Abstract
Habitat fragmentation and forest management have been considered to drastically alter the nature of forest ecosystems globally. However, much uncertainty remains regarding the causative mechanisms mediating temperate forest responses, such as forest physical environment and the structure of woody plant assemblages, regardless of the role these forests play for global sustainability. In this paper, we examine how both habitat fragmentation and timber exploitation via silvicultural operations affect these two factors at local and habitat spatial scales in a hyper-fragmented landscape of mixed beech forests spanning more than 1500 km2 in SW Germany. Variables were recorded across 57 1000 m2 plots covering four habitats: small forest fragments, forest edges within large control forests, as well as managed and unmanaged forest interior sites. As expected, forest habitats differed in disturbance level, physical conditions and community structure at plot and habitat scale. Briefly, diversity of plant assemblages differed across all forest habitats (highest in edge forests) and correlated with integrative indices of edge, fragmentation and management effects. Surprisingly, managed and unmanaged forests did not differ in terms of species richness at local spatial scale, but managed forests exhibited a clear signal of physical/floristic homogenization as species promoted by silviculture proliferated; i.e. impoverished communities at landscape scale. Moreover, functional composition of plant communities responded to the microclimatic regime within forest fragments, resulting in a higher prevalence of species adapted to these microclimatic conditions. Our results underscore the notion that forest fragmentation and silvicultural management (1) promote changes in microclimatic regimes, (2) alter the balance between light-demanding and shade-adapted species, (3) support diverse floras across forest edges, and (4) alter patterns of beta diversity. Hence, in human-modified landscapes edge-affected habitats can be recognized as biodiversity reservoirs in contrast to impoverished managed interior forests. Furthermore, our results ratify the role of unmanaged forests as a source of environmental variability, species turnover, and distinct woody plant communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forest ecosystems have gained unprecedented relevance in the last decades in the context of global sustainability (Lindenmayer et al. 2012; Coomes et al. 2014; Kettle et al. 2014; Haddad et al. 2015). Although much of the attention has been devoted to tropical forests (Taubert et al. 2018), temperate forests play an unquestionable role relative to biodiversity persistence, provision of ecosystem services and social/economic development as they cover nearly 5.2 Mkm2, representing 16% of total remaining forest cover globally (Brunet et al. 2010; Hansen et al. 2010; Paillet et al. 2010). In many regions, temperate forests represent the home for hundreds of native species (from fungi to mammals) and provide key services such as watershed protection, carbon storage and provision of recreational areas (Dixon et al. 1994; Paillet et al. 2010; Millar and Stephenson 2015). For instance, European beech-dominated forests cover nearly 14–15 Mha, support over 300 obligatory forest plant species and serve as huge carbon sink (up to 34 Pg C), especially via soil organic matter, just to mention a few figures (Dixon et al. 1994; Brunet et al. 2010; Schulze et al 2016).
With the exception of remote areas, temperate forests continue to be exposed to human disturbances; i.e. old-growth forest conversion into human-modified landscapes (sensu Tabarelli et al. 2010), with multiple impacts on biological organization from population to ecosystem level (Hansen et al. 2010; Chaudhary et al. 2016). We refer to habitat fragmentation and timber exploitation via forest management as the main drivers of these impacts (Jacquemyn et al. 2003; Schulze et al. 2016). Recently, climate changes have been recognized as an additional source of threats, particularly severe droughts and diseases favored by increasing temperatures (Millar and Stephenson 2015). Isolated or collectively, these pervasive disturbances may depress forest resilience and consequently the ability of human-modified or cultural landscapes to operate as biodiversity repositories and source of key ecosystem services of local and global relevance such as the mitigation of climate changes (Millar and Stephenson 2015; Naudts et al. 2016).
In the case of plants, edge effects resulting from habitat fragmentation have been recognized to alter the nature of both herb and woody plant assemblages, from species richness to functional composition (Lôbo et al. 2011; Pellissier et al. 2013; Magnago 2014). Although some taxa from temperate floras can be considered sensitive to edge effects and fragmentation (Vellend et al. 2006; Pellissier et al. 2013), at community level the establishment of edge-affected habitats (i.e. small forest fragments and forest edges) can be beneficial for biodiversity persistence at landscape scale. Precisely, there is strong evidence that habitats shaped by forest fragmentation, particularly anthropogenic forest edges, support highly diverse plant communities by offering more suitable microclimatic conditions (increased radiation and temperature) as opposed to the moist and shady conditions provided by forest interiors (Ziter et al. 2014). In fact, temperate floras contain a high proportion of light-demanding woody species as plant growth is more limited by energetic constraints (Honnay et al. 2002; Ziter et al. 2014; Smith et al. 2018). In this ecological context, open habitats offer more favorable climatic conditions (Whittaker et al. 2007; Smith et al. 2018). Moreover, light-demanding strategies have been considered to be more tolerant to disturbances and environmental stress; i.e. disturbance-adapted plant species (Bazzaz et al. 2000). Accordingly, human-modified landscapes with high cover of edge-affected forest habitats are expected to benefit several taxa and consequently reorganize plant assemblages at multiple spatial scales, including the occurrence of species-rich assemblages (Hermy et al. 1999; Flückiger et al. 2002; Honnay et al. 2002). To some extent, this potential response to habitat fragmentation might counterbalance those posed by forest management, as these disturbances tend to occur simultaneously across many regions (Ziter et al. 2014).
In fact, silvicultural management is an ancient, pervasive and a typical disturbance imposed on temperate forests, particularly across Central European countries. In this region, forest conversion and exploitation of forest products reached a maximum in the Middle Ages, with only 0.2% of current remaining forest cover to be considered as undisturbed or old-growth forest (Hannah et al. 1995; Williams 2000; Wirth et al. 2009a). This figure is the reality of temperate forests in Central Europe and highlights how important it is to address the impact of human disturbances, as we intend to keep or even improve their ability to provide services, including biodiversity persistence (already threatened by climate change) and forest goods. It is worth highlighting, that in 2011 forestry provided a 485 billion € turnover in Europe (European Commission 2019).
