Abstract
Recent researches suggest that functional diversity represents the response of communities to environmental alterations better than taxonomic diversity. However, there is scarce information about how the functional diversity of freshwater fishes is affected by habitat type and the dominance of non-native species. To address this question, we analysed a large database containing 15 morpho-functional traits of 61 fish species from the Pannon Biogeographic region (Hungary). Based on a fish faunistic list and relative abundance of taxa, we quantified the taxonomic and functional diversity of riverine communities for > 700 sites of six habitat types. We asked how non-native fishes affected the taxonomic and functional diversity in different river types and at the local scale (i.e. at the site level), and how the diversity measures of native fauna elements changes along the invasion gradient. Our results showed that both functional and taxonomic richness increases with habitat complexity, from small headwater streams to large rivers. Therefore taxonomic diversity served as a good proxy for functional diversity along the environmental gradient of river types. Non-natives showed considerable functional diversity relative to their species number in each habitat type. Diversity values of native fauna elements initially increased, and then showed a major decrease along the invasion gradient. River type-specific evaluations highlighted the importance of considering the proliferation of invasive species based on both taxonomic and functional diversity indices. We argue that type-specific action plans are needed in conservation management to preserve the taxonomic and functional diversity of native fishes in Hungary, but also elsewhere.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwaters are among the most endangered ecosystems on Earth (Carpenter et al. 1992; Jenkins 2003; Dudgeon et al. 2006). Their biodiversity is declining considerably (Tickner et al. 2020) due to the mutually reinforcing effects of habitat degradation, climate change and spread of non-native species (Vitousek et al. 1997; Didham et al. 2007). Freshwater fish, the most species rich vertebrate group, is also seriously endangered, thus, their protection requires urgent conservation actions (Lévêque et al. 2008). On the other hand, fishes are among the most important, and the most frequently introduced animal groups in the world (Gozlan et al. 2010). Intentional and unintentional introductions of fishes can be valuable to humankind since they provide important ecosystem services, such as food and recreational fishing. However, non-native fishes can cause serious changes in the native freshwater biota at all organization levels (Leprieur et al. 2008; Martin et al. 2010; Cucherousset and Olden 2011), which can be difficult to quantify comprehensively (Vigliano et al. 2009; Britton et al. 2011; Capps et al. 2015).
At the community level, range expansions of non-natives and range contractions of native species can change the diversity and structure of native communities, a process which has been termed biotic homogenization (Olden et al. 2004). Non-natives have also been shown to decrease the community stability of the recipient native communities (Erős et al. 2020). However, most studies use “traditional” taxonomy-based methods to characterize changes in community level patterns, despite the fact that trait-based approaches seem to be more sensitive in revealing the effect of disturbances (like invasions) on the recipient communities (Violle et al. 2007; Strayer 2012; Gagic et al. 2015; Toussaint et al. 2016).
Morpho-functional traits have increasingly been used for characterizing changes in the functional diversity of fish communities (Villéger et al. 2010; Shuai et al. 2018). Traits such as eye position and oral gape size and position inform on key species-specific functions such as vertical position in the water column and food acquisition. However, the effect of non-native fishes on functional diversity of fish communities has not been revealed in detail in many eco-, and biogeographic regions (Whittaker et al. 2014; Colin et al. 2018; Toussaint et al. 2018), which hinders generalizations about their overall ecological role. It has been shown, for example, that a relatively small increase in the number of non-native fishes could cause a significant increase in the functional diversity of fish communities at large spatial scales (i.e. global or regional; see Toussaint et al. 2018). Moreover, increasing abundance of non-native species was found to negatively affect functional diversity (Matsuzaki et al. 2013; Su et al. 2021), although its intensity depended on stream order (Milardi et al. 2019). However, how functional diversity changes along invasion gradients within and between ecoregions in different running water types is largely unknown.
Therefore, in this study, we present (i) the first comprehensive dataset containing morpho-functional traits of the fish fauna of the Pannon Biogeographic region, Hungary, and examine (ii) how taxonomic and functional diversity of fish communities change in different running water types, and (iii) how “traditional” taxonomic and functional diversity indices of native fauna elements respond to invasion by non-native fishes at both the habitat type and local (sample site) scales. Based on the results of former studies, we hypothesized that non-native fishes would increase both the taxonomic and functional diversity of riverine fish at the entire community level, and that their influence would depend on river type. In this regard, we predicted the largest changes in diversity in lowland streams and rivers compared with other river types, since these habitats were found to contain the most non-native fishes in the Pannon (Takács et al. 2017) and other biogeographic regions (Stewart et al. 2016; Milardi et al. 2019). In addition, we were also interested to examine how the diversity of the native community is influenced by non-native species. Non-native fishes can extirpate native ones in dynamically changing riverine habitats (Baltz and Moyle 1993; Kominoski et al. 2018), but more frequently, influence their relative abundance relationships. Their effects on the diversity of native fish communities is hard to predict for each river type, since this may largely depend on the degree of invasion and specific functional attributes of non-native fishes. This underlines the importance of understanding the influence of non-natives on native communities in a river type specific manner.
