Abstract
Expansion of global commerce has facilitated pathogen pollution via the transportation and translocation of invasive species and their associated parasites and pathogens. In Florida, imported cane toads (Rhinella horribilis) were accidentally and intentionally released on multiple occasions. Early populations were found to be infested with the invasive tick, Amblyomma rotundatum, yet it is unknown if these ticks dispersed with their hosts as cane toads spread throughout much of the state. The objectives of our investigation were to (1) determine if there are fewer tick infestations on toads at the periphery than at the core of their distribution as predicted by founder effect events, and (2) identify if ticks were infected with exotic pathogens. We captured toads from 10 populations across Florida. We collected ticks, vent tissue, and tick attachment site tissue from each toad, then tested samples for bacteria in the genus, Rickettsia. We found that 3/10 populations had toads that were infested with A. rotundatum, and infested individuals were in the earliest introduced populations at the core of their distribution. Pathogen testing confirmed Rickettisa bellii in ticks, but not in toad tissues. Haplotype networks could not clearly distinguish if R. bellii in Florida was more closely related to North or South American strains, but host-tick associations suggest that the pathogen was exotic to Florida. Our investigation demonstrated that an invasive species facilitated the introduction of parasites and pathogens into Florida, yet the invasive tick species encountered limitations to dispersal on this host species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The expansion of global trade and travel has created pathways for invasive species and accelerated their spread worldwide (Sakai et al. 2001; Van Kleunen et al. 2015; Capinha et al. 2017). Once introduced, species with traits that support rapid reproduction and dispersal can become successful invaders. Whether intentional or accidental, these introductions can directly or indirectly alter invaded ecosystems resulting in decreased biodiversity, ecosystem function, and ecosystem services (Floerl et al. 2009; Mooney and Cleland 2001).
One way invasive species alter ecosystems is via the co-introduction of non-native parasites and pathogens, i.e. pathogen pollution (Cunningham et al. 2003). An emerging focus of invasion ecology is the study of how host-parasite dynamics change with the introduction of invasive species to a novel area. Parasites and pathogens are ubiquitous in biological communities and can influence both the invasion success of non-native species and the fitness of native species (Prenter et al. 2004). Parasites and pathogens can negatively impact the fecundity of hosts via energy trade-offs that allocate the host’s energy to immune response instead of dispersal, growth, or reproduction (Luong et al. 2017), in turn regulating host population levels (Prenter et al. 2004).
Following the establishment of an invasive host species, parasites and pathogens may be distributed unevenly across the invasive host range due to a variety of ecological processes. Peripheral host populations, those that are on the leading edge of the expansion front, may have lower rates of parasitism due to the stochastic infestation rates of immigrant individuals which may lead to lower rates of parasitism in colonizing populations (Phillips et al. 2010; Barnett et al. 2018). Alternatively, but not mutually exclusively, core host populations, those that established early in the invasion process, may have more parasites than populations along the expanding peripheral edge because habitat at the site of establishment may be of higher quality than habitat in isolated, peripheral populations, leading to higher host and parasite densities at the distribution core. In addition, cross-species transmission of parasites from native or previously established non-native species to the invading species (spill-back) may also facilitate uneven or patchy distribution of parasitism across the landscape (Chalkowski et al. 2018). Thus, patterns of parasite distribution on invasive host species can provide insight into the underlying processes driving host-parasite relationships.
While some parasite populations may struggle to persist during the invasion process, those that successfully become established often share generalist phenotypic traits. Parasites that are host generalists (Ewen et al. 2012) can tolerate variable climatic conditions (Polo et al. 2021) and typically have simple life cycles that require few host species or can reproduce asexually. Many tick species (Ixodida) exemplify these traits and are frequently successful invaders (Barré and Uilenberg 2010). Multiple tick species have successfully invaded and established in the United States (Burridge 2011) including Amblyomma rotundatum, which originated from Central and South America. Amblyomma rotundatum is a 3-host tick species, yet the same host species may support all three tick life stages. A. rotundatum reproduces parthenogenically, which streamlines population growth by eliminating the need to search for a mate. Amblyomma rotundatum is the one of the most prolific generalist feeders reported in the literature, with 59 reported herpetological host species (De Jesus 2021) and 16 mammal host species (Guglielmone and Nava 2010). This combination of traits has made it a highly successful invasive species.