Impacts posed by silviculture on Central European forests have long been addressed and it is worth to mention changes in the natural disturbance regime (i.e. treefall gap dynamics), forest microclimate, particularly moisture and light availability (Decocq et al. 2005; Paillet et al. 2010; Boch et al. 2013; Duguid and Ashton 2013), with consequences on species distribution and abundance, community organization, ecological functions and ecosystem services (Emmer et al. 1998; Hahn and Fanta 2001). Precisely, managed forests have been documented to support impoverished plant assemblages and promote community-level homogenization at local and landscape spatial scales due to (1) even-aged cultivation of selected tree species, and (2) by favoring a small set of shade-adapted tree species (Hahn and Fanta 2001; MUF 2002; Decocq et al. 2005). Conversely, unmanaged forests can progressively move to the old-growth forest stage as exposed to a natural disturbance regime that usually promotes woody plant diversity and trait variability due to high habitat heterogeneity; i.e. community-level taxonomic, phylogenetic and functional diversity (Bauhus et al. 2009; Brunet et al. 2010).
Despite of such a broad perspective, to what extent forest management alters forest physical habitats and reorganizes plant assemblages relative to the taxonomic, phylogenetic and functional dimension remains incomplete and partly controversial (e.g. Paillet et al. 2010; Boch et al. 2013; Schulze et al. 2016; Braunisch et al. 2019). Accurate knowledge is crucial to evaluate the role played by managed forests as biodiversity repositories, source of ecosystem services, as well as forest resilience for ongoing climate changes (Millar and Stephenson 2015; Naudts et al. 2016). Information deficit is aggravated by aspects such as (1) difficulties in classifying forests into categories due to the variety of historic and present management policies (Hahn and Fanta 2001), (2) potential interactions with fragmentation effects (Avon et al. 2013), and most importantly, (3) the severe lack of unmanaged, pristine control or old-growth forests, as even large-scale research projects fail to detect certain basic old-growth features in unmanaged forests (Bauhus et al. 2009; Blaser et al. 2013).
In this paper, which is based on the dissertation of the first author (Bähner 2016), we examine how both forest fragmentation and management via silvicultural operations affect forest physical environment and the structure of woody plant assemblages at local and habitat spatial scales, with potentially important implications for biodiversity persistence across human-modified landscapes in Europe. We expected tangible changes in the physical environment, particularly light availability, and correlated changes across a comprehensive set of community-level attributes (such as species richness and diversity, as well as taxonomic and functional composition) of assemblages inhabiting a hyper-fragmented forest landscape in SW Germany. First, we describe and compare disturbance- and microclimate-related variables across four forest habitats (forest fragments, edges, as well as managed and unmanaged continuous forests). Second, woody plant assemblages are described and their attributes correlated with potential explanatory variables via integrative quantitative indices reflecting fragmentation, edge and management effects as well as microclimatic requirements. Finally, we update the present knowledge about forest responses to edge effects and silviculture and highlight potential implications for forest management and biodiversity persistence in temperate, human-modified landscapes.
Methods
Study landscape, forest habitat types, and study plots
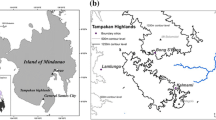
The study landscape is located in the Northern Palatinate highlands (49° 36′ N and 7° 44′ E), a low, undulating mountain range (250–687 m asl) of Permian origin covering an area of 1556.4 km2 in Southwest Germany (Fig. 1). The region is characterized by a temperate, sub-oceanic climate (mean annual precipitation: 800 mm; mean annual temperature: 9.4 °C, 1970–2010, Deutscher Wetterdienst 2013). We studied deciduous, broad-leaved forests, whose cores can be phytosociologically classified as Carpino-Fagetalia mixed forests with varying transitional degrees of Fagion and Carpinion betuli stands. While we lack details about ancient management regimes, extensive deforestation occurred in the Middle Ages, mainly in sand and siltstone-dominated valleys, while the agriculturally less valuable igneous hilltops were mostly forested. This has led to a landscape of hyper-fragmented forests, embedded in a matrix of cultivated fields, pastures and meadow orchards. Forest cover of this landscape (34%) is representative for Germany (31%, MUF 2002). Despite its high fragmentation degree with over 2,900 forest fragments ranging from 0.1 to 5616 ha (ca. 85% of them < 10 ha) and a total edge length of over 5700 km, the region still harbors large forest tracts exceeding 1000 ha (Bähner et al. 2017).
Geographical localization of study sites and landscape, with respect to Europe (A) and Rhineland-Palatinate, SW Germany (B). The Northern Palatinate highlands (C) encompass > 2900 forest fragments (grey polygons). Situated within these remnants are the individual 57 study plots (black circles). Letters indicate study plots of the respective habitats (E forest edge, F forest fragment, M managed forest interior, U unmanaged forest interior). Larger municipalities are divided into larger and smaller than 10,000 inhabitants (larger/smaller black boxes)
Study sites (20 × 50 m; 0.1 ha) were chosen across four habitat types differently affected by forest fragmentation and management: (1) Small forest fragments (n = 10): ranging between 1.6 ha and 176.2 ha (34 ± 52 ha, mean ± sd) and entirely surrounded by open matrix. Plots were situated in the fragment center. (2) Forest edges (n = 19): peripheral areas within 50 m of the physical border of large forest tracts (continuous control forests, the three largest forest tracts in the study region, 1,155 ha, 3,537 ha and 5,289 ha). (3) Managed forest interior (n = 12): core areas of control forests beyond 100 m of the border, as this is the pertinent distance beyond which many edge effects approach minimal intensity (Laurance et al. 2002). The plots represented current silvicultural management practices dominated by age-class forests, with varying management regimes, intensities, policies, and small-scale choices by foresters. Only mature stands were chosen, with trees > 30 cm diameter dominating the canopy. (4) Unmanaged forest interior (n = 16): core areas of large forest tracts beyond 100 m of the border and without detectable edge influence. Mean distance to the closest edge was 439 m. These sites included natural forest reserves (state property, unmanaged since 1972), as well as privately owned forests. The latter were preselected on the criteria of seclusion, (small) property size and visual inspection (e.g. regarding amounts of coarse woody debris) to minimize the probability of forest use. Low levels (mean wood extraction < 4 m3 ha−1 a−1) or absence of management, as well as forest history in privately owned forests were verified via historic maps and personal interviews with forest owners. Time since last wood extraction varied (29 ± 25 a) and was not available for two out of six sites.
Fragment, edge and managed plots were chosen at random across the study region. This design reflects the landscape configuration available to us and follows similar studies (Girão et al. 2007; Valladares et al. 2012). Inter-plot distance ranged from 0.1 km to 70.1 km with 18.8 ± 14.0 km and plots ranged in altitude from 277 to 640 m with 406 ± 89 m. In 2013, within each plot all woody plant individuals > 1.3 m height and with diameter at breast height > 1 cm were identified to species level, totaling 4139 plant individuals from 34 species and 15 families.