Materials and methods
Study area and the characteristics of its fish fauna
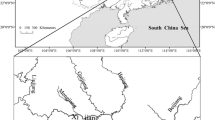
The study area is situated in Hungary, Central Europe. The whole area (93,030 km2) of Hungary lies in the Carpathian Basin and belongs to the catchment of the River Danube (catchment area 796,250 km2; length 2847 km). Since ca. 70% of the country’s area is lowland (Fig. 1), lowland streams and rivers constitute the majority of the river network. The extensive—more than 40,000 km long—artificial drainage and irrigation canal system increases the length of lowland watercourses further (Martonné Erdős 2004). This area belongs to the Pannon biogeographic Region, which supports ~ 80 fish species that show considerable diversity (Kottelat and Freyhof 2007; Sály 2007). The region’s fish fauna is dominated by widespread Eurasian species, but a number of Danubian endemics, and species with Ponto-Caspian origin (Lévêque et al. 2008), enrich the species pool of the area. Besides the diverse native fish fauna, the number of non-native fauna components increased exponentially in the last several decades (Takács et al. 2017).
Map of the study area showing the 738 sampling sites distributed among the six river types (a). Blue circle: submontane stream (SS), orange circle: highland stream (HS), green circle: highland river (HR), red circle: lowland stream (LS), purple rectangle: lowland river (LR), ligh grey filled triangle: River Danube (D). Geographic position of Hungary in Europe is indicated in the insert b. Colour figure online
Fish survey data
Our data set consists of fish distribution and relative abundance data, collected during a countrywide fish survey between 2011 and 2015 using standardized electrofishing protocols (Erős 2007; Sallai 2019). In wadeable watercourses (i.e. streams), a battery-powered electrofishing device was used to sample a 150-m long reach at each site by slowly wading upstream and single pass fishing the whole stream width. Non-wadeable river habitats and the Danubian sites were sampled by boat electrofishing using engine powered devices, slowly moving downstream and electrofishing one (in case of highland and lowland river sites) to ten (in Danube sites) 500 m long near shore sections, depending on the size of the habitat (Erős 2007). Comprehensive recent surveys provided relative abundance data for 738 sampling sites, belonging to six major running water types according to Erős (2007): (i) submontane streams (SS); (ii) highland streams (HS); (iii) highland rivers (HR); (iv) lowland streams and canals (LS); (v) lowland rivers (LR); and (vi) the main channel of the River Danube. Due to the geographic conditions of Hungary most sites belonged to LS (n = 307), followed by HS (n = 223), LR (n = 110), SS (n = 44) and HR (n = 42), while the Danube was represented by 12 sites (Fig. 1). Here, we analysed a subset of the entire data set (see Takács et al. 2017), including samples in which the number of species exceeded three and the calculation of functional diversity measures was possible see below and (Laliberté et al. 2014).
Mesurement of morpho-functional traits
To characterize the functional structure of fish communities, we collected data on morpho-anatomical features (Fig. 2) that reflected key functions such as food acquisition or locomotion types (Villéger et al. 2010). Most individuals used for the morpho-anatomical measurements were collected during our fish surveys, but data for some rare species were obtained from fisheries and conserved specimens deposited in institutional (e.g.: Balaton Limnological Research Institute, and Danube Research Institute) and museum (e.g. Hungarian Natural History Museum) collections. In these cases only the morphometric data of well-preserved specimens were used. Multiple morphologic variables were measured directly on the studied fish specimens, e.g. body weight, oral gape width and depth, using scale and digital callippers, while others were measured on photos taken from the specimens. For this latter process, each fish was placed flat on a table surface and the left side was photographed from a perpendicular angle using a tripod mounted Nikon D5300 digital camera. The area and distance measurements were conducted by imageJ software (Rasband 2012). To eliminate intermeasurer variability (Takács et al. 2016), all measurements were made by the same person. For each surveyed species, morphometric data of five adult individuals were recorded and averaged. From the measured 20 continuous morphometric variables, 15 functional traits (axes) were created (Table 1) according to Villéger et al. (2010).
