Abstract
Examining context-dependency of ecological impacts of alien plant invasions is critical to further understand the mechanisms driving impacts. We examined how different regional and local habitat contexts influence the abundance-impact associations of an invasive ground-creeper, Tradescantia fluminensis, in native forests across eastern New South Wales (NSW), Australia. Invader impacts were assessed using surveys of resident vegetation at 97 monitoring plots (5 m × 2 m) located at 14 sites, representing a gradient of T. fluminensis abundance. We modelled the association of T. fluminensis invasion with native species richness (number per plot) and foliage cover across two different habitat types (remnant vs replanted forests), two vegetation community types (wet sclerophyll vs river oak forests), and two regions (northern vs. southern NSW). We also modelled variation in native species responses amongst different functional growth forms. The negative associations of T. fluminensis and native species communities was more strongly explained by local site variables (i.e., habitat type, community type, plant growth form) than regional scales. Native richness reduced with invasion in river oak but not wet sclerophyll forest. Native richness also declined in remnant forest, although no effect of invasion was observed in replanted forest. Surprisingly, native species growth forms most like T. fluminensis (ground layer herbs, ferns) were more resistant to reductions in native richness compared to divergent forms (shrub, tree and woody vine recruits). This study highlights the need to explicitly consider local community and habitat context and functional representation of resident species when considering invader impacts and site-level management plans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alien plant invasions threaten ecosystems globally by disrupting key ecosystem functions and processes (Ehrenfeld 2010), and reducing native biodiversity across multiple scales and taxonomic groups (Gaertner et al. 2009; Hejda et al. 2009; Vilà et al. 2011). With regards to native vegetation, alien plants can homogenise community composition, causing reductions in species richness, population abundance and fitness (Gaertner et al. 2009; Vilà et al. 2011). Impact mechanisms typically include direct competition for limited resources (e.g., light, soil nutrients, mutualist services) (Zhang et al. 2019; Traveset and Richardson 2014; Petri and Ibañez 2023; Liao et al. 2008), allelopathic interference (e.g., allelopathy) (Zhang et al. 2019) and modification of disturbances, such as fire (Gaertner et al. 2014). To improve predictive capacity in invasive plant impact mechanisms, enabling more optimal management, there is an increasing research focus on context dependency in invasion—for example, variation in impacts amongst invasive plant taxa and recipient communities (Beaury et al. 2023; Vilà et al. 2006), and how impacts are moderated by other disturbance processes, such as land development and climate change (Didham et al. 2005, 2007; Gooden and French 2014).
Context-dependent impacts of alien plant invasions are defined as situations where the direction and shape of impact relationships vary depending on the conditions under which they are observed (Catford et al. 2022). Context dependence of relationships within ecology is an emerging issue and is increasingly being invoked in ecological studies (Catford et al. 2022). Studies of alien plant invasions are increasingly considering how environmental factors such as habitat type, disturbance, region, micro-habitat, season and landscape context interact with the invader to alter the impacts on native plant communities (e.g., Bieberich et al. 2020; Fried and Panetta 2016; Gooden and French 2014; O’Loughlin et al. 2021). For example, it is well-established that greater rates of landscape disturbance or modification is strongly associated with increased abundance of alien plant species (Butcher and Kelly 2011; González-Moreno et al. 2013, 2014; Vilà et al. 2007). In some cases, the interaction of invasion with a disturbance effect, such as logging or proximity to modified landscapes, significantly alters the shape of abundance-impact relationships (Sokol et al. 2017), whereas in other cases, the disturbance will have limited influence on abundance-impact relationships with native plant communtities (Gooden and French 2014; O’Loughlin et al. 2021). This variation in abundance-impact relationships due to the interaction between alien plant species and landscape context highlights the need for further research into how these interactions vary across a broad range of invasive species and contexts at broad spatial scales.
Alien plant impacts can also vary amongst resident native plant species within invaded communities as a function of the resident species’ functional traits (e.g., Fried et al. 2019; Gooden et al. 2009a, b), which can lead to significant changes in the functional structure of invaded ecosystems (Castro-Díez et al. 2016; Fernandez et al. 2021; Forey et al. 2023; Sodhi et al. 2019). Plant functional traits are the morphological, physiological or phenological features that represent ecological strategies that affect plant fitness or modulate their response to environmental factors (Pérez-Harguindeguy et al. 2016). Testing how the effects of alien plant invasion vary amongst different native plant species’ functional traits can elucidate the mechanisms by which invaders modify recipient communities and explain why certain native species are more at risk of decline from invasion pressures than others (Forey et al. 2023; Fried et al. 2019; Sodhi et al. 2019). Previous studies have shown that native species with niches that strongly overlap with an invader are more likely to share traits and be competitively excluded under resource limited conditions (Fernandez et al. 2021; Sodhi et al. 2019). Likewise, native species with functional traits that diverge from an invader are more likely to persist due to increased niche complementarity (Fried et al. 2019; Sodhi et al. 2019). As such, the trajectory of vegetation community responses to invasion can be the product of both processes operating at the landscape scale and at the local plant community level.
Tradescantia fluminensis (commonly named ‘wandering trad’) is a creeping, herbaceous groundcover that has widely invaded temperate and sub-tropical forests of eastern Australia and New Zealand. It forms dense, sprawling mats in moist, shaded contexts, such as stream embankments and gullies, where it has been shown to reduce native vegetation abundance and richness (McAlpine et al. 2015; O’Loughlin et al. 2021; Standish et al. 2001). Invasion by T. fluminensis is also considered a significant threat to regeneration of planted forests, hampering efforts to restore vulnerable ecosystems (Standish et al. 2001). However, studies to date have been undertaken in one or a few habitat types and over small spatial scales (e.g., McAlpine et al. 2015; O’Loughlin et al. 2021; Standish et al. 2001). Our study aimed to examine the impacts of T. fluminensis amongst different vegetation community types, different levels of habitat rehabilitation and over broader landscape and regional spatial scales in eastern Australia. We also sought to determine how different functional growth traits moderate native species’ responses to invasion, to better predict trajectories of community compositional change with alien plant invasion.