Indices for disturbance and microclimate
Following a recent trend in fragmentation research, we opted to use comprehensive indices to quantify anthropogenic disturbance and microclimatic regimes, in synergy with categorical variables (Fardila et al. 2017). Indices for anthropogenic disturbances (fragmentation, edge, and management index) as well as for community-wide microclimatic requirements in forest stands (henceforth microclimatic index, MCI) were adopted following Martorell and Peters (2005) and as used e.g. by Ribeiro et al. (2016). This was done by first normalizing all explanatory variables related to a given index and then performing a principal component analysis (PCA) with them. The plot scores on the first PCA axis are then rescaled to range from 0 (low disturbance/cold, moist, dark microclimates) to 100 (high disturbance/warm, dry, light microclimates), hence generating the index in question. Further information on how the different variables were measured can be found in the supplementary material (Online Resource, Table A1).
Metrics used to create the fragmentation index (FI) span those related to fragment size and shape (fragment area (ha), core area (ha), perimeter/area ratio (m−1) and shape index) and isolation (proximity index, compare Lang and Tiede (2003) for a further description of indices). Core area was defined as the remaining forest area assuming a pertinent edge buffer of 100 m (Broadbent et al. 2008). The shape index calculates the deviation of a forest fragment shape from a perfect circle. The proximity index is a measure for patch isolation. Axis 1 of the PCA (FI before rescaling) explained 66% of the variation of these variables and was significantly correlated with all of them (mean R2 = 0.86 and mean p < 0.001).
The edge index (EI) incorporates the euclidian distance of a plot to the nearest forest edge (m), as well as the relative forest cover in a 100 m buffer around it (%). Axis 1 of the PCA explained 79% of the variation of these variables and was significantly correlated with all of them (mean R2 = 0.87 and mean p < 0.001).
While we had no information on historic (i.e. medieval) management regimes in our plots, we created the management index (MI) to quantify contemporary management intensity. The MI consisted of variables representing past logging events (number of stumps per 0.1 ha), forest ownership/classification (either public or private ownership, or natural forest reserve), amounts of coarse woody debris (m3 ha−1), including lying coarse woody debris, standing coarse woody debris and stump volume (m3 ha−1) as well as unextracted living tree volume (basal area of trees; m2 0.1 ha−1). Axis 1 of the PCA explained 48% of variation of these variables and was significantly correlated with all of them (mean R2 = 0.52 and mean p < 0.001).
The microclimatic index (MCI) was based on measures of thermophily, photophily, and xerophily, reflecting niche requirements of woody plants that commonly depend on site-specific microclimates and therefore are highly autocorrelative. In order to quantify microclimatic requirements of the studied woody plant communities, we integrated these three aspects into a synthetic index, using Ellenberg’s indicator values (EIVs) for light (ordinal scale ranging from 1 to 9, i.e., shade to high light conditions), temperature (from 1 to 9, i.e., cold to hot), and moisture (1 to 12, i.e., dry to submersed conditions). Mean indicator values (MEIV) were calculated for each plot similarly to Meyer et al. (2013) by first multiplying species dominance in a given plot with the corresponding EIV available in the literature (Ellenberg and Leuschner 1996). Then the sum over these products gives the MEIV (i.e. for all species in one plot). Any given MEIV thus represents the overall woody plant community in a given plot in terms of thermophily, photophily, and xerophily, respectively. Woody plant species with EIVs given as ‘indifferent’ were left out of the calculation (6 for Ellenberg temperature and 11 for Ellenberg moisture) and only contributed 13.7% and 13.8% to the data set in terms of abundance. Axis 1 of the subsequent PCA explained 55% of variation of the three MEIVs and was significantly correlated with all of them (mean R2 = 0.55 and mean p < 0.01).
Statistical analysis
All analyses were performed using R version 3.0.2 (R Core Team 2013). To evaluate the performance of aforementioned indices (fragmentation, edge, and management index, as well as MCI), we made habitat-wise comparisons, employing one-way ANOVAs (with Tukey’s test as post hoc tests) where appropriate, and Kruskal–Wallis tests (with Nemenyi test as post hoc test) where ANOVA-assumptions could not be met with transformations.
Likewise, habitat-wise differences in species richness and Shannon diversity were assessed using Kruskal–Wallis test and one-way ANOVA, respectively.
As the effects of forest fragmentation and management are likely to interact in complex ways, we opted to supplement our categorical analyses with a correlative approach. Hence, fragmentation, edge and management effects on woody plant diversity (Shannon index) and on MCI were assessed using multiple linear regressions with fragmentation, edge, and management indices as explanatory variables and Shannon index and MCI as response variables. Partitioning (relative importance) of global R2 was assessed with the relaimpo package following Grömping (2006) using the lmg metric. We report the mean and 95% CI of each partial R2 for each variable based on 1000 bootstraps (function boot.relimp, Grömping 2006). Sufficiency of sampling intensity was assessed by comparing recorded species richness with expected species richness, estimated from calculation of saturation levels of species area curves, following Moreno and Halffter (2000).
Community segregation across forest habitats was examined using similarity values in a non-metric multidimensional scaling (NMDS, Bray–Curtis dissimilarity of square-root transformed abundance data, function metaMDS, (vegan package, Oksanen et al. 2015)) and performing an ADONIS permutation test (9999 permutations) with forest habitat as a grouping variable. As a post hoc test we performed pairwise ADONIS procedures and adjusted p-values for multiple testing (Bonferroni-correction). In order to access possible bias of spatial plot location on community composition, we conducted a Mantel test with Spearman rank correlation and log transformed physical distances. To illustrate how community composition is structured by microclimate-related functional traits, we superimposed aforementioned NMDS with a heat map, in which the MCI’s microclimatic signals correspond to a color code (ranging from blue (dark/cold/moist) via green–yellow–orange to red (light/warm/dry) conditions. This signal was calculated for each plot and then interpolated across the ordination space. Hence, a visible color gradient in the ordination suggests that microclimatic conditions covary and may therefore be interpreted as shaping community composition. Microclimate/color interpolations between sites were calculated with interp function in the akima package (Akima and Gebhardt 2015). In order to further validate the role of microclimatic regimes, we fitted the microclimatic index, as well as its components (MEIVs for light, temperature and moisture) as environmental variables onto community composition in the NMDS and further compared the relationship between MEIVs and community composition with corresponding null models analogously to Zelený and Schaffers (2012).