Morphometric data recorded on the studied species. Head depth (Hd), eye diameter (Ed), distance from the top of the mouth to the bottom of the head along the head depth axis (Mo), body depth (Bd), eye height (Eh), caudal fin depth (Cfd), caudal peduncle depth (Cpd), pectoral fin insertion (Pfi), body height at the pectoral fin insertion (Pfb), pectoral fin length (Pfl), pectoral fin surface (PFs), caudal fin length (CFs), body width (Bw), mouth depth (Md) and width (Mw) (After Villéger et al. 2010). Colour figure online
Diversity quantification
Quantification of functional richness and diversity was made at the entire species-pool level of the studied sites (i.e. 61 species), and separately for the six different river types as well. Functional richness at the native, non-native and entire (i.e. natives and non-natives pooled together) community levels was characterised using the convex hulls’ volume on PCA plots, which was derived from the log-transformed 15 morpho-functional traits of fish species. The area of each polygon was expressed in the percentage of the convex hull’s volume containing the entire species pool. Additionally, the functional and taxonomic diversity of the entire, native and non-native species pools were expressed in the percentage of the total functional and taxonomic diversity of the entire species pool (i.e. 61 species).
Taxonomic and functional diversity values were computed for each site using abundance-weighted and non-weighted indices. For taxonomic diversity, we used species richness (S) as a non-abundance weighted metric, and the Shannon diversity index (H’) as an abundance-weighted metric. We used the Functional richness (Fric), given by the volume occupied by the entire species pool in the multidimensional trait space (Villéger et al. 2008), as a non-abundance weighted functional diversity measure. The Rao’s quadratic entropy (RaoQ) was employed for an abundance weighted index, which takes into account the abundance of each species and measures the pairwise functional difference among species (Botta‐Dukát 2005). Calculations of Fric and RaoQ were performed with R software version 3.6.0 (R Core Team 2015) using the FD package (Laliberté et al. 2014). Values of the four diversity measures were visualized on boxplots for each habitat type, and tested for significant habitat type specific differences by non parametric Kruskal–Wallis tests using the PAST 2.17 statistical software (Hammer et al. 2001).
The relationships between diversity measures (S, FRic, H’, RaoQ) of native fish communities and the relative abundance of non-native fishes were tested by generalized additive models (GAM) using the mgcv R package (Wood 2011). GAM models were compared with linear models based on Akaike’s information criterion (AIC). Since we were interested in the general shape of the relationships, we first set river types as random factors (function: bs = ”re”). As river types also affected S and FRic of native fish communities significantly on top of the relative abundance of non-native fishes, we further detailed the relationships in each individual river type. To meet assumptions of normality, the relative abundance of non-native fishes was cubic root-transformed, species richness (S) was ln-transformed, while functional richness (FRic) was square root-transformed.
Results
Altogether 200,750 individuals classified into 61 fish species and hybrids were recorded (Fig. 1, Table 2). Of these, 16 non-native species were found, which gave 18.2% of the total catch. The lowest number of non-native species was recorded in submontain streams (5 species), while the highest number of non-native species was found in lowland rivers (13 species). Average values of the computed 15 morpho-functional traits showed large variations among the surveyed species (Supplementary Table 1). The first two PCA axes explained 54% of variance in functional characteristics among species within the entire species pool (i.e. 61 species; Fig. 3A).
Morpho-functional diversity of the investigated 61 Middle Danubian fish species (A) and the species pools of the studied six river types (B). The presented 2-dimensional space made by first and second principal component (PC) axes summarizing the log transformed 15 morpho-functional trait attributes of the studied 61 species. The top right corner subfigure on A shows the correlations among the 15 functional traits used. Species and functional trait codes are shown in the Tables 1 and 2. The non-native species are marked by red dots and highlighted by red. The convex hulls of the entire species pools are indicated by grey dotted lines in each subfigure. The polygons showing the entire and native species pools of the studied six river types on subfigure B are enframed by dashed and solid lines respectively. Polygons of non natives are enframed by red dotted lines, and filled with pink colour. Abbreviations and colour codes of the river types correspond with Figs. 1, 3 and 4. The percentage of variance explained by each PC axis is given in brackets. Colour figure online
PCA plots showed that polygons enframing the functional attributes of native and non-native fishes overlap considerably. At the same time many non-native species showed extreme morpho-functional trait values in several variables, especially in oral gape surface and gut length (Fig. 3A). Results showed that the polygons defined by native and non-native species covered 65% and 69% of the convex hull area of the total species pool (Supplementary Table 2). With respect to the different river types, the covered area varied between 52.0% (SS) and 93.5% (LR) at the entire community level, and ranged from 43.0% (SS) to 64.6% (LR) for the native, and from 19.1% (D) to 60.5% (LS) for the non-native species, respectively (Fig. 3B, Supplementary Table 2). The functional and taxonomic diversity showed strong linear correlation within the native fish and pooled data sets (Fig. 4). However, non-native species showed relatively high functional diversity and low taxonomic diversity relative to native species, especially in the HS, LS and LR habitat types.