Methods
Study area and habitat context
Full-floristic vegetation surveys were undertaken at 97 plots nested in 14 sites (described below in survey design, Sect. “Survey design”) between July and October 2020 along the eastern slopes and foothills of the Great Dividing Range, New South Wales, Australia (~ 800 km latitudinal gradient). Sites were situated on sheltered, deeply shaded riparian embankments with remnant, intact overstorey canopies of mature native trees and no recent history of deforestation (site details listed in Online Resource Table S1). Sites were selected with the assistance of natural resource managers within each local region in areas where T. fluminensis invasion had been observed over several years with no commensurate disruption of forest canopies by logging, fire or other disturbances.
Sites were clustered into two regions: (1) northern NSW, with sites distributed between the townships of Coffs Harbour to Port Macquarie; (2) southern NSW, situated between the Shoalhaven-Illawarra and the Bega Valley (Online Resource Figure S1). The northern region is characterised by a subtropical climate, with higher minimum (~ 12.8 °C) and maximum temperatures (~ 23 °C) and higher total annual rainfall (~ 1600 mm) compared to the cool-warm temperate southern region (Online Resource Figure S1). In contrast, the southern region is characterised by a more temperate climate and has cooler average minimum (~ 10.5 °C) and maximum temperatures (~ 21 °C) and a lower average total annual rainfall (~ 1300 mm) (Online Resource Figure S1).
In each region, surveys were undertaken in two contrasting vegetation community types that differed in the dominant canopy species, vegetation structure and topographic context (a list of dominant tree species is provided in Online Resource Table S1). (1) Wet sclerophyll forest: present on eastern escarpment slopes and foothills; comprising a tall, open canopy dominated by species within the genus Eucalyptus, with a dense (often closed) mid-storey dominated by a highly species-rich mesophyllous rainforest flora, vine thickets and a dense ground layer of ferns and herbs. (2) River oak forest: present along freshwater streams in coastal or escarpment valleys; dominated by a single species, Casuarina cunninghamiana subsp. cunninghamiana, with a sparse shrub layer and grassy ground layer. Examples of community type are presented in Online Resource Figure S2.
Eight sites were positioned in remnant forests whilst six sites had been revegetated by replanting a diverse mix of nursery-grown native tree species over several decades and had since become dominated in the understorey by T. fluminensis (Online Resource Table S1). The selection of both remnant and replanted sites (ie. revegetated) interspersed across the latitudinal gradient allowed us to test whether the impacts of T. fluminensis on native vegetation vary between different habitat contexts, and the potential constraints on vegetation restoration trajectories imposed by its invasion.
Survey design
The association of T. fluminensis invasion with resident vegetation richness was assessed using a multi-site comparative approach (described below), following a similar method deployed by Gooden et al. (2009a, b) and O’Loughlin et al. (2021). Full-floristic surveys of vascular plant taxa were undertaken within 97 rectangular plots (2 m × 5 m) allocated across the 14 sites (4–8 plots per site) that were nested in the two regions (n = 7 sites per region) (Online Resource Figure S3). Within each site, plots were distributed across a gradient of T. fluminensis foliage cover, including at least one or two reference plots that were dominated by native vegetation with very little or no resident T. fluminensis plants. Plots with different levels of T. fluminensis cover were interspersed across each site, and positioned in areas of similar overstorey canopy cover, distance to the nearby stream and elevation on the riparian embankment with no evidence of canopy or soil disturbance.
The corners of each plot were marked with stakes, GPS coordinates were recorded, and representative photographs taken as a baseline record of vegetation condition. Photographs were taken of the overstory canopy above each plot as evidence of the intact nature of the mature vegetation. Overstorey canopy cover was visually estimated for each plot. Each plot was coded by its habitat type (remnant canopy (n = 74) vs replanted canopy (n = 23)) and community type (wet sclerophyll forest (n = 41) vs river oak forest (n = 56)).
Quantifying T. fluminensis abundance
Each plot was split into 10 × 1 m2 subplots (Online Resource Figure S3) to improve accuracy for visually estimating T. fluminensis abundance. The abundance of T. fluminensis was visually estimated as the projected percentage foliage cover within each of 10 sub-plots. Five measurements of the height of T. fluminensis foliage were recorded per subplot using a hand-held tape measure to the nearest cm. The volume of space occupied by T. fluminensis per plot was subsequently calculated by first converting foliage cover from a percentage to m2, then multiplying cover by average height m (O’Loughlin et al. 2021; Standish et al. 2001).
To determine whether T. fluminensis foliage cover or volume were better predictors of T. fluminensis biomass, T. fluminensis biomass was harvested from fourteen 1-m2 quadrats at two sites in August 2020. The foliage cover of T. fluminensis was visually estimated and five measurements of foliage height were recorded from each quadrat before harvesting all above ground T. fluminensis plant matter at the soil surface. The wet biomass was weighed and then the harvested above ground matter was oven dried at 70 °C for 3 days. The dry weight was weighed and recorded.
Vegetation surveys
Detailed surveys of resident vascular plant species were undertaken for plants growing under 2 m height that were rooted within or overhanging each plot, as these were deemed most likely to directly interact with and be impacted by T. fluminensis invasion within the understorey (O’Loughlin et al. 2021; Standish et al. 2001). The abundance of all plant species was visually estimated as the projected percentage foliage cover within each of the plots. Species nomenclature followed Flora of NSW (PlantNET 2022). Seedlings and saplings of shrubs and trees growing under 2 m at the time of sampling, but which can reach > 2 m at maturity, were also recorded to ascertain impacts of T. fluminensis invasion on canopy species recruitment. Several specimens could be confidently identified to genus (Oxalis, Solanum, Galium and Passiflora) but not species level. Given these genera comprise a variety of co-occurring native and alien species across eastern NSW, it was therefore not possible to assign them to an origin category. These unidentified taxa were rarely encountered and thus excluded from subsequent analyses.