To compare multivariate heterogeneity of the assemblage compositions across forest habitats, we calculated Bray–Curtis dissimilarity between plot-pairs and compared it across forest habitats (Kruskal–Wallis tests with Nemenyi test as post hoc test).
Finally and complementarily, identification of indicator species for certain habitats was performed using a Dufrêne-Legendre indicator species analysis (Dufrêne and Legendre 1997) in the labdsv package (Roberts 2015). Indicator values (IV) range from 0 (no habitat association) to 1 (perfect habitat association).
Results
As expected, the investigated forest habitats experienced different and characteristic levels of anthropogenic disturbance and microclimatic regimes, respectively (Fig. 2). Most importantly, both managed and unmanaged forest habitats exhibited major differences relative to forest edges and fragments, particularly in terms of edge effects and microclimate (Fig. 2B, D). Plots in unmanaged forests indeed displayed substantially lower management intensity, on average more than threefold, compared to any other habitat. Furthermore, dead wood density was 88% higher in unmanaged stands (72.1 m3/ha), compared with managed ones (38.3 m3/ha; t-test, t = 2.6734, df = 25.947, p < 0.05). Unmanaged interior forests showed a higher fragmentation degree than e.g. edge habitats (Fig. 2A), as plots in unmanaged sites included privately owned forests, which on average were situated in slightly smaller forests than the other interior sites.
Habitat-wise comparison of all anthropogenic disturbance indices, as well as the microclimatic index (MCI), presented by box and whisker plots. Edge and management index and MCI: one-way ANOVA with Tukey’s test as post hoc test. Fragmentation index: Kruskal–Wallis test with Nemenyi test as post hoc tests. Whiskers incorporate maximum values which do not exceed 1.5 times interquartile range. *p < 0.05, **p < 0.01, ***p < 0.001
Across these four habitats a total of 4139 woody plant individuals from 34 species and 15 families were recorded. All species were typical for forests or forest edges and none were characteristically associated with agricultural or other habitats. One non-native species (Robinia pseudoacacia) occurred, but with minor abundance (19 individuals in one plot). At plot level, forest edges supported more enriched and diverse assemblages (Fig. 3). Precisely, forest edge floras were twofold more speciose as compared to both unmanaged and managed forests. Accordingly, woody plant diversity was positively related to forest edge effects (Fig. 3B, Table 1). However, there was no major influence either by fragmentation effects or by silvicultural management.
Effects of anthropogenic disturbance (forest fragmentation and silvicultural management) on species richness and diversity of woody plant species in the Northern Palatinate highlands. A Species richness (Kruskal–Wallis test with Nemenyi test as post hoc test, Χ2(53,3) = 18.855, p < 0.001, untransformed data is shown). B Shannon diversity (ANOVA with Tukey’s test as post hoc test, F(53,3) = 3.602, p < 0.05). More specific anthropogenic influences are represented by: small forest fragments, edges of large forests, unmanaged interior of large forests, and managed interior of large forests. Whiskers incorporate maximum values which do not exceed 1.5 times interquartile range. *p < 0.05, **p < 0.01, ***p < 0.001
Moving to habitat scale, there was further evidence for enriched edge floras, as species richness in forest edges was 103% and 77% higher than in managed and unmanaged interior forests and 77% higher than in small forest fragments, when rarefied to a same sample size of n = 10 plots (i.e., 1 ha, Fig. 4). These effects were further reflected by variable ratios between observed and expected species richness: The sampled flora of small forest fragments, edges, and of managed and unmanaged interior forests was represented by, respectively, 65%, 73%, 79%, and 59% of the expected species pool, also indicating that the sampling effort did not fully capture the woody plant flora across the forest habitats.
Effects of anthropogenic disturbance (forest fragmentation and silvicultural management) on species-area relationships of small forest fragments (diamonds, blue), forest edges of large control forests (squares, red), and managed (triangles, yellow) and unmanaged (circles, green) interior forests in the Northern Palatinate highlands. Rarefied species richness is plotted against cumulative plot area (0.1 ha). White symbols and black line: mean of 100 iterations. Polygons: 95% confidence interval. The endpoints represent actual species richness in the respective habitat
While there was no large-scale spatial effect on taxonomic similarity across the 57 plots (Mantel test, r = − 0.01, p = 0.55), several forest habitats differed in terms of plot-level taxonomic composition (Table 2). Woody plant community segregation was particularly driven by compositional differences between fragmented vs. managed and managed vs. unmanaged forests. Furthermore, there was striking evidence for biotic homogenization, as managed communities showed higher levels of community similarity (Fig. 5). This pattern was further confirmed by 46% higher community dissimilarity of unmanaged woody plant communities over managed ones (Kruskal Wallist test, Fig. 5). The highest levels of heterogeneity were found in forest edge communities (66% larger than managed interior forests), indicating large compositional variability. While statistically non-significant, cross-habitat differences in the species pool seemed more related to occurrence of rare species (< 5% dominance; e.g. high occurrence in forest edges) than to changes in the relative contribution of dominant species (Online Resource, Fig. A1).