While it has been hypothesized that A. rotundatum was introduced into Florida with its native host, the cane toad (Rhinella horribilis formerly R. marina, Acevedo et al. 2016, Mittan-Moreau et al. 2022, Oliver et al. 1993), the origins of A. rotundatum in Florida are not well understood and are likely complicated. Multiple invasive reptile species that originated from Central and South America have been introduced in the decades after cane toads became established (Krysko et al. 2011; Nava et al. 2017) providing additional potential source populations of A. rotundatum, and other non-native reptiles including the Burmese python (Python bivittatus) from Southeast Asia and the Peter’s Rock agama (Agama picticauda) from Africa (Corn et al. 2014, pers. obs.) have been observed in Florida with A. rotundatum infestations. Additionally, cases of parasite spillover of A. rotundatum have been reported on native snake species like the southern black racer (Coluber constrictor priapus) in southernmost Florida and cottonmouth (Agkistrodon piscivorus conanti) (Hanson et al. 2007; Corn et al. 2014; Lillywhite and Sheehy 2019) from northwest peninsular Florida suggesting that this invasive tick species is distributed throughout Florida.
Cane toads are an infamous invasive species that were introduced into subtropical and tropical climates worldwide in failed biological control attempts for sugar cane beetles (King and Krakauer 1966; Lever 2001). Cane toads were originally introduced into Florida cane sugar fields in Palm Beach County during the 1930s and 1940s, although those populations did not become established (King and Krakauer 1966). Instead, cane toads likely became established in Florida in the 1950s after an accidental mass-release of toads imported to the Miami Airport from Colombia (Krakauer 1968; Oliver et al. 1993). Then in the 1960s, animal dealers intentional released additional cane toads from Colombia in Pembroke Park near Ft. Lauderdale in Broward County and from Suriname in Kendall near Miami in Miami-Dade County (King and Krakauer 1966). Thus, two populations were established approximately 60 km from each other in southeast Florida (Fig. 1). Since these introductions, cane toads have utilized canals and urbanized habitats to establish expanding populations across southern Florida and northwards into central Florida (Meshaka et al. 2006; Wilson 2016). Amblyomma rotundatum were first identified infesting invasive cane toads in Miami, Florida in the late 1970’s (Oliver et al. 1993).
Ticks are associated with numerous bacterial pathogens. Bacteria in the genus Rickettsia are the most ubiquitous tick-borne pathogen reported globally (Parola et al. 2013; Piotrowski and Rymaszewska 2020). Rickettsia bacteria vary in their pathogenicity from causing severe human and animal illnesses to nonpathogenic endosymbionts (Parola et al. 2013; El Karkouri et al. 2022). Rickettsia are likely candidates for pathogen pollution because of their unique transmission properties. Unlike some tick-borne pathogens, Rickettsia can be transmitted transovarially, i.e., adult female ticks transmit Rickettsia bacteria to their eggs and the resulting larvae emerge infected (Horta et al. 2006; Laukaitis and Macaluso 2021). The subsequent life stages can then maintain that infection between molts, referred to as transstadial transmission (Labruna 2009; Parola et al. 2013). Transovarial and transstadial bacterial transmission facilitate transmission from one life stage to another without the tick taking a bloodmeal from an infected host; therefore pathogens can be imported in infected ticks even if those ticks are infesting an uninfected host. All life stages disseminate Rickettsia (Parola et al. 2013), although some species of Rickettsia require infected hosts to maintain infection in the tick population. Nonetheless, transovarial transmission provides exotic rickettsial species with fewer barriers to overcome during the invasion process than bacteria with other transmission routes.
Rickettsia species have been reported in A. rotundatum within the cane toad’s native range. One commonly reported species is Rickettsia bellii (Labruna et al. 2004; Luz et al. 2018; Sánchez-Montes et al. 2019) which has been reported in 19 tick species across the Americas and is considered nonpathogenic (Krawczak et al. 2018). Rickettsia bellii infections are maintained by both transovarial and transstadial transmission. This species also has distinct clades for North and South American strains (Krawczak et al. 2018). Rickettsia bellii provides a unique model system to observe how Rickettsia respond to the biological invasions processes of their tick host.
Cane toads; the tick, Amblyomma rotundatum; and the bacterial species, Rickettsia belli provide a unique host-parasite-microbe system to empirically observe geographic patterns of parasite infestation and microbe infection that inform parasite ecology within the distribution of an invasive species. The objectives of our investigation were to (1) determine if core-periphery patterns of tick infestation rates occurred in cane toads. We predicted that there would be fewer tick infestations in toads at the periphery of the invasion than at the core of their distribution and (2) identify whether these invasive ticks were infected with exotic Rickettsia species not native to Florida and (3) determine if the pattern of a core-periphery distribution was maintained in the exotic bacteria found in ticks or cane toads. To conduct this study, we surveyed cane toad populations along a core to periphery distributional gradient for the presence of ticks. We then screened ticks and cane toads for infection with rickettsial bacteria.