To evaluate whether functional traits explain variation in native vegetation responses to T. fluminensis invasion, each plant species was assigned to the following seven growth categories: (1) shrubs (typically multi-stemmed, growing to < 2 m tall at maturity), (2) mesophyllous trees (mainly rainforest species with large, shade-adapted leaves), (3) sclerophyll trees (typically leathery leaves adapted to high-light conditions and associated with upper canopy species within the genera Eucalyptus, Syncarpia, Casuarina and Acacia), (4) ferns, (5) graminoids (including grasses within the family Poaceae and grass-like plants, such as sedges and rushes), (6) herbs and (7) climbers. Graminoids and herbs were further subdivided into spreading (rhizomatous, stoloniferous) and tufted forms. Climbers were also subdivided into scramblers/twiners (often with thin, herbaceous stems) that typically grow alongside T. fluminensis within the understorey ground and shrub layers versus woody climbers that grow mainly in the canopies of overstorey trees at maturity. Growth form categories were assigned based on descriptions within the Flora of NSW and direct observations of species growth habits in the field.
Statistical analysis
All models were fitted in RStudio version 2023.06.01 (Posit team 2023).
Associations amongst T. fluminensis volume, cover and biomass
Linear regressions were fitted to dry T. fluminensis biomass with T. fluminensis foliage cover and volume as predictor variables, using the ‘lm’ function in the “lme4” package (Bates et al. 2015). The predicted values were calculated using the ‘predict’ function in the “car” package (Fox and Weisberg 2019). Tradescantia fluminensis volume (R2 = 0.89, \({F}_{1, 12}\) = 104.5, P < 0.0001) was a very strong predictor of T. fluminensis biomass, as found by Standish et al. (2001), compared to T. fluminensis cover which explained 13% less variation in biomass than volume (R2 = 0.76, \({F}_{1, 12}\) = 43.54, P < 0.0001) (Online Resource Figure S4). Furthermore, T. fluminensis foliage cover was a strong positive predictor of volume (R2 = 0.84, \({F}_{1, 12}\) = 65.36, P < 0.0001) (Online Resource Figure S4). As such, T. fluminensis volume was selected as the most suitable surrogate for T. fluminensis biomass in all subsequent analyses.
Previous studies in New Zealand showed that T. fluminensis abundance is negatively associated with overstorey canopy cover (Standish et al. 2001). However, linear models for our data found no such association with canopy cover, which was generally > 60% across all sites (Online Resource Figure S5). As such, overstorey canopy cover was not included as a covariate predictor in subsequent analyses.
Responses of native and alien species richness and cover to T. fluminensis invasion across different spatial, habitat and community vegetation contexts
The richness of alien (excluding T. fluminensis) and native species was calculated as the total number of different species present in each 10 m2 plot. The foliage cover of each species per plot was calculated as the average cover across the ten subplots. The cover values of all native and alien species were then combined to derive a single foliage cover value for native and then again for alien species. Generalised linear mixed models (GLMM) were fitted to alien and native species richness with Poisson error distribution and log link functions. Generalised linear mixed models were fitted to alien species and native species foliage cover with a beta family error distribution and logit link functions as recommended by Douma and Weedon (2019). For native species, one plot had a total cover of 111.6% foliage cover due to several species having overlapping foliage; to fit a beta model, native species cover in this plot was converted to 100%. Beta models require the response variable to range between 0 and 1. Native and alien species cover data both included zero values; therefore, we transformed the dataset using the following equation: cover* = (cover(n − 1) + ½)/n, with n being the number of observations to ensure all values were between 0 and 1 (Douma and Weedon 2019).
Cover and richness were modelled as a function of T. fluminensis volume and its two-way interaction with each of three predictor variables: region (north vs south), vegetation community type (wet sclerophyll forest vs river oak forest) and habitat type (remnant vs replanted forest). Three-way interactions between predictor variables were not evaluated as we did not have sufficient statistical power, and a smaller number of replanted wet sclerophyll forest sites meant our replanted habitat treatment had a small but significant association with river oak forest (Cramer’s V = 0.22, p = 0.03). “Site” was included as a random effect in our full model to account for site-level variation associated with this design imbalance, spatial autocorrelation, and the non-independent clustering of plots within each site. Fit of the model, overdispersion and zero-inflation were assessed using several tests from the “DHARMa” package (Hartig 2022).
Using the ‘dredge’ function, subsets of the full model were ranked and the best-fit models which were within 2 Akaike’s Information Criterion (AIC) of each other were selected. If more than one model was selected, the coefficients from these models were averaged using the ‘model.avg’ function from the “MuMIn” package (Barton 2023). The use of function ‘dredge’ on full models can result in the loss of interaction terms, or entire predictor variables in the final best-fit models. Confidence intervals for the coefficients were estimated using the ‘confint’ function in the “stats” package (R Core Team 2023). The predicted values for each significant predictor and their significant interactions from the model averaged GLMMs were generated using the ‘emmeans’ function in the “emmeans” package (Lenth 2023).
For all further analyses, four non-invaded reference plots from the southern region were excluded, as most of their resident species (> 80% foliage cover) were identified as other alien plant species. Furthermore, a single plot was excluded from analyses as it had three times higher T. fluminensis volume than any other plot dominated by the weed. As such, for all subsequent analyses, n = 92.
Impacts of T. fluminensis invasion on the richness and cover of different native plant growth forms
A series of generalised linear mixed models were used to determine the effect of T. fluminensis volume on native species richness and foliage cover within each of the ten different native species growth form categories. The percentage of zero values for native species total cover across the 10 growth forms across the 92 plots ranged between 32 and 80%. To be able to account for that many zeros in the data set, zero-inflated beta regression (ZIBR) models were fitted with logit-link functions using ‘glmmTMB’ function from the “glmmTMB” package (Brooks et al. 2017). Models included T. fluminensis volume as the predictor and site nested within region as random effects. We did not include habitat or community type as predictors in the functional trait analyses, as we were interested in exploring generalisable trends in trait responses at broad scales, independent of species identities contributing to community composition. Poisson error distribution with log-link functions were fitted to species richness in each growth form using ‘glmmTMB’ function from the “glmmTMB” package (Brooks et al. 2017). For all models, site nested within region was included as a random effect. Model assumptions were tested as above. The predicted values were calculated using the ‘predict’ function in the “car” package (Fox and Weisberg 2019).