Effects of anthropogenic disturbance (forest fragmentation and silvicultural management) on beta diversity of woody plant communities in the Northern Palatinate highlands. Beta diversity of the woody plant communities was measured as Bray–Curtis dissimilarity of plot pairs; Kruskal–Wallis test with Nemenyi test as post hoc test, Χ2(3) = 53.646, p < 0.001
Prevailing microclimates shape community structure of the woody flora in the Northern Palatinate highlands. NMDS ordination (square-root transformed Bray–Curtis dissimilarities, stress = 0.18) was superimposed with the microclimatic index (MCI) by interpolating the color-coded MCI between site-specific communities (letters). Red hues indicate thermo-, photo-, and xerophilic communities, blue hues indicate cold- and shade tolerant, as well as hygrophilic communities. Overlaid vectors represent the correlation of environmental factors (MCI and its components) with the ordination. Vector arrows indicate the direction of environmental factors, and the length of each vector is proportional to R2. *p < 0.05, **p < 0.01, ***p < 0.001. F small forest fragments, E forest edges, M managed forest interior, U unmanaged forest interior
Regarding woody plant functional signatures, prevailing microclimates correlated with neither species richness nor abundance (Spearman-rank correlations, all p > 0.05). However, plot-level taxonomic composition was found to strongly covary with the prevailing microclimatic conditions, as evidenced by: (1) a correlation between MEIV for temperature and community composition (confirmed by null model comparison), (2) the clearly visible color gradient of the heat map along the ordination space (Fig. 6), and (3) a pronounced correlation between the microclimatic index and community composition since this index acted as a dominant explanatory variable for community distribution from one extreme point in the NMDS ordination to the other (Fig. 6). In other words, plots that were very different in terms of taxonomic composition of woody plants were also very likely to differ in their microclimatic requirements of their communities. Furthermore, a plot’s response in respect to microclimatic conditions (MCI) significantly increased with increasing intensity of fragmentation and edge effects, while forest management showed no influence (multiple linear regression, global R2 = 0.37, Table 3). In synthesis, in our plots managed interior forests were coined by shade-adapted woody plant communities, while unmanaged, and even more so edge floras, exhibited the widest variability in microclimatic requirements. Woody floras in small fragments displayed the overall strongest response to microclimatic conditions in our study (Fig. 2). These findings are further highlighted by identification of habitat indicator species. Sambucus nigra, a shade-intolerant shrub, was found to be an indicator species for small forest fragments (IV = 0.30, p < 0.01). Likewise, all three edge indicator species are well known termophilous and/or photophilous trees and shrubs of open habitats (Prunus avium, IV = 0.30, p < 0.05; Prunus spinosa, IV = 0.26, p < 0.01; Sorbus aucuparia, IV = 0.21, p < 0.05). Correspondingly, using character species, we were able to identify phyto–sociological associations typically linked to forest edges, e.g. Sambucetum racemosae, Crataego-Prunetum spinosae and Rubo fruticosi-Coryletum avellanae (Schubert et al. 1995). Finally, indicator species of managed interior forests were trees with either pronounced shade tolerance (e.g. Fagus sylvatica, IV = 0.37, p < 0.01) or history of deliberate plantation in this study region (Larix decidua, IV = 0.27, p < 0.05).
Discussion
Our results suggest that main habitats of contemporary European beech forest are completely distinct in terms of human disturbance and microclimatic regime, with forest fragments and forest edges as the most distinct ones as compared to forest interiors (both managed and unmanaged). In this perspective it is worth to mention the presence of more illuminated and heterogeneous microhabitats across forest edges and fragments. Forest habitats support a relatively diverse flora with cross-habitat differences through an extensive set of plant assemblage attributes, including the occurrence of plant groups considered indicators of particular microclimatic conditions. In these human-modified forest landscapes, forest edges appear to support the most diverse assemblages at local and landscape spatial scales due to high levels of species turnover and the co-occurrence of several plant groups. On the other hand, managed forests are floristically less diverse and more homogeneous at landscape spatial scale. By favoring light-demanding and thermophilic plant species, habitat fragmentation and the establishment of edge-affected habitats appears to be more pervasive than silvicultural management in terms of species distribution and species assembly, although all these forest habitats can be considered complementary in terms of physical conditions and species occurrence.
Our findings reinforce the notion that human disturbances represent a key driver operating at multiple levels of ecological organization, from population to ecosystem level and across human-modified forest landscapes (Foley et al. 2005; Fardila et al. 2017). Precisely, we add additional support for the general idea that habitat fragmentation, including the establishment of forest edges, as well as forest management are able to reorganize plant assemblages taxonomically and functionally. Precisely, we observed the emergence of species-rich assemblages across more illuminated and/or climatically diverse habitats such as forest edges, fragments and unmanaged forest stands. Such “positive” effects posed by the establishment of forest edges has long been recognized in temperate forests (Coch 1995; Ziter et al. 2014), while few have documented increased microhabitat heterogeneity and diverse plant assemblages associated to unmanaged forest interiors at landscape scale.
Although we have not explicitly examined the underlying mechanisms reorganizing plant assemblages in our focal landscape, here we shall address a basic mechanism: microclimatic changes due to the creation of forest edges and silvicultural management. Forest edges and small forest fragments have been long recognized to represent more illuminated, warm and desiccated habitats in temperate forests (Ziter et al. 2014; Smith et al. 2018), although our findings suggest that, additionally, they are more heterogeneous, especially compared to managed interior forests. These habitats apparently favor a relatively diverse flora consisting of more heat/light/drought-adapted plant species (e.g. Prunus spinosa), but also allow for the presence of shade-adapted ones (e.g. Fagus sylvatica), probably in the core zones of fragments or forest edges less exposed to sunlight. As small forest fragments do not retain extensive core areas, they are not as floristically diverse at multiple spatial scales as forest edges. On the other hand, the forest interior represents the irreplaceable microhabitat for shade-tolerant trees such as Fagus sylvatica. In this perspective, unmanaged forests, while equally rich in species as their managed counterparts at local scale, support higher beta diversity and a tendency toward increased microclimatic index. This can be interpreted as first signs of old-growth formation, as natural treefall gaps greatly differ in size, resulting in higher variation of light dynamics (Bauhus et al. 2009). Consequently, unmanaged forest interiors are able to assemble a higher number of species from different ecological groups at landscape level.
Concluding, human disturbances, including forest management, alter the natural balance between illuminated and shaded forest habitats at landscape level, with cascading effects on species distribution and plant assemblage structure. This phenomenon relies on the fact that in temperate climates plant biodiversity is ultimately constrained by energy availability, as plants need to cope with seasonality and energy intake maximization (Hawkings et al. 2003; Whittaker et al. 2007; Hawkins et al. 2014; Smith et al. 2018). Hence, closed, energy-limited temperate forest interiors sustain few shade-adapted plant species, whereas open habitats maintain higher biodiversity, thereby explaining the reversed edge/interior diversity gradient observed at tropical latitudes (Tabarelli et al. 2008; Bartish et al. 2010; Smith et al. 2018). Moreover, typical edge assemblages are complemented by commercially used trees, which usually appear within the first ten meters from the forest margins, due to cultivation by foresters (Coch 1995; Bartsch and Röhrig 2016). Finally, silvicultural best practices shape forest edge communities. More precisely, forestry institutions aim at promoting taxonomic richness and structural complexity along forest edges (Coch 1995; Bartsch and Röhrig 2016). This may explain the occurrence of many rare woody plant species across the forest edges in our focal landscape, including species considered vulnerable concerning their genetic resources (Acer campestre, Sorbus torminalis, and Ulmus glabra, Bundesanstalt für Landwirtschaft und Ernährung 2018).