Materials and methods
Cane toad and tick collection
We collected cane toads from 10 populations across their invasive range in Florida (Fig. 1) from April–June 2021. Sampling dates overlapped with the cane toad breeding season, which occurs from March through September in Florida (Krakauer 1968). We determined survey locations based on prior studies and citizen science reports through EDDMapS (Mittan & Zamudio 2019; Rubio et al. 2020; EDDMapS 2024). We conducted surveys near water bodies in urban areas at night, when and where cane toads actively forage (Wilson 2016). We captured toads by hand and placed them into individual ventilated plastic containers until ticks could be removed; animals were held in containers for < 24 h (University of Florida IACUC #202111387).
Toads were visually inspected for all life stages of attached ticks prior to euthanasia. If ticks were present, they were removed from the toad using fine tip forceps. Once removed, ticks were stored in 100% molecular-grade ethanol until they could be identified and extracted for DNA. We identified ticks to species morphologically using taxonomic keys from the United States, Central America, and South America (Keirans and Oliver 1993; Guzman-Cornejo et al. 2011; Nava et al. 2017).
After ticks were removed, toads were humanely euthanized through dermal application of 20% benzocaine gel (American Veterinary Medical Association 2020, IACUC #202111387). Following euthanasia, we collected morphological data (i.e., snout-urostyle length (SUL), mass, and sex) and tissue biopsies from all toads. When ticks were found on the toads, we took a skin biopsy at the site of tick attachment, referred to hereafter as attachment tissue. We collected vent tissue from all toads to assess if Rickettsia bacteria could be collected from highly vascularized tissues (Levin et al. 2016). Tissue samples were placed in 100% molecular grade ethanol and then stored at − 20 °C until they were extracted for DNA.
DNA extractions
Tick and tissue samples were rinsed once with PBS buffer and twice with DI water to remove debris or benzocaine gel before DNA was extracted. DNA was then extracted from ticks and cane toad tissue samples using the Qiagen Gentra Puregene Kit (Valenica, CA, USA) with the manufacturer protocol. We extracted DNA from individual adults and nymphal ticks, but aggregated larvae into pools of 25 ticks per host. We cut vent and attachment site tissue into 10 ng pieces before extraction (Qiagen 2014). We stored eluted DNA at − 20 °C until PCR protocols were conducted.
PCR and sequencing
To screen for Rickettsia, we initially targeted the gltA gene, as it can broadly detect Rickettsia species (Roux et al. 1997). We further analyzed Rickettsia positive samples using primers that amplified portions of two additional genes: atpA and coxA because they have previously been shown to successfully amplify R. bellii (Weinert et al. 2009; Krawczak et al. 2018). All three genes were used to differentiate between strains of R. bellii. We ran a positive and negative control for each PCR assay. We used a positive control from a Rickettsia sp. collected from an Ixodes scapularis specimen. Our negative control was PCR grade water. All PCR products were run on a 1.5% agarose gel with RedView Stain (Genecopoeia, Rockville, MD) and visualized on UVP gel documentation system (Analytik-Jena, Beverly, MA). We considered samples positive if they had the appropriate band size. All PCR products were cleaned with SAP/Exonuclease and sent to a commercial lab for Sanger sequencing (Functional Biosciences, WI, USA). Consensus sequences were constructed from forward and reverse primers for atpA and gltA. For coxA only forward sequences were used due to a bacterial coinfection of Chryseobacterium sp. that was co-amplified with the reverse primer (Supplementary Materials). Sequences were then aligned using Geneious software (2019.1.3) (Biomatters Ltd., Auckland, New Zealand) and compared to sequences in GenBank using NCBI BLAST.
Phylogenetic and network analyses
We concatenated and assembled our three gene sequences in Geneious and compared them with sequence data from R. bellii isolates and field collected specimens deposited in GenBank (OP650115–OP650207). We aligned our samples with GenBank samples using ClustalW (Thompson et al. 1994) in MEGA X (Kumar et al. 2018) software. Each alignment was visually examined to make sure all sequences aligned.
To determine the relationship between our Rickettsia samples and other Rickettisial species, we constructed a tree using the Tamura-Nei genetic distance model and the UPGMA tree building method with 500 bootstrap. We then examined each target gene and all 3 genes concatenated in a minimum spanning network in PopArt (Leigh and Bryant 2015) in order to compare our samples from haplotypes in North America and South America. Additional sequences from Krawczak et al. (2018) and GenBank (Supplementary Table 1) supplemented the haplotype network.