Using the predicted values, the percentage change of each variable between absent (0 m3) and maximum observed (3.86 m3) T. fluminensis volume was calculated for each growth form category for both native species richness and cover. Of the 242 native vascular plants species included in previous analyses, 181 were included in the functional growth form analyses, excluding epiphytic ferns, epiphytic orchids and one Ranunculus species (present in a single plot) that could not be assigned to a growth form category.
Results
Summary of vegetation composition
A total of 242 different vascular plant taxa (hereafter species) was recorded; of these, 76% (185) were native and 24% (57, not including T. fluminensis) were alien (species list provided in Online Resource Table S2). On average, each of the 242 identifiable species was detected in only 4.3% (± 5.1 SD) of the 97 plots, and 85 species were found in only one plot, indicating a high degree of compositional heterogeneity across the broad latitudinal gradient. The most common species included the native prostrate grass Oplismenus aemulus (39%), the native prostrate herb Commelina cyanea (27%), alien shrub-small tree Ligustrum sinense (26%) and native shrub-small tree Ficus coronata (26%).
Responses of native and alien species richness and cover to T. fluminensis invasion across different spatial, habitat and community vegetation contexts
Native species richness
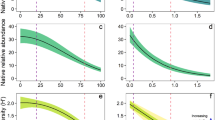
Three top models for native species richness were selected and averaged. There was no significant interactive effect of T. fluminensis volume and region on native species richness. The effect of T. fluminensis volume on native species richness differed between habitats (significant interactive effect), with strong negative association in native species richness observed in remnant forest, whilst native richness remained stable in replanted forest as T. fluminensis volume increased (Fig. 1a; Table 1). On average, across all levels of T. fluminensis volume, native richness was significantly higher in remnant forests compared to replanted forests.
a Relationship between native species richness and Tradescantia fluminensis volume (m3) across two different habitat types: remnant (n = 71) and replanted (n = 21). b Relationship between native species richness and Tradescantia fluminensis volume (m3) across two different community types: river oak forest (n = 51) and wet sclerophyll forest (n = 41). Points represent raw data and solid lines represent the model averaged predictions bound by shaded 95% confidence intervals from the top three generalised linear mixed models
The effects of T. fluminensis volume on native species richness also differed significantly between the two community types (significant interactive effect, Fig. 1b; Table 1). On average, native species richness was negatively associated with increasing T. fluminensis volume in the river oak forest, whilst native richness was not associated with T. fluminensis volume in the wet sclerophyll forest. On average, native richness was two-times greater in wet sclerophyll forest compared with river oak forests at all levels of T. fluminensis volume.
Native species cover
A single top ranked model was identified for native species cover. In replanted forests, native species cover was generally low and remained constant over the gradient of T. fluminensis volume, whilst native species cover was strongly negatively associated with increasing T. fluminensis volume in remnant forest (note that average native species cover was significantly higher overall in plots with remnant compared with replanted canopies, Fig. 2a; Table 1). On average, at very high volumes of T. fluminensis (> 3 m3), native species cover was similar in plots with remnant canopies and replanted canopies. Native cover was significantly lower on average across all T. fluminensis volumes in river oak forests compared to wet sclerophyll forests (Fig. 2b; Table 1). Native cover had a strongly negative association with increasing T. fluminensis volume in both the northern and southern regions, although the magnitude of difference between low and high T. fluminensis volume tended to be higher in the southern region (steeper slope, Fig. 2c; Table 1). At volumes greater than 2.6 m3, native species cover was lower in the southern region compared to the northern region.
a Relationship between native species total cover and Tradescantia fluminensis volume (m3) across two different habitat types: remnant (n = 71) and replanted (n = 21). b Difference in native species total cover between two community types: river oak forest (n = 51) and wet sclerophyll forest (n = 41), bars representing the prediction from the generalised linear mixed model and bars representing ± standard error. Note. An interaction term between Tradescantia fluminensis volume and community type was not included in the final best-fit model. The main effect of community type was significant and is presented. c Relationship between native species total cover and Tradescantia fluminensis volume (m3) across two different regions: north (n = 44) and south (n = 48). In plots a and c, points represent raw data and solid lines represent the prediction bound by shaded 95% confidence intervals from a single generalised linear mixed model
Alien species richness and cover
Six top models for alien species richness were selected and averaged. Alien species richness was not influenced by region, habitat type, T. fluminensis volume or the interaction between T. fluminensis volume and habitat type (Table 1). Four top models were selected for alien species cover and averaged. Alien species cover was ~ 50% lower on average in the southern compared with northern region but was not significantly related to any other predictor variable including T. fluminensis volume, habitat type, community type nor the interaction between habitat type and T. fluminensis volume (Fig. 3; Table 1).
Impacts of T. fluminensis invasion on the richness and cover of different native plant growth forms
Species richness was strongly negatively associated with increasing T. fluminensis volume for all growth form categories for which significant effects were detected (Fig. 4; Table 2). Species richness was significantly lower (> 80%) at high T. fluminensis volume (3.86 m3) on average for the two climbing growth forms and woody species (shrubs and mesophyllous trees). Native species richness of ferns and spreading graminoids (i.e., grass-like plants with either a stoloniferous or rhizomatous habit) was also significantly lower (~ 69–78%) at high T. fluminensis volume. There was no significant change in species richness for herbs, tufted graminoids or sclerophyllous trees with increasing T. fluminensis volume.