There has been a lot of debate concerning the impact of silvicultural management on the biodiversity of Central European forests (Paillet et al. 2010; Hobi et al. 2015; Schulze et al. 2016). Major issues include whether silvicultural interventions are able to mimic natural disturbances thus facilitating biodiversity or whether the cessation of management might lead to monotonous beech stands of low biodiversity. In our focal landscape, managed forest stands diverged little from unmanaged forests in terms of woody plant species richness or diversity at plot level, what might be attributable to the young age of unmanaged control forests (< 50 a), which are still developing old-growth features (see Bengtsson et al. 2000; Bauhus et al. 2009; Wirth et al. 2009a). However, our managed forests exhibited lower plant beta diversity and, to a lesser degree, altered microclimatic regimes, with managed forest communities being much more constrained/homogenized and having slightly higher shade/cold tolerance. Such a physical and biotic homogenization probably results from two main drivers. First, the structural and, consequently, micro-climatic simplification experienced by managed forest stands due to the elimination of treefall gap dynamics, i.e. a key driver for microclimatic heterogeneity (Brunet et al. 2010), as indicated by the low volume of dead wood in managed stands (see Wirth et al. 2009b). Among many practices, management often results in the implementation of even-sized tree stands and a shift in forest stand dominance towards a few commercial tree species, thus reducing the variety of tree structural types and associated microclimatic variation (see Paillet et al. 2010; Duguid and Ashton 2013; Penone et al. 2019). In addition to reduced forest structural complexity, favoring economically important timber species reduces diversity of plant assemblages per se. Among 85 tree species in Central Europe, only 28 are commercially used (Schulze et al. 2016) and only 4 genera constitute 73% of Germany’s forests (MUF 2002). Similarly, in our study 93% of individuals in managed forests were either beech, oak, or hornbeam. Furthermore, many of these timber species exhibit pronounced shade/cold tolerance, such as beech (F. sylvatica), which also was an indicator species of managed forests in this study. The fact that larch (L. decidua) was identified as an additional indicator species is likely due to deliberate tree planting, as this is a photophilous species. This implies that managed forest stands are not a naturally suitable habitat for small-statured tree species and shrubs requiring for more illuminated and/or warmer microhabitats such as Prunus spinosa or Sorbus aria. Concluding, we have documented that timber-oriented forest management generates a strong signal of floristic homogenization at the spatial scale of habitats.
In summary, human disturbances operate as an important driver of species assembly, able to reorganize plant species assemblages at local and landscape level in Central European beech forests. While the establishment of forest fragments and forest edges increase habitat heterogeneity via more illuminated/warmer habitats, forest management represents a driving force behind the homogenization of both microclimatic conditions and plant assemblages at landscape scale. Forest edges represent the most species-rich habitat and increase forest diversity by promoting light-demanding species via century-long deforestation and fragmentation. In this view, edges and small fragments may serve as reservoirs for light-adapted woody plants especially in managed forest landscapes with strong physical/floristic homogenization, while unmanaged forests retain key components of floristic biodiversity, partly due to heterogeneous microclimatic regimes formed by natural disturbances (Paillet et al. 2010; Duguid and Ashton 2013; Penone et al. 2019). As timber-oriented forest management reduces environmental variability and plant species diversity at landscape scale, such drivers of homogenization must be operated with caution (Duguid and Ashton 2013), as the conservation value of these human-modified landscapes relies on habitat complementarity. Nevertheless, further studies are required to illuminate this matter.
Change history
12 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10531-021-02307-3
References
Akima H, Gebhardt A (2015) akima: interpolation of irregularly and regularly spaced data. R package version 0.5-12
Avon C, Dumas Y, Bergès L (2013) Management practices increase the impact of roads on plant communities in forests. Biol Conserv 159:24–31
Bähner KW (2016) Plants, herbivores, and their interactions in human-modified landscapes. PhD Thesis, University of Kaiserslautern, Kaiserslautern, Germany
Bähner KW, Zweig KA, Leal IR, Wirth R (2017) Robustness of plant–insect herbivore interaction networks to climate change in a fragmented temperate forest landscape. Bull Entomol Res 107:1–10
Bartish IV, Hennekens S, Aidoud A, Hennion F, Prinzing A (2010) Species pools along contemporary environmental gradients represent different levels of diversification. J Biogeogr 37:2317–2331
Bartsch N, Röhrig E (2016) Waldökologie: Einführung für Mitteleuropa. Springer Spektrum, Berlin, Heidelberg
Bauhus J, Puettmann K, Messier C (2009) Silviculture for old-growth attributes. For Ecol Manag 258:525–537
Bazzaz FA (2000) Plants in changing environments: linking physiological, population, and community ecology. Cambridge University Press, Cambridge
Bengtsson J, Nilsson SG, Franc A, Menozzo P (2000) Biodiversity, disturbances, ecosystem function and management of European forests. Forest Ecol Manag 132:39–50
Blaser S, Prati D, Senn-Irlet B, Fischer M (2013) Effects of forest management on the diversity of deadwood-inhabiting fungi in Central European forests. For Ecol Manag 304:42–48
Boch S, Prati D, Müller J, Socher S, Baumbach H, Buscot F, Gockel S, Hemp A, Hessenmöller D, Kalko EKV, Linsenmair KE, Pfeiffer S, Pommer U, Schöning I, Schulze E-D, Seilwinder C, Weisser WW, Wells K, Fischer M (2013) High plant species richness indicates management-related disturbances rather than the conservation status of forests. Basic Appl Ecol 14:496–505