Taxonomic (TD) and functional (FD) facets of fish biodiversity in the studied six river types. TD and FD are expressed in the percentage of the total functional and taxonomic diversity of the Hungarian fish fauna, respectively. The solid line represents the identity line FD = TD. Black square: entire species pool of the six river types, blue circle: native species pool, red triangle: non-native species pool. River type codes correspond with Fig. 1. Colour figure online
At the sample site level, the taxonomic and functional diversity measures at the entire community level varied largely both within and among river types (Fig. 5, Supplementary Table 3 and 4). The lowest values were recorded at the SS habitat type in all cases. In the case of S and Fric, a significant increase was observed along the gradient from SS to the Danube. At the same time, the abundance-weighted diversity values showed only a moderate increase.
Boxplots showing the values of A species richness (S), B functional richness (Fric), C Shannon diversity (H’), and D Rao quadatic diversity metrics of the entire assemblage in the studied six habitat types. For river type codes see Table 2. Each box represents the 25% and 75% quartiles of the dataset, the band in the box is the median. The whiskers are drawn from the top of the box up to the largest data point less than 1.5 times the box height from the box (the"upper inner fence"), and similarly below the box. Values outside the inner fences are shown as circles values further than three times the box height from the box (the "outer fences") are shown as black stars. Boxplots marked with the same letters do not differ significantly based on non-parametric Kruskal–Wallis pairwise comparisons (p˂0.05). The median and min–max values of each datasets and the results of all pairwise comparisons are presented in the Supplementary Tables 3 and 4. Colour figure online
Results of GAM analyses made using the entire assamblege and the studied habitat types’ data are presented in Figs. 6 and 7. The number of native fish species (S), as well as their functional richness (Fric), showed a hump-shaped relationship with the increasing relative abundance of non-native taxa (GAM, Fig. 6A, B). In case of RaoQ the relationship showed an initial increase to 0.5, and beyond this value the curve showed quick decline. Among the four diversity measures, only the Shannon diversity (H’) of native fish taxa did not show a significant relationship with either the relative abundance of non-native taxa (Fig. 6C), or river types (Fig. 7C). Habitat type level analyses show, that the course of the certain diversity curves can show large differences. They generally show decreasing values after an initial rise, with the exception of RaoQ data for lowland watercourses, which show a slight increase with increasing relative abundance of invasive species (Fig. 7D).
Relationship between A species richness (S), B functional richness (Fric), C Shannon diversity (H’), and D RaoQ of native fish communities and the relative abundance of non-native fish taxa based on generalized additive models (GAM, trend line from the smooth function ± SE, n = 630). n.s. non-significant, * < 0.1, ** < 0.5, *** < 0.001
Relationships between A species richness (S), B functional richness (Fric), C Shannon diversity (H’), and D RaoQ of native fish communities in the studied six habitat types, based on generalized additive models (GAM, trend line from the smooth function ± SE). n.s. non-significant, * < 0.1, ** < 0.5, *** < 0.001. See Table 1 for river type codes. Note: to improve visibility only the curves with significant correlations are shown. Colour figure online
Discussion
In this study, we established a morpho-functional trait data base of fishes of the Middle Danubian fish fauna, and quantified how taxononomic and functional diversity measures are distributed in six characteristic riverine habitat types. Moreover, we quantified how non-native species influence these metrics in the different river types. Analyses conducted on aggregated and sample site-level data sets showed that functional diversity corresponds well with taxonomic diversity along the examined riverine habitat gradients. Non-natives influenced the taxonomic and functional diversity metrics at both the entire and native community levels. However, their effect depended on both the diversity metric and the river type considered.
Although non-native species made up only 26.2% of the total species pool, their convex hull covered a larger area in the functional trait space (69% vs. 66%, respectively) than the area of native fauna elements (Fig. 3 and Supplementary Table 2). Analyses at the habitat type level showed that the non-native fauna components had greater functional diversity relative to their species richness than native species (Fig. 4). Additionally, the functional characteristics of non-native and native species showed only moderate overlap. Being functionally different from native competitors is one of the possible reasons for the success of invasive species (Vila-Gispert et al. 2005; Shuai et al. 2018). This statement is well-supported by our data, since many non-native species separated from natives in several morpho-functional traits. For example, the Ponto-Caspian gobies, which are already among the most common species in Danubian rip-rap habitats (Copp et al. 2005; Erős 2005; Brandner et al. 2013), differ largely from the native species both in their food aquisition and locomotion attributes. Carp species such as the silver/bighead carp (Hypophthalmichthys) hybrids and the gibel carp (Carassius gibelio ) are also among the most successful invaders of Hungarian large lowland rivers and small streams (Takács et al. 2017; Vitál et al. 2017). Moreover, gobies and carp species are functionally different community members, indicated by their opposite orientation in the multidimensional trait space (shown on the PC1 axis), primarily due to their specific locomotion types and food acquisition characteristics (Fig. 3). This latter finding may also explain why the convex hulls of native and non-native fauna elements show a moderate overlap in the morpho-functional trait space, and potentially underlies why non-native species increase the functional diversity of the entire community, at least relative to their low contribution to taxonomic diversity. At the same time there are several non-natives in the Hungarian fish fauna [e.g. black bullhead: Ameiurus melas, pumpkinseed: Lepomis gibbosus, amur sleeper: Perccottus glenii ] whose morpho-functional characteristics do not differ largely from those of native species (Fig. 3). Yet, these species can be considered as successful invaders (Takács et al. 2017). Certainly, non-native species might also have some further specific functional characteristics that make them successful (e.g. behaviour, life history or reproduction), uncovered in our analysis. While analysing behaviour and life history traits might shed further light on the ecological roles on non natives, our approach highlights the importance of analysing basic traits, such as locomotion and food acquisition, to identify relevant characteristics with significant ecological effects.