Relationship between Tradescantia fluminensis volume and two response variables: native species richness and total native species cover (%) for ten different native species growth forms. Solid lines represent predictions from the fitted model bound by shaded 95% confidence intervals. Asterisks denote significant effects of Tradescantia fluminensis volume (in the zero-inflated beta regression models this represents that either the predictors in the ZI or BR model were significant, or both) and percentage values represent the magnitude of change in the response variable from 0 m3 Tradescantia fluminensis to 3.86 m3 Tradescantia fluminensis, (n = 92)
In contrast to richness, increasing T. fluminensis volume was strongly negative associated with total foliage cover for all growth forms (by > 90%), except for sclerophyllous trees (Fig. 4; Table 2). Climbers in the canopy layer had the largest difference in cover (cover reduced by 99% and richness by 96% between 0 and 3.86 m3 T. fluminensis volume, Fig. 4). Although, species categorised as climbers in the canopy layer had much lower total foliage cover overall compared to other growth forms such as ferns, their cover was estimated to be reduced to less than 0.1% at the highest observed volumes of T. fluminensis.
Discussion
General findings and consideration of invasion impact mechanisms
This study revealed that T. fluminensis invasion in native forest communities is strongly negatively associated with native species richness and abundance. These observed relationships, however, varied between the functional groups in the resident community. Our results are consistent with impacts of T. fluminensis observed in temperate rainforest communities in southern Australia and in lowland podocarp/broad-leaved forest remnant in the North Island of New Zealand (O’Loughlin et al. 2021; Standish et al. 2001). However, our data have revealed that specific spatial, habitat and community vegetation contexts can alter the magnitude and shape of abundance-impact relationships of T. fluminensis invasion on recipient native plant species (discussed below). Our results therefore highlight the value and need to consider environmental context in studies investigating patterns of biodiversity change associated with alien plant invasion (Catford et al. 2022).
The strength of our study was to use a classical multi-site comparative analysis by extensively field-sampling a range of sites representing a broad gradient of invasion at a very large, regional spatial scale, across diverse habitat and community contexts. These data are thus consistent with global meta-analyses, that seek to relate weed invasion to changes in native biodiversity based on correlative field data (e.g., Vilà et al. 2011; Pyšek et al. 2011). Nevertheless, in our correlative study, the drivers of native vegetation associations to T. fluminensis invasion were not examined mechanistically (i.e., through manipulative experimentation), and it remains somewhat uncertain whether invasion by T. fluminensis was the proximate driver or rather a passenger of native vegetation community change. A key question to first address is thus: is T. fluminensis an opportunistic invader, colonising areas where the resident vegetation has been previously disturbed (e.g., overstorey canopy removal, tree-fall)? A recent study by O’Loughlin et al. (2021) indeed sought to untangle the relative contributions of T. fluminensis and anthropogenic disturbances to native vegetation communities; they found that the detection of T. fluminensis and its negative associations with native plant species richness were not amplified by disturbances, such as proximity to roadside, footpaths, suburban gardens, etc. They concluded that the strongest driver of local vegetation diversity decline was the local abundance of T. fluminensis.
Furthermore, our surveys targeted areas of dense, well-established, and mature native canopy that had not been recently disturbed. Site visits with land managers identified areas where T. fluminensis had actively invaded intact forest (either by spreading from nearby suburban gardens, by stem fragments deposited by flowing streams or illegal dumping of garden waste, consistent with findings by Butcher and Kelly (2011)), without any evident pre-emptive disturbance. Indeed, T. fluminensis is shade-tolerant and competitive under low-light conditions and has been observed to proliferate in closed forests elsewhere in Australia (Dugdale et al. 2015; O’Loughlin et al. 2021). We therefore speculate that the negative association between T. fluminensis abundance and native species richness and cover was driven by competitive interference, due to its formation of deep swards that reduce light levels at the ground surface, suppressing native seedling recruitment (Kelly and Skipworth 1984; Standish et al. 2001). Even if reduced native species richness and T. fluminensis abundance were coincidental at early stages of its invasion (responding oppositely to fine scale disturbances, such as tree-fall), the predominance of T. fluminensis likely suppresses species recovery and maintains low vegetation diversity over time.
Impacts of T. fluminensis on native and alien species across different spatial, habitat and community vegetation contexts
Forests with remnant and replanted upper native tree canopies were both equally susceptible to invasion, with similar ranges of T. fluminensis volume. The abundance-impact relationship of the habitat types, however, varied significantly, with strong negative associations detected in remnant forests while remaining unchanged with increasing T. fluminensis volume in replanted forests. The negative association of native species richness and T. fluminensis foliage cover in remnant forest patches was consistent with the results from O’Loughlin et al. (2021) (T. fluminensis volume and native species richness and total cover) and Standish et al. (2001) (T. fluminensis biomass and native species richness). In contrast, native species richness in remnant lowland forest in New Zealand declined after a threshold of 0.85–0.90 m3 T. fluminensis volume had been exceeded (McAlpine et al. 2015).
The differences in the shape of the abundance-impact relationships between the remnant and replanted forest communities was likely due to legacy effects of land use. We found that replanted forests that had previously been cleared and subsequently revegetated had significantly lower understorey native plant richness compared to remnant forests, even in the absence of T. fluminensis. This suggests that other historical disturbances were the primary cause of native species decline in the replanted forests, with no evidence that T. fluminensis invasion further reduced native vegetation richness in the replanted forests. This may be because the native understorey species present within the replanted forests were either resistant to historical land clearing (residual species) or able to recolonise the forest patches following tree planting. Such species traits enabling persistence and/or recolonisation may also have enabled co-existence amidst T. fluminensis invasion into replanted forests. We did not have sufficient power to examine variation in functional trait representation of understorey vegetation assemblages between replanted and remnant forests and thus our speculations here must be considered cautiously. Nevertheless, our results demonstrate that site historical effects can significantly alter the shape and direction of impacts of alien plant invasion on native vegetation communities. Given the low levels of species richness in non-invaded replanted forests, removal of T. fluminensis is unlikely to facilitate passive restoration of planted areas without further reintroductions of target native species.