Braunisch V, Roder S, Coppes J, Froidevaux JSP, Arlettaz R, Bollmann K (2019) Structural complexity in managed and strictly protected mountain forests: effects on the habitat suitability for indicator bird species. For Ecol Manag 448:139–149
Broadbent EN, Asner GP, Keller M, Knapp DE, Oliveira PJC, Silva JN (2008) Forest fragmentation and edge effects from deforestation and selective logging in the Brazilian Amazon. Biol Conserv 141:1745–1757
Brunet J, Fritz Ö, Richnau G (2010) Biodiversity in European beech forests–a review with recommendations for sustainable forest management. Ecol Bull 53:77–94
Bundesanstalt für Landwirtschaft und Ernährung (2018) Information system on genetic ressources. https://blag-fgr.genres.de. Accessed 15 Jun 2018
Chaudhary A, Burivalova Z, Koh LP, Hellweg S (2016) Impact of forest management on species richness: global meta-analysis and economic trade-offs. Sci Rep 6:23954
Coch T (1995) Waldrandpflege. Grundlagen und Konzepte, Neumann, Radebeul
Coomes DA, Burslem DFRP, Simonson WD (2014) Forests and global change. Cambridge University Press, Cambridge
Decocq G, Aubert M, Dupont F, Bardat J, Wattez-Franger A, Saguez R, de Foucault B, Alard D, Delelis-Dusollier A (2005) Silviculture-driven vegetation change in a European temperate deciduous forest. Ann For Sci 62:313–323
Deutscher Wetterdienst (2013) Mean annual climate data. https://www.dwd.de. Accessed 15 Jun 2018
Dixon RK, Brown S, Houghton RA, Solomon AM, Trexler MC, Wisniewski J (1994) Carbon pools and flux of global forest ecosystems. Science 263:185–191
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Duguid MC, Ashton MS (2013) A meta-analysis of the effect of forest management for timber on understory plant species diversity in temperate forests. For Ecol Manag 303:81–90
Ellenberg H, Leuschner C (1996) Vegetation Mitteleuropas mit den Alpen. UTB, Stuttgart
Emmer I, Fanta J, Kobus A, Annemieke K, Sevink J (1998) Reversing borealization as a means to restore biodiversity in Central-European mountainforests–an example from the Krkonoše Mountains, Czech Republic. Biodivers Conserv 7:229–247
European Commission (2019) Forest-based industries. https://ec.europa.eu/growth/sectors/raw-materials/industries/forest-based_en. Accessed 15 Nov 2019
Fardila D, Kelly LT, Moore JL, McCarthy MA (2017) A systematic review reveals changes in where and how we have studied habitat loss and fragmentation over 20 years. Biol Conserv 212:130–138
Flückiger VPF, Bienz H, Glünkin R, Iseli K (2002) Vom Krautsaum bis ins Kronendach – Erforschung und Aufwertung der Waldränder im Kanton Solothurn. Mitteilungen der Naturforschenden Gesellschaft des Kantons Solothurn 39:9–39
Foley JA, Defries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Coe MT, Daily GC, Gibbs HK, Helkowski JH, Holloway T, Howard EA, Kucharik CJ, Monfreda C, Patz JA, Prentice IC, Ramankutty N, Snyder PK (2005) Global consequences of land use. Science 309:570–574
Girão LC, Lopes AV, Tabarelli M, Bruna EM (2007) Changes in tree reproductive traits reduce functional diversity in a fragmented Atlantic forest landscape. PLoS ONE 2:1–12
Grömping U (2006) Relative importance for linear regression in R: the package relaimpo. J Stat Softw 17:1–27
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD, Cook WM, Damschen EI, Ewers RM, Foster BL, Jenkins CN, King AJ, Laurance WF, Levey DJ, Margules CR, Melbourne BA, Nicholls AO, Orrock JL, Song D-X, Townshend JR (2015) Habitat fragmentation and its lasting impact on Earth's ecosystems. Sci Adv 1(2):e1500052
Hahn K, Fanta J (2001) Contemporary beech forest management in Europe: working report 1. Copenhagen
Hannah L, Carr J, Lankerani A (1995) Human disturbance and natural habitat: a biome level analysis of a global data set. Biodiv Cons 4:128–155
Hansen MC, Stehman SV, Potapov PV (2010) Quantification of global gross forest cover loss. PNAS 107:8650–8655
Hawkins BA, Field R, Cornell H, Currie DJ, Guégan J-F, Kaufman DM, Kerr JT, Mittelbach GG, Oberdorff T, O’Brien EM, Porter EE, Turner JRG (2003) Energy, water, and broad-scale geographic patterns of species richness. Ecology 84:3105–3117
Hawkins BA, Rueda M, Rangel TF, Field R, Diniz-Filho JAF (2014) Community phylogenetics at the biogeographical scale: cold tolerance, niche conservatism and the structure of North American forests. J Biogeogr 41:23–38
Hermy M, Honnay O, Firbank L, Grashof-Bokdam C, Lawesson JE (1999) An ecological comparison between ancient and other forest plant species of Europe, and the implications for forest conservation. Biol Conserv 91:9–22
Hobi ML, Commarmot B, Bugmann H (2015) Pattern and process in the largest primeval beech forest of Europe (Ukrainian Carpathians). J Veg Sci 26:323–336
Honnay O, Verheyen K, Hermy M (2002) Permeability of ancient forest edges for weedy plant species invasion. For Ecol Manag 161:109–122
Jacquemyn H, Butaye J, Hermy M (2003) Influence of environmental and spatial variables on regional distribution of forest plant species in a fragmented and changing landscape. Ecography 26:768–776
Kettle CJ, Koh LP (2014) Global forest fragmentation. CABI, Oxfordshire
Lang S, Tiede D (2003) vLATE Extension für ArcGIS—vektorbasiertes Tool zur quantitativen Landschaftsstrukturanalyse. In: ESRI Anwenderkonferenz 2003, Insbruck
Laurance W, Lovejoy T, Vasconcelos HI, Bruna EM, Didham RK, Stouffer PC, Gascon C, Bierregaard RO, Laurance SG, Sampaio E (2002) Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Conserv Biol 16:605–618
Lindenmayer DB, Franklin JF, Lõhmus A, Baker SC, Bauhus J, Beese W, Brodie A, Kiehl B, Kouki J, Martínez Pastur G, Messier C, Neyland M, Palik B, Sverdrup-Thygeson A, Volney J, Wayne A, Gustafsson L (2012) A major shift to the retention approach for forestry can help resolve some global forest sustainability issues. Conserv Lett 5:421–431
Lôbo D, Leão T, Melo FPL, Santos AMM, Marcelo T (2011) Forest fragmentation drives Atlantic forest of northeastern Brazil to biotic homogenization. Divers Distrib 17:287–296
Magnago LFS, Edwards DP, Edwards FA, Magrach A, Martins SV, Laurance WF (2014) Functional attributes change but functional richnessis is unchanged after fragmentation of Brazilian Atlantic forests. J Ecol 102:475–485