Results
Cane toad and tick collections
We collected a total of 234 cane toads from 10 populations across south and central Florida. Surveys yielded 54 females and 180 males, including 212 adult toads and 22 juveniles. Snout-urostyle length ranged from 6.8 to 13.3 cm with an average of 10.8 ± 1.6 cm. Of all cane toads collected, 56/234 (23.9%) toads were infested with ticks (Table 1). Toads that were infested with ticks were collected from 3/10 populations: Homestead, Miami, and West Palm Beach (Fig. 1). Among the three infested toad populations, ticks were found on toads 60.0% (57/95 toads) of the time. All ticks were identified as A. rotundatum. In total we collected 495 A. rotundatum and toads were infested with 5.2 ± 1.5 ticks/toad (Table 1).
Rickettsia screening
We tested ticks and tissue from vent and attachment sites for the presence of Rickettsia bacteria. Of the toads infested with ticks, 27/56 toads (48.2%) had ticks infected with Rickettsia (Table 2). Homestead, Miami, and West Palm Beach all had ticks with rickettsial infections. We did not detect Rickettsia in any vent or tick attachment tissue samples.
Phylogenetic and haplotype network analyses
Examining the gltA gene we found that our samples grouped with R. bellii and not with other rickettsial species (Fig. 2). We found that all three target genes from A. rotundatum also closely matched with R. bellii sequences from both North and South America (Table 3).
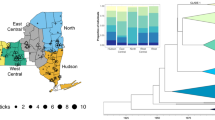
A phylogenetic tree for the gltA gene of Rickettsia species was constructed using the Tamura-Nei genetic distance model and the UPGMA tree building method with 500 bootstrap. Sequences from this study are designated as Tick #. Previously published sequences are listed by Rickettsia species and accession number. Blue dots indicate R. bellii collected from South America, and red dots are North America
Using the minimum spanning network based on the atpA gene, we found that our samples shared the same haplotype as two samples from South America detected in Amblyomma dissmile (Fig. 3) (Supplementary Materials Table 1). However, there was not a clear distinction between North and South American haplotypes for any sequence or when all three sequences were concatenated (Fig. 3).
Discussion
Our investigation aimed to determine whether tick infestation in cane toads and bacterial infection in ticks decreased from the core to the periphery of the cane toad’s distribution in Florida. We found evidence that cane toads at sites near the initial establishment were infested with A. rotundatum. Cane toads collected from Homestead, Miami, and West Palm Beach were infested with A. rotundatum (3/10 locations surveyed). These locations were along the south Atlantic coast of Florida where toad populations were introduced in the 1950’s and 1960’s (King and Krakauer 1966; Krakauer 1968; Wilson 2016). Interestingly, cane toads from Fort Lauderdale lay between two infested populations yet this population did not have ticks, which exemplifies the stochastic nature of ectoparasite infestation among host populations. None of the cane toad populations > 120 km from the core of the introduction contained toads that were infested with ticks. In relation to the core population, these uninfested, peripheral populations lay to the west on Gulf Coast side of Florida or to the north in West Palm Beach. Cane toad populations in these locations did not become established until the early 2000’s and were geographically distant from the founder populations (Wilson 2016). The lack of tick infestation along the invasion front is consistent with founder effects and provides support for the Enemy Release Hypothesis (Torchin et al. 2003). Invasive species can establish populations without parasites via stochastic processes or lose their parasites as they advance along the expansion front due to low host densities or unfavorable environmental conditions for the invasive tick species (Torchin et al. 2003; Phillips et al. 2010).
Unfavorable environmental conditions for parasites at the periphery of an expanding distribution can further facilitate founder effects, but we did not find evidence for this additive effect. We hypothesized that because cane toads in Florida have been shown to prefer disturbed and urbanized habitats that may hinder tick survival (Meshaka et al. 2006; Wilson 2016), it could limit the expansion of this tick species on cane toads. Amblyomma rotundatum is a 3-host tick, meaning it must drop from its host after feeding, then molt and wait for another bloodmeal. The environment into which ticks drop is crucial to their survival because ticks spend more than 99% of their lifetime off-host (Needham 1991, Diuk-Wasser et al. 2021). Cane toads are commonly found in manmade ponds, canals, and residential yards (Meshaka et al. 2006; Wilson 2016, pers. Obs.), and these urban habitats may not have the appropriate microclimate to prevent tick desiccation (Needham 1991). Urbanized habitats often lack preferred vegetation cover, moist soil, or leaf litter where ticks can take cover (Burtis et al. 2019; Diuk-Wasser et al. 2021). Studies in French Guiana (part of the native range of both cane toads and their ticks) have documented that urbanized habitat led to a loss of ticks on cane toads despite having sufficient toad populations to support tick transmission. In these cases, abiotic constraints in the urban environment prevented ticks from establishing (Devore et al. 2020). Nonetheless, we found ticks in the heavily urbanized core population of cane toads that have been established for more than 60 years, suggesting that local environmental conditions were not the driving factor limiting expansion on cane toad populations.