The functional characteristics of the fish communities showed considerable overlap among the six river types, being a common pattern for both native and non-native species. However, non-natives contributed considerably to the functional diversity of LS and LR river types. Here, the differences between polygon areas of the entire and native communities exceeded 25% (see Supplementary Table 2). This result corresponds well with the finding that the effect of non-natives on the taxonomic and functional diversity indices was most pronounced in lowland habitats (e.g., Takács et al. 2017; Milardi et al. 2019). The proliferation of non-native species in lowland areas may predominantly be determined by the distribution of fish ponds (Takács et al. 2017; Milardi et al. 2019).
The non-native fish taxa were expected to affect the composition and functional characteristics of the native fauna components. This effect was detectable not only at the level of the entire fish community, but at the site level as well (Figs. 6, 7). Here, both the taxonomic and functional diversity measures were sensitive indicators of the effects of non-native species. At the same time RaoQ, a functional measure which also accounts for abundance differences among taxa, highlighted significant effects of non-native species on the communities of native species, in the case of multiple river types. While functional richness measures scale largely with taxonomic richness in general (Petchey and Gaston 2002; Erős et al. 2009), our data confirms that the community-level functional effect of invasive species can only be revealed if both the functional attributes and the relative abundance of taxa are considered jointly. Overall, these results suggest that taxonomic information alone can be a reliable first proxy for functional diversity, at least for comparisons among river types, in a similar way found for invasive aquatic plants and macroinvertabrates (Michelan et al. 2010; Martello et al. 2018). However, to consider potential and detailed functional effects of non-native fishes, the use of trait-based and abundance-weighted indices may be more advantageous.
Interestingly, we found an increase in both taxonomic and functional diversity indices of native fauna components at low levels of invasion, which was followed by a considerable decrease in the index values along the invasion gradient. However, our data also justifies that responses were rather river-type specific, and, within specific types, index-dependent. It is likely that the increase in diversity values at very low stages of invasion (0–10%) are related more to environmental conditions of the habitat than to the effect of non-native species. For example, small and remote streams in the riverine network had very low species richness and diversity values. With increasing habitat size (i.e. width and depth), habitat complexity increases, providing suitable habitats for both native and non-native fishes (Gorman and Karr 1978; Schlosser 1982). This habitat effect can be one of the main reason why most diversity values increased with increasing invasion rate at native community levels in lowland habitat types (Fig. 7). Alternatively, an increase in species richness and diversity can also be related to the escape of native fish from fish ponds and reservoirs (Gozlan et al. 2010; Takács et al. 2017). Furthermore, these artificial waterbodies serve as a source for not only non-native species, but native fauna elements too, which are at the same time not characteristic for the given waterbody (e.g. carp—Cyprinus carpio (Linnaeus, 1758), pike—Esox lucius (Linnaeus, 1758) or pikeperch- Sander lucioperca (Linnaeus, 1758) in submontane and highland streams). Their occurrence may correspond well with the appearence of non-native species in some habitat types, especially in highland and lowland streams (Fig. 7). These native, but not type-specific and accidentally-escaped species present an additional problem in the conservation management of freshwaters, and can negatively impact taxonomic and functional diversity of fish communities (Economidis et al. 2000; Perdikaris et al. 2016; Milardi et al. 2020). Moreover, we also note the negative impact of intentional releases, since economically exploited native species are often introduced into various habitats. An example is the common carp which is one of the most important fish species in the world’s aquaculture (FAO 2012), and also intensively stocked in Hungary, and which was recorded in all river types (Table 2).