The negative associations between T. fluminensis invasion and native vegetation also varied between the different vegetation communities. Overall, the wet sclerophyll forest community was more resistant to reductions of native species in association with T. fluminensis abundance, maintaining high species richness on average with increasing T. fluminensis abundance. Conversely, native species richness was negatively associated with increasing T. fluminensis abundance in the river oak forest. Wet sclerophyll forests also had fewer sites with high volumes of T. fluminensis (> 2.5 m3) and on average had higher native species richness and abundance compared to river oak forest. The vulnerability of a plant community to alien plant invasion, and subsequent impacts of invasion on resident native species, have been associated with low biotic resistance coupled with high levels of invader propagule pressure (Ibáñez et al. 2021; Petri and Ibañez 2023). We speculate that wet sclerophyll forest has relatively higher biotic resistance to T. fluminensis invasion because of greater diversity and abundance of resident native species, with fewer vacant niche spaces available to support T. fluminensis establishment and its competitive dominance. Furthermore, the river oak forest community is characterised by frequent flood events (Roberts et al. 2016) that select for a reduced number of flood-adapted native species. We speculate that these forest communities have more available space for T. fluminensis invasion by water-dispersed stem fragments.
We had originally predicted that the abundance-impact relationships of T. fluminensis and native vegetation would vary regionally, given the strong divergence in climate (temperature and rainfall) across the broad latitudinal gradient. Although region (north vs south) was retained in the best-fit models, regional variation in the associations between T. fluminensis volume and native species richness and cover were surprisingly weak. This result contrasts with previous studies; for example, Bieberich et al. (2020) identified light and season and Ibáñez et al. (2023) identified temperature and rainfall as key variables driving differences in impacts of alien plant species at broad scales. This study found that the abundance-impact relationships of T. fluminensis on native vegetation were consistent between the two regions thus demonstrating that the negative associations of T. fluminensis invasion with native species is maintained at regional scales.
Impact of growth forms on native species response to T. fluminensis invasion
Tradescantia fluminensis invasion was associated with significant reductions in the cover of all native plant growth forms (> 90% on average) except sclerophyllous trees. However, its impacts on species richness were modulated by growth form, with the magnitude of decline in response to invasion greater for species with more divergent growth forms compared with T. fluminensis. The recruits of woody climbers that grow within the upper canopy (lianas) had > 90% decline in richness. Shrubs and mesophyllous, understorey rainforest trees had > 80% reductions in richness in association with T. fluminensis abundance. Ferns and grasses that co-occur with T. fluminensis had < 80% reductions in richness, whereas herbs (including those with stoloniferous habits that are most similar in form to T. fluminensis) exhibited no significant reductions in richness with increasing T. fluminensis volume.
This trend was unexpected, given that previous research and niche theory suggests that species with similar growth forms to an invader are more likely to competitively interact strongly for limited resources and experience more significant declines in richness and abundance (Fernandez et al. 2021; Sodhi et al. 2019). There are several potential mechanisms driving these patterns. Native herb species (e.g., Commelina cyanea, Aneilema spp., Viola spp. Hydrocotyle spp., Centella asiatica, etc.) with the same prostrate growth habitat as T. fluminensis may be better able to resist its invasion by precedence in access to limited resources (e.g., Mason et al. 2012). Their rapid, spreading (stoloniferous and rhizomatous) growth habit may also respond dynamically to competition with T. fluminensis by spreading into areas not presently occupied by the invader, enabling co-existence at the community scale. Although woody climbers, shrubs and rainforest trees do not likely interact strongly with T. fluminensis at maturity, our surveys included data for co-occurring resident seedlings and juvenile saplings growing < 2 m within the understorey layer. Increasing T. fluminensis biomass has been found to reduce native seedlings abundance in remnant forests in New Zealand (Standish et al. 2001), likely due to competition for light that inhibits seed germination and recruitment. The apparent vulnerability of these woody species to T. fluminensis at recruitment and juvenile growth stages signals a broader impact of its invasion on the structure of the resident vegetation community.
No evidence for co-invasion by other alien species
Alien species richness and cover were very low across plots and varied regionally, with higher abundances observed in the northern region compared to the southern region. We found that alien species richness and abundance did not vary with increasing T. fluminensis volume or across the two different habitats and community types. This suggests that T. fluminensis invasion and its associations with native vegetation were not moderated or confounded by co-invasion by other alien plant species.
Conclusion
Our study has demonstrated that native vegetation responses to alien plant invasion are strongly context-dependent and cannot easily be generalised without accounting for habitat and community condition. By sampling vegetation across a broad latitudinal gradient, we found that patterns of vegetation richness and abundance are more strongly explained by community type and historical disturbances of the canopy at the local site scale with little variation explained at very broad regional scales. Indeed, at the local site scale, invasion was a strong filter of community structure, with effects on richness varying across different native plant growth forms.
Data on context-dependent invasive plant impacts could improve management of invaded communities, for example by predicting which communities and resident species are more susecptible to declines and targeting vulnerable species groups for rehabilitation following invasive plant control. Our results highlight the need for invasive plant management plans to be customised at the site scale, by accounting for the condition and composition of resident native communities, the abundance of the target invader and potential for community recvovery following its control.
Data availability
The data that supports this study can be downloaded from the CSIRO Data Access Portal (https://data.csiro.au/collection/csiro:61215).