The core to periphery gradient in tick infestation of cane toads did not support the hypothesis that this distribution was driven by spillback of A. rotundatum to toads. The widespread distribution of this tick on multiple reptile hosts sympatric to peripheral cane toad populations provided opportunity for spillback in the northern and western populations of cane toads, yet these populations were uninfested. Because the pattern of infestation was structured along a core to periphery gradient, it was unlikely a result of spillback of ticks from other established or native species. Indeed, cane toads were likely the initial hosts and one of the dispersal vectors of A. rotundatum that provided the mechanism for spillover of this tick to multiple native and non-native reptile species throughout Florida (Oliver et al. 1993).
We found evidence of a likely exotic bacterial microbe throughout the tick populations that we sampled. We detected R. bellii in A. rotundatum at all three field sites where ticks were found. Thus, while tick dispersal among host toad populations may have been hindered, it appears that Rickettsia have successfully dispersed with their tick vector as predicted by their endosymbiont status and transovarial and transstadial transmission routes.
Previous investigations into the phylogenetics of R. bellii found that isolates collected from North America and South America formed separate clades and grouped together by tick genus and host preference (Krawczak et al. 2018). In North America, R. bellii has primarily been reported in Dermacentor species (Krawczak et al. 2018), while in South America it has been shown to infect ticks in the genera Ixodes, Haemaphysalis and Amblyomma (Krawczak et al. 2018). Even though North American and South America Rickettsia bellii form distinct clades, there are few polymorphisms that differentiate isolates (< 0.5%) (Krawczak et al 2018). We sequenced three rickettsial genes that each contained polymorphisms found in R. bellii. The atpA gene matched closely to Rickettsia sequences from Amblyomma dissimile in French Guiana, another avid reptile feeder in South America (Table 3). Vector-pathogen affiliation and haplotype data from this atpA gene suggest R. bellii originated from South America. However, the coxA and gltA genes resembled strains recovered from different tick species and locations (Table 3). This combination of gene sequence data could not clarify whether or not our R. bellii originated from South America. Thus, while the atpA gene is suggestive of a South American origin, further genomic analysis is needed to definitively determine the origin of R. bellii in Florida.
Studies of Rickettsia and toads thus far have only identified pathogens in ticks but not toad tissues (Luz et al. 2013; Horta et al. 2006). We did not detect Rickettsia species in attachment site or vent tissue collected from cane toads in our study. Tick attachment tissue examined in previous studies on cane toads in South America found that A. rotundatum bites can result in skin lesions (Luz et al. 2013). Whether or not these lesions can harbor tick-borne pathogens is still unknown. Future studies should collect tissue from lesions and attachment sites and test them for tick-borne pathogens.
Conclusions
Overall, we found that A. rotundatum infests cane toads in areas < 120 km from the initial introduction sites in southern Florida, but not in populations further north or west. Cane toad populations in Homestead and Miami, sites at or near the initial introductions, continue to have tick infestations with A. rotundatum. The expansion of this tick species on cane toads into peripheral populations may have been restricted by demographic stochasticity associated with founder events rather than changes in habitat conditions from core to periphery. The core to periphery decline in infestation suggests that these processes are a more likely explanation than spill-back from established host populations because peripheral populations were sympatric with other reptiles hosting A. rotundatum. Our surveys found that A. rotundatum from cane toads were frequently infected with Rickettsia bellii, which was likely a South American strain that arrived in Florida with cane toads. This invasion system provides insight into how Rickettsia bacteria can be successfully transported during a host invasion and maintained during the expansion phase. Because many Rickettsia species are pathogenic, these findings suggest that pathogen pollution with exotic Rickettsia can impact human or animal welfare globally.
Data availability
Sequence data with metadata have been deposited in NCBI Genbank.