Overall, although other studies also found a decrease in taxonomic and functional diversity along invasion gradients, diversity responses were also reported to be largely river type- or region-specific. For example, Shuai et al. (2018) found a hump-shaped response of functional richness (Fric) and a linear response of functional divergence to increasing invasion in the Pearl River hydrosystem, China. However, they did not observe any specific relationships between species richness and the intensity of invasion. Milardi et al. (2020) found a negative relationship between functional dispersion and the degree of invasion in the Po River basin, Italy. Consequently, we conclude that while taxon-based diversity indices can serve as a good proxy for functional diversity among river types (i.e. along long environmental gradients), index- and river type-specific evaluations are required for a better understanding of the ecological effects of invasive species on the taxonomic and functional diversity of native communities. Our database on the morpho-functional traits of freshwater fishes will potentially help identify if a newly emerging alien species could damage native fishes in the future, based solely on functional characteristics. In other words, consideration of functional traits of fishes are expected to help improve risk assessment and management. Similarly, such a database would help analyze the community responses of fishes to human-induced negative effects (e.g. overexploitation, flow modification, destruction of habitats, pollution and eutrophication), not only from a traditional taxonomic point of view, but also from a functional and ecosystem functioning perspective.
Data availability
All data are available in the ms.
Code availability
The R code used in the ms is freely available.
References
Baltz DM, Moyle PB (1993) Invasion resistance to introduced species by a native assemblage of California stream fishes. Ecol Appl 3:246–255
Botta-Dukát Z (2005) Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. J Veg Sci 16:533–540. https://doi.org/10.1111/j.1654-1103.2005.tb02393.x
Brandner J, Cerwenka AF, Schliewen UK, Geist J (2013) Bigger is better: characteristics of round gobies forming an invasion front in the Danube River. PLoS ONE. https://doi.org/10.1371/journal.pone.0073036
Britton JR, Gozlan RE, Copp GH (2011) Managing non-native fish in the environment. Fish Fish 12:256–274. https://doi.org/10.1111/j.1467-2979.2010.00390.x
Capps KA, Ulseth A, Flecker AS (2015) Quantifying the top-down and bottom-up effects of a non-native grazer in freshwaters. Biol Invasions 17:1253–1266. https://doi.org/10.1007/s10530-014-0793-z
Carpenter SR, Fisher SG, Grimm NB, Kitchell JF (1992) Global change and freshwater ecosystems. Annu Rev Ecol Syst 23:119–139
Colin N, Villéger S, Wilkes M et al (2018) Functional diversity measures revealed impacts of non-native species and habitat degradation on species-poor freshwater fish assemblages. Sci Total Environ 625:861–871. https://doi.org/10.1016/j.scitotenv.2017.12.316
Copp GH, Bianco PG, Bogutskaya NG et al (2005) To be, or not to be, a non-native freshwater fish? J Appl Ichthyol 21:242–262. https://doi.org/10.1111/j.1439-0426.2005.00690.x
Cucherousset J, Olden JD (2011) Feature : introduced fish and ecology ecological impacts of non-native freshwater fishes. Fish Bethesda 36:215–230
Didham RK, Tylianakis JM, Gemmell NJ et al (2007) Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol Evol 22:489–496. https://doi.org/10.1016/j.tree.2007.07.001
Dudgeon D, Arthington AH, Gessner MO et al (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev Camb Philos Soc 81:163–182. https://doi.org/10.1017/S1464793105006950
Economidis PS, Dimitriou E, Pagoni R et al (2000) Introduced and translocated fish species in the inland waters of Greece. Fish Manag Ecol 7:239–250. https://doi.org/10.1046/j.1365-2400.2000.00197.x
Erős T (2005) Life-history diversification in the Middle Danubian fish fauna—a conservation perspective. Large Rivers 16:289–304. https://doi.org/10.1127/lr/16/2005/289
Erős T (2007) Partitioning the diversity of riverine fish: the roles of habitat types and non-native species. Freshw Biol 52:1400–1415. https://doi.org/10.1111/j.1365-2427.2007.01777.x
Erős T, Heino J, Schmera D, Rask M (2009) Characterising functional trait diversity and trait-environment relationships in fish assemblages of boreal lakes. Freshw Biol 54:1788–1803. https://doi.org/10.1111/j.1365-2427.2009.02220.x
Erős T, Comte L, Filipe AF et al (2020) Effects of nonnative species on the stability of riverine fish communities. Ecography 43:1156–1166. https://doi.org/10.1111/ecog.04985
FAO (2012) The State of world fisheries and aquaculture 2012. FAO Publisher, Rome, p 209
Gagic V, Bartomeus I, Jonsson T et al (2015) Functional identity and diversity of animals predict ecosystem functioning better than species-based indices. Proc R Soc B. https://doi.org/10.1098/rspb.2014.2620
Gorman OT, Karr JR (1978) Habitat structure and stream fish communities. Ecology 59:507–515
Gozlan RE, Britton JR, Cowx I, Copp GH (2010) Current knowledge on non-native freshwater fish introductions. J Fish Biol 76:751–786. https://doi.org/10.1111/j.1095-8649.2010.02566.x
Hammer Ø, Harper DA, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9
Jenkins M (2003) Prospects for biodiversity. Science 302:1175–1177. https://doi.org/10.1126/science.1088666
Kominoski JS, Ruhí A, Hagler MM et al (2018) Patterns and drivers of fish extirpations in rivers of the American Southwest and Southeast. Glob Chang Biol 24:1175–1185. https://doi.org/10.1111/gcb.13940
Kottelat M, Freyhof J (2007) Handbook of European Freshwater Fishes. Publications Kottelat, Cornol.
