Abstract
Novel interactions between invaders and native species that have evolved in their absence may impose strong selective pressures that drive species to extinction or prompt rapid co-evolution and learning. Here, we report on the effects that invasive cane toads, a toxic prey species, have had on freshwater crocodile populations in 7 waterholes of the Victoria River, Australia, before and up to 14 years after toads invaded. We recorded observations of crocodiles attacking toads, dissected dead crocodiles to determine if they had eaten toads and indexed the abundances of cane toads, live crocodiles and dead crocodiles. Following toad-invasion we observed crocodiles attacking cane toads. Dead crocodiles were only observed following the invasion of toads and 62% of the 71 dead crocodiles we dissected had toads in their stomachs. Counts of dead crocodiles showed a humped relationship with time since toad invasion and declined markedly after 3 years post-toad invasion. Live crocodile abundance declined sharply following toad-invasion, but this decline attenuated approximately 4 years post-invasion. The pulse of crocodile mortality and attenuation of the rate of crocodile population decline suggests that crocodiles have evolved or learned to enable co-existence with toads. However, crocodile populations have shown no sign of recovery in the 8–14 years post toad invasion. Our findings highlight that adaptation by native species to the presence of invaders may be imperfect and thus may not necessarily entail numerical recovery of populations to pre-invasion levels, but instead downward shifts to new equilibria due to ongoing interactions with invaders.
Similar content being viewed by others
Introduction
Invasive species rank as one of the greatest threats to biodiversity. Novel interactions between invaders and predatory species that have evolved in their absence may impose strong selective pressures that can drive species to extinction or prompt rapid evolutionary change (Savidge 1987; Suarez and Tsutsui 2008). However, despite widespread recognition that invasive species often have profound impacts on native species (Mack et al. 2000; Vitousek et al. 1996), most studies on the ecological effects of invaders have been of short duration or have focused on single time points during the invasion (Strayer et al. 2006). Consequently, for most ecological systems there is little information on how interactions between native species and invaders change over time (Strayer et al. 2006). Hence, the true extent and time-course of invasive species’ impacts can be difficult to deduce (Parker et al., 1999; Sanders et al., 2003, Dominguez Almela et al. 2021).
If invaders impose strong selection pressures on native vertebrates, it is likely that their impacts on native species will change over time as a result of co-evolution and learning (Mooney and Cleland 2001; Strayer et al. 2006) Similarly, the traits of invaders and their populations may change through time due to adaptation to their new environment and density dependent processes such as competition and parasitism (Alves et al. 2019; Phillips et al. 2008). Consequently, studies investigating the impacts of invasive species at the time of the invasion may yield very different results to those that are undertaken long-after (Strayer et al. 2006).
The cane toad Rhinella marina L. is an anuran native to South America that has been progressively invading Australia since they were introduced in the 1930s (Shine 2010). Many native Australian predators lack evolutionary exposure to toads and thus have little physiological resistance to the novel toxins possessed by cane toads (Ujvari et al. 2013a, 2013b). Consequently, many predators, including northern quolls, varanid lizards, freshwater crocodiles, and snakes die after attacking or consuming cane toads (Covacevich and Archer 1975; Smith and Phillips 2006). The invasion of cane toads has caused significant population declines of goannas (Varanus spp.), northern quolls (Dasyurus hallucatus) and freshwater crocodiles (Crocodylus johnstoni) (Doody et al. 2009; Letnic et al. 2008; Ujvari and Madsen 2009; Woinarski et al. 2010). However, no native predators have gone extinct as a result of toad invasion (Shine 2010).
Nethertheless, understanding of the cane toad’s longer-term impacts is limited because few studies have assessed predator populations for extended periods post-toad invasion (Doody et al. 2017; Fukuda et al. 2016). Evolutionary theory predicts that when novel prey are toxic, natural selection should favour traits in predators that result in avoidance of toxic prey or physiological resistance to novel toxins (Brodie and Brodie 1999; Carlsson et al. 2009). Evidence that toads have imposed selective pressure on native predators is provided by a study conducted well after toad invasion showing that red-bellied black snakes (Pseudechis porphyriacus) and green tree snakes (Dendrelaphis punctulatus, Colubridae) have smaller heads in areas long-colonised by toads than in areas where cane toads were absent (Phillips and Shine 2006). The shift in head size of snakes was assumed to be a product of selection against animals with larger gapes capable of consuming large toads. Similarly, varanid lizards with a prediliction for consuming amphibians before the invasion of toads were more likely to die as a result of consuming cane toads post-invasion than individuals that did not consume amphibians (Ward‐Fear et al. 2020). Predators may also learn to avoid cane toads via conditioned taste aversion. For example, laboratory studies show that planigales (Planigale maculata), freshwater crocodiles and northern quolls that ingest cane toads may subsequently avoid attacking toads (O’Donnell et al. 2010; Somaweera et al. 2011; Webb et al. 2008).