References
Acevedo AA, Lampo M, Cipriani R (2016) The cane or marine toad, Rhinella marina (Anura, Bufonidae): two genetically and morphologically distinct species. Zootaxa 4103(6):574–586. https://doi.org/10.11646/zootaxa.4103.6.7
American veterinary medical association. (2020). AVMA guidelines for the euthanasia of animals: 2020.0.1 edition. 121 pp
Barnett LK, Phillips BL, Heath ACG, Coates A, Hoskin CJ (2018) The impact of parasites during range expansion of an invasive gecko. Parasitology 145(11):1400–1409. https://doi.org/10.1017/S003118201800015X
Barré N, Uilenberg G (2010) Spread of parasites transported with their hosts: case study of two species of cattle tick. Rev Sci Tech 29(1):149
Burridge, MJ (2011) Non-native and invasive ticks. University Press of Florida
Burtis JC, Yavitt JB, Fahey TJ, Ostfeld RS (2019) Ticks as soil-dwelling arthropods: an intersection between disease and soil ecology. J Med Entomol 56(6):1555–1564. https://doi.org/10.1093/jme/tjz11
Capinha C, Seebens H, Cassey P, García-Díaz P, Lenzner B, Mang T, Moser D, Pyšek P, Rödder D, Scalera R, Winter M, Dullinger S, Essl F (2017) Diversity, biogeography and the global flows of alien amphibians and reptiles. Divers Distrib 23(11):1313–1322. https://doi.org/10.1111/ddi.12617
Chalkowski K, Lepczyk CA, Zohdy S (2018) Parasite ecology of invasive species: conceptual framework and new hypotheses. Trends Parasitol 34(8):655–663. https://doi.org/10.1016/j.pt.2018.05.008
Corn JL, Mertins JW, Hanson B, Snow S (2014) First reports of ectoparasites collected from wild-caught exotic reptiles in Florida. J Med Entomol 48(1):94–100. https://doi.org/10.1603/ME10065
Cunningham AA, Daszak P, Rodriguez JP (2003) Pathogen pollution: defining a parasitological threat to biodiversity conservation. J Parasitol 89(Suppl):S78–S83
DeVore JL, Shine R, Ducatez S (2020) Urbanization and translocation disrupt the relationship between host density and parasite abundance. J Anim Ecol 89(4):1122–1133. https://doi.org/10.1111/1365-2656.13175
Diuk-Wasser MA, VanAcker MC, Fernandez MP (2021) Impact of land use changes and habitat fragmentation on the eco-epidemiology of tick-borne diseases. J Med Entomol 58(4):1546–1564. https://doi.org/10.1093/jme/tjaa209
EDDMapS. 2024. Early Detection & Distribution Mapping System. The University of Georgia - Center for Invasive Species and Ecosystem Health
El Karkouri K, Ghigo E, Raoult D, Fournier PE (2022) Genomic evolution and adaptation of arthropod-associated Rickettsia. Sci Rep 12(1):1–15. https://doi.org/10.1038/s41598-022-07725-z
Ewen JG, Bensch S, Blackburn TM, Bonneaud C, Brown R, Cassey P, Clarke RH, Pérez-Tris J (2012) Establishment of exotic parasites: the origins and characteristics of an avian malaria community in an isolated island avifauna. Ecol Lett 15(10):1112–1119. https://doi.org/10.1111/j.1461-0248.2012.01833.x
Floerl O, Inglis GJ, Dey K, Smith A (2009) The importance of transport hubs in stepping-stone invasions. J Appl Ecol 46(1):37–45. https://doi.org/10.1111/j.1365-2664.2008.01540.x
Guglielmone AA, Nava S (2010) Hosts of Amblyomma dissimile Koch, 1844 and Amblyomma rotundatum Koch, 1844 (Acari: Ixodidae). Zootaxa. https://doi.org/10.11646/ZOOTAXA.2541.1.2
Guzman-Cornejo C, Robbins RG, Guglielmone AA, Montiel-Parra G, Pérez TM (2011) The Amblyomma (Acari: Ixodida: Ixodidae) of Mexico Identification keys, distribution and hosts. Zootaxa 2998(1):16–38. https://doi.org/10.11646/ZOOTAXA.2998.1.2
Hanson BA, Frank PA, Mertins JW, Corn JL (2007) Tick paralysis of a snake caused by Amblyomma rotundatum (Acari: Ixodidae). J Med Entomol 44(1):155–157. https://doi.org/10.1093/jmedent/41.5.155
Horta MC, Pinter A, Schumaker TTS, Labruna MB (2006) Natural infection, transovarial transmission, and transstadial survival of Rickettsia bellii in the tick Ixodes loricatus (Acari: Ixodidae) from Brazil. Ann N Y Acad Sci 1078:285–290. https://doi.org/10.1196/annals.1374.053
De Jesus, C.E. 2021. Surveillance and ecology of tick-borne pathogens and tick-host associations of reptiles and amphibians in Florida. Dissertation Thesis. University of Florida
Keirans JE, Oliver JH Jr (1993) First description of the male and redescription of the immature stages of Amblyomma rotundatum (Acari: Ixodidae), a recently discovered tick in the U.S.A. J Parasitol 79(6):860–865. https://doi.org/10.2307/3283722