References
Bartoń K (2023) MuMIn: multi-model inference. R package version 1.47.5. https://CRAN.R-project.org/package=MuMIn
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Soft 67:1–48. https://doi.org/10.18637/jss.v067.i01
Beaury EM, Sofaer HR, Early R, Pearse IS, Blumenthal DM, Corbin JD, Diez J, Dukes JS, Barnett DT, Ibáñez I, Petri L, Vilà M, Bradley BA (2023) Macroscale analyses suggest invasive plant impacts depend more on the composition of invading plants than on environmental context. Global Ecol Biogeogr 32:1964–1976. https://doi.org/10.1111/geb.13749
Bieberich J, Feldhaar H, Lauerer M (2020) Micro-habitat and season dependent impact of the invasive Impatiens glandulifera on native vegetation. NeoBiota 57:109–131. https://doi.org/10.3897/neobiota.57.51131
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400. https://doi.org/10.32614/RJ-2017-066
Butcher ER, Kelly D (2011) Physical and anthropogenic factors predict distribution of the invasive weed Tradescantia fluminensis. Austral Ecol 36:621–627. https://doi.org/10.1111/j.1442-9993.2010.02196.x
Castro-Díez P, Pauchard A, Traveset A, Vilà M (2016) Linking the impacts of plant invasion on community functional structure and ecosystem properties. J Veg Sci 27:1233–1242. https://doi.org/10.1111/JVS.12429
Catford JA, Wilson JRU, Pyšek P, Hulme PE, Duncan RP (2022) Addressing context dependence in ecology. Trends Ecol Evol 37:158–170. https://doi.org/10.1016/j.tree.2021.09.007
Didham RK, Tylianakis JM, Hutchison MA, Ewers RM, Gemmell NJ (2005) Are invasive species the drivers of ecological change? Trends Ecol Evol 20:470–474. https://doi.org/10.1016/j.tree.2005.06.010
Didham RK, Tylianakis JM, Gemmell NJ, Rand TA, Ewers RM (2007) Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol Evol 22:489–496. https://doi.org/10.1016/j.tree.2007.07.001
Douma JC, Weedon JT (2019) Analysing continuous proportions in ecology and evolution: a practical introduction to beta and Dirichlet regression. Methods Ecol Evol 10:1412–1430. https://doi.org/10.1111/2041-210X.13234
Dugdale T, McLaren D, Conran J (2015) The biology of Australian weeds 65. “Tradescantia fluminensis” Vell. Plant Prot Q 30:116–125
Ehrenfeld JG (2010) Ecosystem consequences of biological invasions. Annu Rev Ecol Evol Syst 41:59–80. https://doi.org/10.1146/annurev-ecolsys-102209-144650
Fernandez RD, Castro-Díez P, Aragón R, Pérez-Harguindeguy N (2021) Changes in community functional structure and ecosystem properties along an invasion gradient of Ligustrum lucidum. J Veg Sci 32:e13098. https://doi.org/10.1111/JVS.13098
Forey E, Lodhar SYF, Galvin SD, Lowry JH, Gopaul S, Hanson G, Carboni M, Chauvat M, Boehmer HJ (2023) Alien palm invasion leads to selective biotic filtering of resident plant communities towards competitive functional traits. Biol Invas 25:1489–1508. https://doi.org/10.1007/s10530-022-02991-4
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd edn. Sage, Thousand Oaks CA
Fried G, Panetta FD (2016) Comparing an exotic shrub’s impact with that of a native life form analogue: Baccharis halimifolia vs Tamarix gallica in Mediterranean salt marsh communities. J Veg Sci 27:812–823. https://doi.org/10.1111/JVS.12407
Fried G, Carboni M, Mahaut L, Violle C (2019) Functional traits modulate plant community responses to alien plant invasion. Perspect Plant Ecol Evol Syst 37:53–63. https://doi.org/10.1016/j.ppees.2019.02.003
Gaertner M, Den BA, Hui C, Richardson DM (2009) Impacts of alien plant invasions on species richness in mediterranean-type ecosystems: a meta-analysis. Prog Phys Geogr 33:319–338. https://doi.org/10.1177/0309133309341607
Gaertner M, Biggs R, Te Beest M, Hui C, Molofsky J, Richardson DM (2014) Invasive plants as drivers of regime shifts: Identifying high-priority invaders that alter feedback relationships. Divers Distrib 20:733–744. https://doi.org/10.1111/ddi.12182
González-Moreno P, Pino J, Gassó N, Vilà M (2013) Landscape context modulates alien plant invasion in Mediterranean forest edges. Biol Invas 15:547–557. https://doi.org/10.1007/s10530-012-0306-x
González-Moreno P, Diez JM, Ibáñez I, Font X, Vilà M (2014) Plant invasions are context-dependent: multiscale effects of climate, human activity and habitat. Divers Distrib 20:720–731. https://doi.org/10.1111/ddi.12206
Gooden B, French K (2014) Non-interactive effects of plant invasion and landscape modification on native communities. Divers Distrib 20:626–639. https://doi.org/10.1111/DDI.12178
Gooden B, French K, Turner PJ (2009a) Invasion and management of a woody plant, Lantana camara L., alters vegetation diversity within wet sclerophyll forest in southeastern Australia. For Ecol Manag 257:960–967. https://doi.org/10.1016/j.foreco.2008.10.040
Gooden B, French K, Turner PJ, Downey PO (2009b) Impact threshold for an alien plant invader, Lantana camara L., on native plant communities. Biol Conserv 142:2631–2641. https://doi.org/10.1016/J.BIOCON.2009.06.012
Hartig F (2022) DHARMa: residual diagnostics for hierarchical (Multi-level/mixed) regression models. R package version 0.4.6. https://CRAN.R-project.org/package=DHARMa
Hejda M, Pyšek P, Jarošík V (2009) Impact of invasive plants on the species richness, diversity and composition of invaded communities. J Ecol 97:393–403. https://doi.org/10.1111/J.1365-2745.2009.01480.X
Ibáñez I, Liu G, Petri L, Schaffer-Morrison S, Schueller S (2021) Assessing vulnerability and resistance to plant invasions: a native community perspective. Invasive Plant Sci Manag 14:64–74. https://doi.org/10.1017/inp.2021.15
Ibáñez I, Petri L, Barnett DT, Beaury EM, Blumenthal DM, Corbin JD, Diez J, Dukes JS, Early R, Pearse IS, Sorte CJB, Vilà M, Bradley B (2023) Combining local, landscape, and regional geographies to assess plant community vulnerability to invasion impact. Ecol Appl 33:e2821. https://doi.org/10.1002/eap.2821