If the invasion of toads imposes selective pressure on a predator population, it should operate like a filter that removes individuals that are most susceptible to being killed by toads. Once selection has occurred, populations of native predators should be able to coexist and thus achieve a new demographic equilibrium with cane toads (Shine 2010). However, the paucity of long-term studies makes it difficult to predict what this new equilibrium should look like. In theory, it is possible that predator populations could recover to pre-invasion levels if toads are no longer a significant source of mortality. In contrast, if adaptation is imperfect, then predator populations should not fully recover. For example, populations of varanid lizards and northern quolls have shown little sign of recovery to pre-toad levels in the post-toad era (Doody et al. 2017; Jolly et al. 2018). Thus, invaders may have chronic effects on populations of native species (Strayer et al. 2006).
Here, we report on the time-course of the impacts that the invasion of cane toads has had on local populations of an endemic Australian predator, the freshwater crocodile, using data collected before and up to 14 years following the invasion of toads on the Victoria River in Australia’s Northern Territory (Fig. 1). Freshwater crocodiles prey upon cane toads (Fig. 2a), are susceptible to cane toad toxins (Fig. 2c) and their populations have declined following the invasion of toads (Britton et al. 2013; Fukuda et al. 2016; Letnic et al. 2008). In this study, we documented the date of arrival and abundance of cane toads, interactions between crocodiles and cane toads, mortality rates of crocodiles and the abundance of crocodiles before and after toads invaded. Because cane toad-induced mortality could impose strong selection on crocodiles to have greater resistance to toad toxins or avoid toads, and exposure to toads could result in crocodiles learning to avoid toads, we hypothesised: (1) that evidence of crocodile mortality would initially increase following the invasion of toads and then decline because surviving crocodiles would be less likely to be killed by toads; and (2) that crocodile populations would initially decline following the invasion of toads and then begin to recover with increasing time since invasion.
Map showing location of survey sites (open circles), the town of Kalkarindji (closed circle) and date of toad invasion at sites along the Victoria River where crocodile populations were monitored before and after the invasion of cane toads. The inset shows the location of the Victoria River catchment (shaded) in Australia
a A small (approximately 100 cm TL) freshwater crocodile holding a cane toad with toxic secretions exuding from its parotid gland illustrated with arrow. b A large (220 cm TL) dead crocodile that was subsequently dissected. The crocodile’s stomach contained a cane toad. c) A small (approximately 90 cm TL) crocodile on the edge of the river. Six toads (circled) are also present on the river’s edge in this photograph
Materials and methods
Study area
We surveyed crocodile populations in seven sections of the Victoria River in the Northern Territory, Australia (Fig. 1; Table 1). The climate of the Victoria River District of the Northern Territory is semi-arid and monsoonal and is characterised by a hot humid wet season (December–March) and a hot dry season (April–November). On average, 286 days exceed 30 °C each year at Victoria River Downs (16° 24′ 00″ S, 131° 00′ 36″), located 10 km from our Victoria River/Wickham River Junction survey site (Australian Bureau of Meteorology 2019). The Victoria River has a highly seasonal flow regime, with peak flows during the wet season (December-March) when monsoonal rains bring > 80% of the annual precipitation. During the late dry-season, the upper reaches of the Victoria River consist of a series of deep and permanent pools, separated by exposed sand or rock bars. A semi-arid landscape dominated by savannah grassland surrounds the Victoria River in this region.
We conducted surveys for crocodiles and toads in seven permanent pools in the upper reaches of the Victoria River where navigable water is present throughout the dry season. The survey sections (Fig. 1) from downstream to upstream were Victoria Gorge, Victoria River-Wickham River Junction (hereafter referred to as Wickham), Pigeonhole, Longreach Lagoon, Rifle Hole, an unnamed waterhole (hereafter referred to as No Name), and Mucka (Fig. 1). The width of the river in each survey section ranged from 20 to 70 m. The mean annual rainfall received at the survey sections decreased from north to south and was greatest at Victoria River Gorge (947 mm, Victoria River Road House; 15.62° S, 13.13° E) and least at Mucka (612 mm at Riveren 17° 54′ 14° S, 130 13′ 37″ E, source, Australian Bureau of Meteorology).
