004) Are invasive species a major cause of extinctions? Trends Ecol Evol 19:470–474. https://doi.org/10.1016/j.tree.2004.07.005
Hänel C, Chown SL (1998) The impact of a small, alien invertebrate on a sub-Antarctic terrestrial ecosystem: Limnophyes minimus (Diptera, Chironomidae) at Marion Island. Polar Biol 20:99–106. https://doi.org/10.1007/s003000050282
Heriot S, Asher J, Williams MR, Moro D (2019) The eradication of ungulates (sheep and goats) from Dirk Hartog Island Shark Bay World Heritage Area Australia. Biol Invasions 21(5):1789–1805. https://doi.org/10.1007/s10530-019-01937-7
IUCN/SSC. 2013. Guidelines for reintroductions and other conservation translocations. Version 1.0. Gland, Switzerland: IUCN Species Survival Commission
Jiménez A, Weigelt B, Santos-Guerra A, Caujapé-Castells J, Fernández-Palacios JM, Conti E (2017) Surviving in isolation: genetic variation, bottlenecks and reproductive strategies in the Canarian endemic Limonium macrophyllum (Plumbaginaceae). Genetica 145:91–104. https://doi.org/10.1007/s10709-017-9948-z
Juan A (2002) Estudio sobre la morfología, variabilidad molecular y biología reproductiva de Medicago citrina (Font Quer) Greuter (Leguminosae). Bases para su conservación. PhD Dissertation. Universitat de Valencia
Juan A, Crespo MB (1999) Comportamiento fitosociológico de Medicago citrina (Font Quer) Greuter (Leguminosae), endemismo mediterráneo-iberolevantino. Acta Bot Malacitana 24:221–229. https://doi.org/10.24310/abm.v24i0.8532
Kessler CC (2001) Eradication of feral goats and pigs from Sarigan Island, Commonwealth of the Northern Mariana Islands. In: Veitch CR, Clout MN. Turning the tide: the eradication of invasive spcies. IUCN SCC Invasive Species Specialist Group. Glan, Switzerland and Cambridge, pp 132–140
Laguna E, Jiménez J (1995) Conservación de la flora de las islas Columbretes (España). Ecologia Mediterranea 21:325–336. https://doi.org/10.3406/ecmed.1995.1784
Laguna E, Sáez L, Mus M, Juan A, Crespo MB (2017) Medicago citrina. In: Pasta S, Perez-Graber A, Fazan L, Montmollin B (eds) The Top 50 Mediterranean Island Plants UPDATE 2017. IUCN/SSC/Mediterranean Plant Specialist Group. Neuchâtel, Switzerland
Leica Geosystems (2013) Leica RX1200 user manual. http://www.geoaxxis.de/manuals/tps1200/rx1220_user_en.pdf
Latorre L, Larrinaga AR, Santamaría L (2013) Combined impact of multiple exotic herbivores on different life stages of an endangered plant endemism, Medicago citrina. J Ecol 101:107–117. https://doi.org/10.1111/1365-2745.12005
Lefi E, Medrano H, Cifre J (2004) Water uptake dynamics, photosynthesis and water use efficiency in field-grown Medicago arborea and Medicago citrina under prolonged Mediterranean drought conditions. Ann Appl Biol 144:299–307. https://doi.org/10.1111/j.1744-7348.2004.tb00345.x
Lenth RV (2016) Least-squares means: The R package lsmeans. J Stat Softw 69:1–33
Mateu A (2011) Flora de les reserves naturals des Vedrà, es Vedranell i els illots de Ponent d’Eivissa. Master Thesis. Universitat de les Illes Balears
Mayol J, Mayol M, Domenech O, Oliver J, McMinn M, Rodríguez A (2012) Aerial broadcast of rodenticide on the island of Sa Dragonera (Balearic Islands, Spain). A promising rodent eradication experience on a Mediterranean islands. Aliens: The Invasive Species Bulletin 32
Meyer JY, Butaud JF (2009) The impacts of rats on the endangered native flora of french Polynesia (Pacific Islands): drivers of plant extinction or coup de grâce species? Biol Invasions 11:1569–1585. https://doi.org/10.1007/s10530-008-9407-y
Nogales M, Rodríguez-Luengo JL, Marrero P (2006) Ecological effects and distribution of invasive non-native mammals on the Canary Islands. Mamm Rev 36:49–65. https://doi.org/10.1111/j.1365-2907.2006.00077.x
North SG, Bullock DJ, Dulloo ME (1994) Changes in the vegetation and reptile populations on Round Island, Mauritius, following eradication of rabbits. Biol Conserv 67:21–28. https://doi.org/10.1016/0006-3207(94)90004-3
Oksanen J, Kindt R, Legendre P, O’Hara B, Simpson GL, Solymos P, Stevens, M. Henry H, Wagner H (2008) Vegan: Community Ecology Package. version 1.15–1
Oliveira A, Peixoto P, Barbosa B, de Faria AP (2021) Reproductive success, herbivory and ex situ conservation of Neoregelia ibitipocensis (Bromeliaceae): an endemic and endangered species from the Atlantic Forest. Aust J Bot. https://doi.org/10.1071/BT21011
Palmer M, Pons GX (2001) Predicting rat presence on small islands. Ecography 24:121–126. https://doi.org/10.1034/j.1600-0587.2001.240202.x
Pasta S, Perez-Graber A, Fazan L. and Montmollin B. de (2017) The Top 50 Mediterranean Island Plants UPDATE 2017. IUCN/SSC/Mediterranean Plant Specialist Group. Neuchâtel (Switzerland). 141 pp. https://top50.iucn-mpsg.org
Pérez-Bañón C, Juan A, Petanidou T, Marcos-García M, Crespo M (2003) The reproductive ecology of Medicago citrina (Font Quer) Greuter (Leguminosae): a bee-pollinated plant in Mediterranean islands where bees are absent. Plant Syst Evol 241:29–46. https://doi.org/10.1007/s00606-003-0004-3
Pielou E (1975) Ecological diversity. Wiley, New York
Pisanu S, Farris E, Filigheddu R, García MB (2012) Demographic effects of large, introduced herbivores on a long-lived endemic plant. Plant Ecol 213:1543–1553. https://doi.org/10.1007/s11258-012-0110-9
QGIS Development Team (2020) QGIS Geographic information system. Open source geospatial foundation project
Quezada JR, DeBach P (1973) Bioecological and population studies of the cottony-cushion scale, Icerya purchasi Mask., and its natural enemies, Rodolia cardinalis Mul. and Cryptochaetum iceryae Will., in Southern California. Hilgardia 41:631–688
R Core Team (2021) R: A language and environment for statistical computing.