Laliberté E, Legendre P, Shipley B (2014) Package ‘FD.’ Meas Funct Divers Mult Traits Other Tools Funct Ecol. https://doi.org/10.3406/rbph.2011.8161
Leprieur F, Beauchard O, Blanchet S et al (2008) Fish invasions in the world’s river systems: when natural processes are blurred by human activities. PLoS Biol 6:0404–0410. https://doi.org/10.1371/journal.pbio.0060028
Lévêque C, Oberdorff T, Paugy D et al (2008) Global diversity of fish (Pisces) in freshwater. Hydrobiologia 595:545–567. https://doi.org/10.1007/s10750-007-9034-0
Martello F, De Bello F, De Castro Morini MS et al (2018) Homogenization and impoverishment of taxonomic and functional diversity of ants in eucalyptus plantations. Sci Rep 8:1–11. https://doi.org/10.1038/s41598-018-20823-1
Martin CW, Valentine MM, Valentine JF (2010) Competitive interactions between invasive nile tilapia and native fish: the potential for altered trophic exchange and modification of food webs. PLoS ONE 5:57–59. https://doi.org/10.1371/journal.pone.0014395
Martonné Erdős K (2004) Magyarország természeti földrajza I. Debreceni Egyetem Kossuth Egyetemi Kiadója, Debrecen
Matsuzaki SIS, Sasaki T, Akasaka M (2013) Consequences of the introduction of exotic and translocated species and future extirpations on the functional diversity of freshwater fish assemblages. Glob Ecol Biogeogr 22(9):1071–1082
Michelan TS, Thomaz SM, Mormul RP, Carvalho P (2010) Effects of an exotic invasive macrophyte (tropical signalgrass) on native plant community composition, species richness and functional diversity. Freshw Biol 55:1315–1326. https://doi.org/10.1111/j.1365-2427.2009.02355.x
Milardi M, Gavioli A, Soininen J, Castaldelli G (2019) Exotic species invasions undermine regional functional diversity of freshwater fish. Sci Rep 9:17921. https://doi.org/10.1038/s41598-019-54210-1
Milardi M, Gavioli A, Soana E et al (2020) The role of species introduction in modifying the functional diversity of native communities. Sci Total Environ 699:134364. https://doi.org/10.1016/j.scitotenv.2019.134364
Olden JD, Poff NLR, Douglas MR et al (2004) Ecological and evolutionary consequences of biotic homogenization. Trends Ecol Evol 19:18–24. https://doi.org/10.1016/j.tree.2003.09.010
Perdikaris C, Koutsikos N, Vardakas L et al (2016) Risk screening of non-native, translocated and traded aquarium freshwater fishes in Greece using Fish invasiveness screening kit. Fish Manag Ecol 23:32–43. https://doi.org/10.1111/fme.12149
Petchey OL, Gaston KJ (2002) Functional diversity (FD), species richness and community composition. Ecol Lett 5:402–411. https://doi.org/10.1046/j.1461-0248.2002.00339.x
R Core Team (2015) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org. Access date: 2020.03.01
Rasband WS (2012) ImageJ. US National Institutes of Health, Bethesda, MD, U.S.A.
Sallai, Varga I & Erős T (2019) Halközösségek monitorozása Magyarország különböző típusú állóvizeiben és vízfolyásokban. In: Váczi O, Varga I & Bakó B (eds.), A Nemzeti Biodiverzitás-monitorozó Rendszer eredményei II. Körös-Maros Nemzeti Park Igazgatóság, Szarvas
Sály P (2007) The system of faunacomponents conception and its application to qualify the degree of naturalness of fish assemblages. Pisces Hungarici 1:93–101
Schlosser IJ (1982) Fish community structure and function along two habitat gradients in a headwater stream. Ecol Monogrpahs 52:395–414
Shuai F, Lek S, Li X, Zhao T (2018) Biological invasions undermine the functional diversity of fish community in a large subtropical river. Biol Invasions 20:2981–2996. https://doi.org/10.1007/s10530-018-1751-y
Stewart DR, Walters AW, Rahel FJ (2016) Landscape-scale determinants of native and non-native Great Plains fish distributions. Divers Distrib 22:225–238. https://doi.org/10.1111/ddi.12383
Strayer DL (2012) Eight questions about invasions and ecosystem functioning. Ecol Lett 15:1199–1210. https://doi.org/10.1111/j.1461-0248.2012.01817.x
Su G, Logez M, Xu J, Tao S, Villéger S, Brosse S (2021) Human impacts on global freshwater fish biodiversity. Science 371(6531):835–838
Takács P (2018) Notes on the taxonomic position and naming problems of the Hungarian stream dwelling gudgeons (Gobio). Pisces Hungarici 12:63-66.