Cane toad arrival and density
During the period (2005–2011) when toads were invading the study area, we conducted surveys of water-bodies throughout the Victoria River catchments for cane toads (Letnic et al. 2014) and consulted residents living along the length of the Victoria River catchment to ascertain if and when cane toads had invaded the area where they live and work. We consider residents’ accounts to be reliable because the invasion of toads was heralded by considerable media coverage and because cane toads are readily distinguishable from native frogs, easily visible on roads at night, and frequently occupy residential areas where they are readily observed because they congregate around sources of water such as garden taps. Cane toads also congregate and are readily observable at artificial watering points such as dams and bore-holes which are used to provide water for cattle (Letnic et al. 2015). These water points are inspected at least weekly by staff on pastoral properties.
For crocodile surveys conducted after September 2008, we quantified the abundance of cane toads on the river-bank (Fig. 2c) in each waterhole. Within each survey section, we counted cane toads within thirty 2 m × 2 m quadrats on the bank (from water’s edge) at random locations along the waterhole. The distances of quadrats from the starting point of each survey were generated before surveys using a random number generator. These locations were converted to coordinates in GIS software and then uploaded into a global positioning system. During surveys we used a GPS to direct us to survey locations.
Mortality of crocodiles
During all surveys we conducted daytime surveys from a boat to detect crocodiles that had died recently due to cane toad ingestion in each survey section. Because dead crocodiles float with the white ventral surface uppermost, they are easily spotted from a boat (Letnic et al. 2008). For each dead crocodile, we classified it as fresh (recently) dead if the flesh of the crocodile was soft and putrid, and as skeletal remains if the flesh was dried and hard or if the remains of the crocodile consisted simply of a skeleton or skull. Where possible we recorded the head length, snout-vent length and total length of dead crocodiles with a tape measure. None of the fresh dead crocodiles had any obvious signs of trauma or injuries (puncture wounds, lacerations, etc.), consistent with cane toad consumption as being the probable cause of death (Letnic et al. 2008). We dissected freshly killed crocodiles if the abdominal cavity was intact and identified any stomach contents. In some instances, we were able to dissect dead crocodiles classified as skeletal crocodile remains where the skin was dried and the abdominal cavity was intact to identify stomach contents.
Within each waterhole, we conducted searches of river-banks on foot to detect crocodile remains estimated to be older than 2 weeks but killed in the calendar year of the survey, that were typically present as intact skeletons. These crocodile carcasses had presumably floated to the edge of the waterholes where they were subsequently stranded by evaporative water loss. Because the Victoria River experiences large floods each wet season which wash away riverside debris that has accumulated in the previous dry season, we judged these animals to have died in the calendar year of the survey. The skulls of all dead crocodiles were removed to avoid double counting. For each skeletal dead crocodile we measured the head length and calculated the TL from this measurement using the equations provided by Webb et al. (1983a).
The snout-urostyle length (SUL) of intact dead toads removed from crocodile stomachs were measured. In many cases, the only undigested parts of toads that remained in crocodile stomachs were their leg bones which due to their distinctive proportions are easily distinguished from those of the only other similarly sized anuran (Litoria caerulea) that is active on the banks of Victoria River during the dry season. In these cases, the tibia length was measured and the toad’s SUL length was estimated from the following regression Eq. (1) calculated by measuring the tibias of 476 live toads collected along the Victoria River.
Crocodile abundance
We indexed crocodile abundance in each waterhole before and after toad invasion by conducting nocturnal counts of crocodiles from a boat (Bayliss 1987). Surveys were conducted once in 2005 prior to the invasion of cane toads at four “downstream” waterholes; Victoria River Gorge, Wickham, Pigeonhole and Longreach and were repeated annually at these waterholes after the invasion of cane toads from 2007 to 2019 (Table 1). Surveys commenced in 2008 prior to the invasion of cane toads at three upstream waterholes, Rifle, No Name and Mucka and were conducted annually until 2018. In 2019, it was not possible to survey Rifle or No Name due to access constraints (Table 1). Data from surveys conducted in 2005 and 2007 reported for the downstream waterholes were previously published in Letnic et al. (2008).