King W, Krakauer T (1966) The exotic herpetofauna of Southeast Florida. Q J Fla Acad Sci 29(2):144–154
Krakauer T (1968) The ecology of the neotropical toad, Bufo marinus. South Fla Herpetol 24(3):214–221
Krawczak FS, Labruna MB, Hecht JA, Paddock CD, Karpathy SE (2018) Genotypic characterization of Rickettsia bellii reveals distinct lineages in the United States and South America. Biomed Res Int 2018:8505483. https://doi.org/10.1155/2018/8505483
Krysko KL, Burgess JP, Rochford MR, Gillette CR, CuevaD Enge KM, Somma LA, Stabile JL, Smith DC, Wasilewski JA, Others, (2011) Verified non-indigenous amphibians and reptiles in Florida from 1863 through 2010: outlining the invasion process and identifying invasion pathways and stages. Zootaxa 3028(1):1–64. https://doi.org/10.11646/zootaxa.3028.1.1
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549. https://doi.org/10.1093/molbev/msy096
Labruna MB (2009) Ecology of Rickettsia in South America. Ann N Y Acad Sci 1166(1):156–166. https://doi.org/10.1111/j.1749-6632.2009.04516.x
Labruna MB, Whitworth T, Bouyer DH, McBride J, Camargo LMA, Camargo EP, Popov V, Walker DH (2004) Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the state of Rondônia, Western Amazon. Brazil J Med Entomol 41(6):1073–1081. https://doi.org/10.1603/0022-2585-41.6.1073
Laukaitis HJ, Macaluso KR (2021) Unpacking the intricacies of Rickettsia–vector interactions. Trends Parasitol 37(8):734–746. https://doi.org/10.1016/j.pt.2021.05.008
Leigh JW, Bryant D (2015) Popart: full-feature software for haplotype network construction. Methods Ecol Evol 6(9):1110–1116. https://doi.org/10.1111/2041-210x.12410
Lever C (2001). The cane toad: The history and ecology of a successful colonist. Westbury Academic & Scientific Pub
Levin ML, Snellgrove AN, Zemtsova GE (2016) Comparative value of blood and skin samples for diagnosis of spotted fever group rickettsial infection in model animals. Ticks Tick Borne Dis 7(5):1029–1034. https://doi.org/10.1016/j.ttbdis.2016.05.011
Lillywhite HB, Sheehy CM III (2019) The unique insular population of cottonmouth snakes at seahorse key. In: Lillywhite H, Martins M (eds) Islands and snakes: isolation and adaptive evolution. Oxford University Press. Islands and Snakes, In Oxford University Press, New York, p 400
Luong LT, Horn CJ, Brophy T (2017) Mitey costly: energetic costs of parasite avoidance and infection. Physiol Biochem Zool 90(4):471–477. https://doi.org/10.1086/691704471
Luz HR, Faccini JLH, Pires MS, da Silva HR, Barros-Battesti DM (2013) Life cycle and behavior of Amblyomma rotundatum (Acari: Ixodidae) under laboratory conditions and remarks on parasitism of toads in Brazil. Exp Appl Acarol 60(1):55–62. https://doi.org/10.1007/s10493-012-9628-8
Luz HR, Silva-Santos E, Costa-Campos CE, Acosta I, Martins TF, Muñoz-Leal S, McIntosh D, Faccini JLH, Labruna MB (2018) Detection of Rickettsia spp. in ticks parasitizing toads (Rhinella horribilis) in the northern Brazilian Amazon. Experimental and Applied Acarology 75(3):309–318. https://doi.org/10.1007/s10493-018-0270-y
Meshaka WE, DeVane J, Marshall SD (2006) An island of cane toads (Bufo marinus) in an ocean of xeric uplands in south-central Florida. Fla Sci 69(3):169–176
Mittan-Moreau CS, Kelehear C, Toledo LF, Bacon J, Guayasamin JM, Snyder A, Zamudio KR (2022) Cryptic lineages and standing genetic variation across independent cane toad introductions. Mol Ecol 31(24):6440–6656. https://doi.org/10.1111/mec.16713
Mittan CS, Zamudio KR (2019) Rapid adaptation to cold in the invasive cane toad Rhinella marina. Conservation Physiol 7(1):coy075. https://doi.org/10.1093/conphys/coy075
Mooney HA, Cleland EE (2001) The evolutionary impact of invasive species. Proc Natl Acad Sci U S A 98(10):5446–5451. https://doi.org/10.1073/pnas.091093398
Nava S, Venzal JM, Acuña DG, Martins TF, Guglielmone AA (2017) Ticks of the Southern Cone of America: diagnosis, distribution, and hosts with taxonomy. Academic Press, Ecology and Sanitary Importance
Needham GR, Teel PD (1991) Off-host physiological ecology of ixodid ticks. Annu Rev Entomol 36:659–681. https://doi.org/10.1146/annurev.en.36.010191.003303
Oliver JH, Hayes MP, Keirans JE, Lavender DR (1993) Establishment of the foreign parthenogenetic tick Amblyomma rotundatum (Acari: Ixodidae) in Florida. J Parasitol 79:786–786
Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, Abdad MY, Stenos J, Bitam I, Fournier PE, Raoult D (2013) Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 26(4):657–702. https://doi.org/10.1128/CMR.00032-13
Phillips BL, Brown GP, Shine R (2010) Life-history evolution in range-shifting populations. Ecology 91(6):1617–1627. https://doi.org/10.1890/09-0910.1
Piotrowski M, Rymaszewska A (2020) Expansion of tick-borne rickettsioses in the world. Microorganisms 8(12):1906. https://doi.org/10.3390/microorganisms8121906
Polo G, Luz HR, Regolin AL, Martins TF, Winck GR, da Silva HR, Onofrio VC, Labruna MB, Faccini JLH (2021) Distribution modeling of Amblyomma rotundatum and Amblyomma dissimile in Brazil: estimates of environmental suitability. Parasitol Res 120(3):797–806. https://doi.org/10.1007/s00436-020-06924-9
Prenter J, Macneil C, Dick JTA, Dunn AM (2004) Roles of parasites in animal invasions. Trends Ecol Evol 19(7):385–390. https://doi.org/10.1016/j.tree.2004.05.002
Qiagen. (2014). Gentra® Puregene® Handbook.
Roux V, Rydkina E, Eremeeva M, Raoult D (1997) Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int J Syst Bacteriol 47(2):252–261. https://doi.org/10.1099/00207713-47-2-252
Rubio AO, French CM Catenazzi A (2020) Morphological correlates of invasion in Florida cane toad (Rhinella marina) populations: Shortening of legs and reduction in leg asymmetry as populations become established. Acta oecologica 109:103652. https://doi.org/10.1016/j.actao.2020.103652
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Annu Rev Ecol Syst 32(1):305–332. https://doi.org/10.1146/annurev.ecolsys.32.081501.114037
Sánchez-Montes S, Isaak-Delgado AB, Guzmán-Cornejo C, Rendón-Franco E, Muñoz-García CI, Bermúdez S, Morales-Diaz J, Cruz-Romero A, Romero-Salas D, Dzul-Rosado K, Lugo-Caballero C, Colunga-Salas P, Becker I (2019) Rickettsia species in ticks that parasitize amphibians and reptiles: novel report from Mexico and review of the worldwide record. Ticks Tick-Borne Dis 10(5):987–994. https://doi.org/10.1016/j.ttbdis.2019.04.013
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673–4680. https://doi.org/10.1093/nar/22.22.4673
Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM (2003) Introduced species and their missing parasites. Nature 421(6923):628–630. https://doi.org/10.1038/nature01346
van Kleunen M, Dawson W, Maurel N (2015) Characteristics of successful alien plants. Mol Ecol 24(9):1954–1968. https://doi.org/10.1002/9781119072799.ch3
Weinert LA, Werren JH, Aebi A, Stone GN, Jiggins FM (2009) Evolution and diversity of Rickettsia bacteria. BMC Biol 7(1):1–15. https://doi.org/10.1186/1741-7007-7-6
Wilson, A. C. (2016). Distribution of cane toads (Rhinella horribilis) in Florida and their status in natural areas. PhD Thesis. University of Florida
Acknowledgements
Special thanks to Yasmin Tavares, Chanakya Bhosale, Nathan Fox, and Natalie Pegg for assisting with cane toad collections. Thanks to Kylie Mendoza, Amber Hanna, Dylan Withee, Nathan Fox, and Samuel Scherneck for assisting with toad biopsies and data collection. Thanks for Savannah Cantrell for assisting with data entry.
Author information
Authors and Affiliations
Contributions
CED coordinated the research project, collected toads, conducted pathogen testing, conducted phylogenetic analyses, wrote, and edited manuscript. MH coordinated the research project, collected toads, collected ticks, conducted toad biopsies, wrote, and edited the manuscript. AS assisted with toad biopsies and edited the manuscript. SB assisted with collection of cane toads and edited the manuscript. CMR supervised the research project, acquired funding, and edited the manuscript. SMW supervised the research project, acquired funding, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial or non-financial conflicts of interest.
Human and animal rights
Collection methods for cane toads and ticks was approved by University of Florida Institutional Animal Care and Use Committee (#202111387).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Jesus, C.E., Harman, M.E.A., Sutton, A. et al. Spatially limited pathogen pollution in an invasive tick and host system. Biol Invasions 26, 2037–2047 (2024). https://doi.org/10.1007/s10530-024-03291-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-024-03291-9