Kelly D, Skipworth JP (1984) Tradescantia fluminensis in a Manawatu (New Zealand) forest: I. Growth and effects on regeneration. N Z J Bot 22:393–397. https://doi.org/10.1080/0028825X.1984.10425270
Lenth R (2023) emmeans: estimated marginal means, aka least-squares means. R package version 1.8.7. https://CRAN.R-project.org/package=emmeans
Liao C, Peng R, Luo Y, Zhou X, Wu X, Fang C, Chen J, Li B (2008) Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis. New Phytol 177:706–714. https://doi.org/10.1111/j.1469-8137.2007.02290.x
Mason TJ, French K, Russell K (2012) Are competitive effects of native species on an invader mediated by water availability? J Veg Sci 23:657–666. https://doi.org/10.1111/j.1654-1103.2012.01393.x
McAlpine KG, Lamoureaux SL, Westbrooke I (2015) Ecological impacts of ground cover weeds in New Zealand lowland forests. N Z J Ecol 39:50–60
O’Loughlin LS, Dane Panetta F, Gooden B (2021) Identifying thresholds in the impacts of an invasive groundcover on native vegetation. Sci Rep 11:20512. https://doi.org/10.1038/s41598-021-98667-5
Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, De VAC, Buchmann N, Funes G, Quétier F, Hodgson JG, Thompson K, Morgan HD, ter Steege H, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, Cornelissen JHC (2016) Corrigendum: New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 64:715–716. https://doi.org/10.1071/BT12225_CO
Petri L, Ibañez I (2023) Assessing the mechanisms and impacts of shrub invasion in forests: a meta-analysis. J Appl Ecol. https://doi.org/10.1111/1365-2664.14496
PlantNET (2022) New South Wales flora online. https://plantnet.rbgsyd.nsw.gov.au/
Posit team (2023). RStudio: integrated development environment for R. http://www.posit.co/
Pyšek P, Vojtêch J, Hulme PE, Pergl J, Hejda M, Schaffner U, Vilà M (2011) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Glob Chang Biol 18:1725–1737. https://doi.org/10.1111/j.1365-2486.2011.02636.x
R Core Team (2023). R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. https://www.R-project.org/.
Roberts J, Colloff MJ, Doody TM (2016) Riverine vegetation of inland south-eastern Australia. In: Capon S, James C, Reid M (eds) Vegetation of Australian riverine landscapes: biology. CSIRO Publishing, Melbourne, pp 177–199
Sodhi DS, Livingstone SW, Carboni M, Cadotte MW (2019) Plant invasion alters trait composition and diversity across habitats. Ecol Evol 9:6199–6210. https://doi.org/10.1002/ece3.5130
Sokol NW, Kuebbing SE, Bradford MA (2017) Impacts of an invasive plant are fundamentally altered by a co-occurring forest disturbance. Ecology 98:2133–2144. https://doi.org/10.1002/ecy.1906
Standish RJ, Robertson AW, Williams PA (2001) The impact of an invasive weed Tradescantia fluminensis on native forest regeneration. J Appl Ecol 38:1253–1263. https://doi.org/10.1046/j.0021-8901.2001.00673.x
Traveset A, Richardson DM (2014) Mutualistic interactions and biological invasions. Annu Rev Ecol Evol Syst 45:89–113. https://doi.org/10.1146/annurev-ecolsys-120213-091857
Vilà M, Tessier M, Suehs CM, Brundu G, Carta L, Galanidis A, Lambdon P, Manca M, Médail F, Moragues E, Traveset A, Troumbis AY, Hulme PE (2006) Local and regional assessments of the impacts of plant invaders on vegetation structure and soil properties of Mediterranean islands. J Biogeogr 33:853–861. https://doi.org/10.1111/j.1365-2699.2005.01430.x
Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708. https://doi.org/10.1111/J.1461-0248.2011.01628.X
Vila M, Pino J, Font X (2007) Regional assessment of plant invasions across different habitat types. J Veg Sci 18:35–42. https://doi.org/10.1111/j.1654-1103.2007.tb02513.x
Zhang P, Li B, Wu J, Hu S (2019) Invasive plants differentially affect soil biota through litter and rhizosphere pathways: a meta-analysis. Ecol Lett 22:200–210. https://doi.org/10.1111/ele.13181
Acknowledgements
This project was proudly funded by the NSW Government’s Environmental Trust. Vegetation surveys were undertaken with approval of site managers under NPWS Scientific Licence number SL102231. We would like to acknowledge and thank all natural resource managers (local government area biosecurity officers, Landcare, members of local ‘friends of’ groups, private landholders, and national parks rangers) for their assistance in monitoring site selection and access. We also wish to acknowledge John Lester for assisting with field work, Gavin Hunter, Caroline Delaisse and Celeste Linde for reviewing a draft of the manuscript, and Jedda Lemmon, Justin Mallee and Les Mitchell for assisting with the identification of some plant specimens from northern NSW. We also thank the anonymous reviewers for their comments and feedback on this manuscript.
Funding
Open access funding provided by CSIRO Library Services. This project was proudly funded by the NSW Government’s Environmental Trust (Project title: Biocontrol Research for Weed Management – Stage 3; Grant Number: 2019/MG/0012) as part of a project focused on the release and evaluation of the approved biological control agent (leaf-smut fungus Kordyana brasiliensis) for Tradescantia fluminensis in New South Wales (July 2020 to June 2023) (https://research.csiro.au/wandering-trad/background/).
Author information
Authors and Affiliations
Contributions
IZR: conceptualization, methodology, formal analysis, writing—original draft; BG: conceptualization, methodology, assistance with analysis, writing—reviewing and editing, supervision; LO: assistance with analysis, writing—reviewing and editing.
Corresponding author
Ethics declarations
Competing interest
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zeil-Rolfe, I., O’Loughlin, L. & Gooden, B. Habitat context and functional growth traits explain alien plant invader impacts on native vegetation communities. Biol Invasions (2024). https://doi.org/10.1007/s10530-024-03338-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10530-024-03338-x