Martorell C, Peters EM (2005) The measurement of chronic disturbance and its effects on the threatened cactus Mammillaria pectinifera. Biol Conserv 124:199–207
Meyer S, Wesche K, Krause B, Leuschner C (1950s) Dramatic losses of specialist arable plants in Central Germany since the 1950s/60s - a cross-regional analysis. Divers Distrib 19:1175–1187
Millar CI, Stephenson NL (2015) Temperate forest health in an era of emerging megadisturbance. Science 349:823–826
Moreno CE, Halffter G (2000) Assessing the completeness of bat biodiversity inventories using species accumulation curves. J Appl Ecol 37:149–158
MUF (2002) Bundeswaldinventur 2. Ministry of Environment and Forestry, Berlin
Naudts K, Chen YC, McGrath MJ, Ryder J, Valade A, Otto J, Luyssaert S (2016) Europe's forest management did not migitate climate warming. Science 351:597–600
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2015) vegan: community ecology package. R package version 2.3-0
Paillet Y, Bergés L, Hjältén J, Odor P, Avon C, Bernhardt-Römermann M, Bijlsma R, de Bruyn L, Fuhr M, Grandin U, Kanka R, Lundin L, Luque S, Magura T, Matesanz S, Mészáros I, Sebastiá M-T, Schmidt W, Standovár T, Tóthmérész B, Uotila A, Valladares F, Vellak K, Rista V (2010) Biodiversity differences between managed and unmanaged forests: meta-analysis of species richness in Europe. Conserv Biol 24:101–112
Pellissier V, Bergès L, Nedeltcheva T, Schmitt MC, Avon C, Cluzeau C, Dupouey J-L (2013) Understorey plant species show long-range spatial patterns in forest patches according to distance-to-edge. J Veg Sci 24:9–24
Penone C, Allan E, Soliveres S, Felipe-Lucia MR, Gossner MM, Seibold S, Schall P, van der Plas F, Manning P, Manzanedo RD, Boch S, Prati D, Ammer C, Bauhus J, Buscot F, Ehbrecht M, Goldmann K, Jung K, Müller J, Müller JC, Pena R, Polle A, Renner S, Ruess L, Schöning I, Schrumpf M, Solly EF, Tschapka M, Weisser WW, Wubet T, Fischer M (2019) Specialisation and diversity of multiple trophic groups are promoted by different forest features. Ecol Lett 22:170–180
R Core Team (2013) R: a language and environment for statistical computing. R package version 3.0.2
Ribeiro EMA, Santos BA, Arroyo-Rodríguez V, Tabarelli M, Souza G, Leal IR (2016) Phylogenetic impoverishment of plant communities following chronic human disturbances in the Brazilian Caatinga. Ecology 6:1583–1592
Roberts D (2015) labdsv: Ordination and multivariate analysis for ecology. R package version 1.7-0
Schubert R, Hilbig W, Klotz S (1995) Bestimmungsbuch der Pflanzengesellschaften Mittel- und Nordostdeutschlands. Gustav Fischer, Jena
Schulze ED, Aas G, Grimm GW, Gossner MM, Walentowski H, Ammer C, Kühn I, Bouriaud O, von Gadow K (2016) A review on plant diversity and forest management of European beech forests. Eur J For Res 135:51–67
Smith IA, Hutyra LR, Reinmann AB, Marrs JK, Thompson JR (2018) Piecing together the fragments: elucidating edge effects on forest carbon dynamics. Front Ecol Environ 16:213–221
Tabarelli M, Lopes AV, Peres CA (2008) Edge-effects drive tropical forest fragments towards an early-successional system. Biotropica 40:657–661
Tabarelli M, Aguiar AV, Ribeiro MC, Metzger JP, Peres CA (2010) Prospects for biodiversity conservation in the Atlantic Forest: Lessons from aging human-modified landscapes. Biol Conserv 143:2328–2340
Taubert F, Fischer R, Groeneveld J, Lehmann S, Müller MS, Rödig E, Wiegand T, Huth A (2018) Global patterns of tropical forest fragmentation. Nature 554:519–522
Valladares G, Cagnolo L, Salvo A (2012) Forest fragmentation leads to food web contraction. Oikos 121:299–305
Vellend M, Verheyen K, Jacquemyn H, Kolb A, van Calster H, Peterken G, Hermy M (2006) Extinction debt of forest plants persists for more than a century following habitat fragmentation. Ecology 87:542–548
Whittaker RJ, Nogués-Bravo D, Araújo MB (2007) Geographical gradients of species richness: a test of the water-energy conjecture of Hawkins et al. (2003) using European data for five taxa. Glob Ecol Biogeogr 16:76–89
Williams M (2000) Dark ages and dark areas: global deforestation in the deep past. J Hist Geogr 26:28–46
Wirth C, Gleixner G, Heimann M (2009a) Old-growth forests. Springer-Verlag, Heidelberg
Wirth C, Messier C, Bergeron Y, Frank D, Fankhänel A (2009b) Old-growth forest definitions: a pragmatic view. In: Wirth C, Gleixner G, Heimann M (eds) Old-growth forests. Springer, Heidelberg
Zelený D, Schaffers AP (2012) Too good to be true: pitfalls of using mean Ellenberg indicator values in vegetation analyses. J Veg Sci 23:419–431
Ziter C, Bennett EM, Gonzalez A (2014) Temperate forest fragments maintain aboveground carbon stocks out to the forest edge despite changes in community composition. Oecologia 176:893–902
Acknowledgements
We would like to express our gratitude to Dr. Patricia Balcar (German Research Institute for Forest Ecology and Forestry in Trippstadt) for guidance, logistic and administrative support, and valuable comments on the manuscript. The establishment of permanent study plots in the Northern Palatinate Highlands by Christoph Dohm was crucial to the completion of this study. We are grateful to Carina Brenner and Julia Hubert for their help during fieldwork and to Max Paul for his assistance with technical GIS issues. This work was supported by a student grant to KB by the Rhineland-Palatinate Ministry for Environment, Agriculture, Nutrition, Viticulture and Forestry.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Daniel Sanchez Mata.
We dedicate this work to eminent ecologist Prof. Dr. Otto Ludwig Lange, who passed away on August 14, 2017, a few days before his 90th birthday.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Forest and plantation biodiversity
The original version of the article was revised due to retrospective open access order.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bähner, K.W., Tabarelli, M., Büdel, B. et al. Habitat fragmentation and forest management alter woody plant communities in a Central European beech forest landscape. Biodivers Conserv 29, 2729–2747 (2020). https://doi.org/10.1007/s10531-020-01996-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-020-01996-6