Takács P, Vitál Z, Ferincz Á, Staszny Á (2016) Repeatability, reproducibility, separative power and subjectivity of different fish morphometric analysis methods. PLoS ONE 11:1–16. https://doi.org/10.1371/journal.pone.0157890
Takács P, Czeglédi I, Ferincz Á et al (2017) Non-native fish species in Hungarian waters: historical overview, potential sources and recent trends in their distribution. Hydrobiologia 795:1–22. https://doi.org/10.1007/s10750-017-3147-x
Tickner D, Opperman JJ, Abell R et al (2020) Bending the curve of global freshwater biodiversity loss: an emergency recovery plan. Bioscience 70:330–342. https://doi.org/10.1093/biosci/biaa002
Toussaint A, Charpin N, Brosse S, Villéger S (2016) Global functional diversity of freshwater fish is concentrated in the Neotropics while functional vulnerability is widespread. Sci Rep 6:1–9. https://doi.org/10.1038/srep22125
Toussaint A, Charpin N, Beauchard O et al (2018) Non-native species led to marked shifts in functional diversity of the world freshwater fish faunas. Ecol Lett 21:1649–1659. https://doi.org/10.1111/ele.13141
Vigliano PH, Beauchamp DA, Milano D et al (2009) Quantifying predation on galaxiids and other native organisms by introduced rainbow trout in an ultraoligotrophic lake in Northern Patagonia, Argentina: a bioenergetics modeling approach. Trans Am Fish Soc 138:1405–1419. https://doi.org/10.1577/t08-067.1
Vila-Gispert A, Alcaraz C, García-Berthou E (2005) Life-history traits of invasive fish in small Mediterranean streams. Biol Invasions 7:107–116. https://doi.org/10.1007/s10530-004-9640-y
Villéger S, Mason NW, Mouillot D (2008) New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89:2290–2301
Villéger S, Miranda JR, Hernández DF, Mouillot D (2010) Contrasting changes in taxonomic vs. functional diversity of tropical fish communities Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol appl 20(6): 1512-1522
Violle C, Navas M, Vile D et al (2007) Let the concept of trait be functional! Oikos 116:882–892
Vitál Z, Józsa V, Specziár A et al (2017) Source of bigheaded carp (Hypophthalmichthys spp.) in Lake Balaton, Hungary: natural recruitment or continuous escapement from aquaculture? Inl Waters 7:218–226. https://doi.org/10.1080/20442041.2017.1319546
Vitousek PM, D’antonio CM, Loope LL et al (1997) Introduced species: a significant component of human-caused global change. N Z J Ecol 21:1–16
Whittaker RJ, Rigal F, Borges PAV et al (2014) Functional biogeography of oceanic islands and the scaling of functional diversity in the Azores. Proc Natl Acad Sci 111:13709–13714. https://doi.org/10.1073/pnas.1218036111
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc 73(1):3–36
Acknowledgements
We would like to express our thanks to Árpád Ferincz, Ágnes Hoitsyné Rieger, Ádám Staszny, Zoltán Szalóky, Judit Vörös, András Weiperth for their help in the sample collections. We are grateful to Andrew Hamer who corrected the grammar of the manuscript.
Funding
Open access funding provided by ELKH Centre for Ecological Research. The faunistic database was built up using the data recorded within the frame of the following projects: OTKA K104279, PD115801, and a KEHOP2015 project of the General Directorate of Water Management. This project was funded by the NKFIH-872 project (Establishment of a National Multidisciplinary Laboratory for Climate Change). Data analyses were supported by the GINOP 2.3.2-15-2016-00004 projects. Péter Takács was supported by the NKFIH OTKA FK131426 grant and Bolyai Fellowship of the Hungarian Academy of Sciences. András Abonyi was supported by the National Research, Development and Innovation Office, Hungary (NKFIH, PD124681).
Author information
Authors and Affiliations
Contributions
PT: sample and data collection, measurements, data visualisation, statistics, ms preparation, BB: sample collection, measurements; AA: statistics, ms preparation; TE: sample and data collection, ms preparation.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no comflicts of intrest.
Ethical approval
This study was carried out following relevant national and international guidelines pertaining to the care and welfare of fish. The manuscript adheres to publisher's Ethical Guidelines.
Consent to participate
The paper is upgraded and approved by all authors.
Consent for publication
The paper is approved by all authors and by the responsible authorities of our Institute.
Additional information
Communicated by Paul Humphries.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takács, P., Abonyi, A., Bánó, B. et al. Effect of non-native species on taxonomic and functional diversity of fish communities in different river types. Biodivers Conserv 30, 2511–2528 (2021). https://doi.org/10.1007/s10531-021-02207-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-021-02207-6