Rita J (2019) Medicago citrina. In: Moreno JC, Bañares A, Blanca G, Güemes J, Ortiz S (eds) Atlas y Libro rojo de la flora vascular amenazada de España. Adenda 2017. Madrid: Ministerio para la Transición Ecológica - SEBICOP, pp 64–65
Sales F, Hedge IC (2000) Medicago L. In: Talavera S, Aedo C, Castroviejo S, Herrero A, Romero C, Salgueiro FJ, Velayos M (eds). Flora iberica. Plantas vasculares de la Península Ibérica e Islas Baleares. Vol. VII(II). Leguminosae (partim). Real Jardín Botánico, CSIC, Madrid, pp 741–775
Santamaría L, Larrinaga AR, Latorre L, Pericàs J (2007) Herbívoros exóticos del Archipiélago de Cabrera: Bases para una estrategia de gestión basada en la minimización de impactos. Proyectos De Investigación En Parques Nacionales 2003–2006:293–306
Schweizer D, Jones HP, Holmes ND (2016) Literature review and meta-analysis of vegetation responses to goat and European rabbit eradications on islands. Pac Sci 70:55–71. https://doi.org/10.2984/70.1.5
Scowcroft PG, Hobdy R (1987) Recovery of goat-damaged vegetation in an insular tropical montane forest. Biotropica 19(3):208–215
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech 27:379–423
Taylor GA, Lovegrove TG (1997) Flora and vegetation of Stanley (Atiu) Island, Mercury Islands. Tane 36:85–111
Traveset A, Richardson DM (2006) Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol Evol 21:208–216. https://doi.org/10.1016/j.tree.2006.01.006
Watson GW, Malumphy CP (2004) Icerya purchasi Maskell, cottony cushion scale (Hemiptera: Margarodidae), causing damage to onramental plants growing outdoors in London. Br J Enomol 17:105–109
Zavaleta ES, Hobbs RJ, Mooney HA (2001) Viewing invasive species removal in a whole-ecosystem context. Trends Ecol Evol 16:454–459. https://doi.org/10.1016/S0169-5347(01)02194-2
Acknowledgements
The authors thank the Natural Reserve staff and Víctor Colomar for collaborating with transport to the area and for their help during fieldwork. We are grateful to Ajuntament de Sant Josep de sa Talaia, and especially to Raül Luna, for their support with the logistics of our stay in Eivissa. Fieldwork was executed with the valuable collaboration of Virginia Picorelli, Antoni Ávila, Antoni J. Far, Francisco Fuster and Jordi Serapio. Víctor Colomar provided the information about the eradication process and the number of rabbits. This study was funded by the Government of the Balearic Islands (Direcció General d'Espais Naturals i Biodiversitat, Conselleria de Medi Ambient i Territori, Govern de les Illes Balears). MC was supported by a PhD fellowship FPI/1925/2016 through Direcció General de Política Universitària i Recerca (Govern de les Illes Balears) and the European Social Dund.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was funded by the Government of the Balearic Islands (Direcció General d'Espais Naturals i Biodiversitat, Conselleria de Medi Ambient i Territori, Govern de les Illes Balears). MC was supported by a PhD fellowship FPI/1925/2016 through Direcció General de Política Universitària i Recerca (Govern de les Illes Balears) and the European Social Fund.
Author information
Authors and Affiliations
Contributions
All authors have contributed equally in the design, sampling, analysis and writing.
Corresponding author
Additional information
Communicated by Daniel Sanchez Mata.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Invasive species.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rita, J., Capó, M. & Cursach, J. Eradication of rabbits from islets is essential for conservation of microinsular vegetation and narrow endangered flora: the case of Medicago citrina (Fabaceae) in s’Espartar islet (Balearic Islands, Western Mediterranean Basin). Biodivers Conserv 31, 779–796 (2022). https://doi.org/10.1007/s10531-022-02362-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-022-02362-4