During surveys, we scanned the water, banks and fringing vegetation with a spotlight, and located crocodiles from their reflective eye shine. All surveys were conducted using the same observer (ML). All crocodiles sighted were approached as closely as possible to estimate total length. However, because crocodiles sometimes submerge when approached, we were unable to estimate the size of every crocodile sighted. Consequently, all crocodiles that were not allocated a size estimate were classified as ‘eyes-only’. Size estimates of crocodiles were periodically validated by estimating the sizes of live/dead crocodiles and then securing and measuring those crocodiles. If crocodiles that were sighted were engaged in feeding behaviour we recorded the type of prey item and the size of the prey item. The size estimates of cane toads were periodically validated by capturing and measuring the snout-urostyle length of toads that were released by crocodiles or sighted on the river-bank after a length estimate had been made. To investigate if the frequency of observations of crocodiles attacking toads changed through time we calculated the proportion of crocodiles attacking toads on each survey occasion by dividing the number of observations of crocodiles attacking toads by the number of crocodiles observed for each survey in each waterhole.
Predictor variables
We considered four variables as potential influences of annual variation in toad density and freshwater crocodile abundance and mortality. The predictor variables were: 1. Years since toad arrival. 2. Pre. vs. post toad arrival—A categorical variable whereby surveys conducted at sites before the arrival of cane toads were classified as before and surveys conducted after toad arrival were classified as after. 3. Antecedent rainfall – cumulative rainfall measured in 12 months before each crocodile survey. Rainfall was hypothesized to influence freshwater crocodile population dynamics because previous studies have indicated that flood events can kill nests and thus limit recruitment (Webb et al. 1983b). Rainfall was hypothesized to influence toad density because toads require ponds to breed in, and the creation of ponds is dependent on rainfall. 4. Calendar year was included as a predictor variable to test the hypotheses that toad density, crocodile mortality and crocodile abundance were correlated with the passage of time independent of the invasion of toads. A 5th variable cane toad density, the index of cane toad abundance derived from boat-based surveys (described above) for each waterhole for each year of survey after toad surveys commenced from 2008 onwards,was also used as a predictor variable for crocodile abundance and mortality.
Cumulative monthly rainfall in the 12 months preceding each survey was calculated for the nearest weather station to each waterhole. For Victoria River Gorge and Wickham, we used rainfall data from the Victoria River Downs weather station. For Longreach and Pigeonhole, we used rainfall data from the Camfield Weather station (17° 2′6″ S, 131° 17′ 41″ E). For Rifle and No Name, we used rainfall data from the Wave Hill weather station (17° 23′ 13″ S, 131° 6′ 59″ E). For Mucka, we used rainfall data from the Riveren rainfall station (17° 54′ 14° S, 130 13′ 37″ E).
Statistical analysis
The effects of the predictor variables on dependent variables representing cane toad abundance, indices of crocodile mortality and crocodile abundance were examined using univariate generalised additive mixed models (GAMM; Wood 2010). This technique allows robust analysis of regression models of non-linear covariate function form with non-normal error terms (Hastie and Tibshirani 1990). GAMM models were fitted with either a Poisson distribution (for counts of crocodile abundance and crocodile mortality measures) and a log canonical link or Gaussian distribution (cane toad density estimate) and an identity link. We used a robust quasi-likelihood error function to minimise effects of outliers on parameter estimates and flexible cubic smoothing splines to model any non-linear functional form between weighted effect size and covariates (e.g., time since toad arrival). Starting crocodile abundance for each waterhole was used as an offset for models of live and dead crocodile abundance measures. To account for repeated measures waterhole was included as a random effect in each model. The mgcv package in R 3.3.1 was used to fit the models to the data (Wood and Wood 2015).
We used the Akaike Information Criterion (AICc) corrected for small sample sizes, Akaike weights (ωi) and model likelihood to identify the most parsimonious model and included a null model (i.e. intercept only) to permit robust inference using a model ranking information theoretic approach (Burnham and Anderson 2003). Akaike weight (ωi) represents the likelihood that a certain model provides the best explanation of the data. The difference in AIC values between the top-ranked model (lowest AIC value) and the remaining models indicates the level of model support. Models with < 2∆AIC from the top-ranked model were considered to be substantially supported, provided that the null model was not similarly supported (Burnham and Anderson 2003).
Results
Cane toad abundance
Cane toads invaded the study area progressively from north to south and were first observed by residents at Victoria River Gorge and Wickham in 2005 and 2006, respectively (Fig. 1; Table 1). Residents advised that toads invaded Pigeonhole and Longreach in the early months of 2007. Residents advised that toads invaded the town of Kalkarindji in 2010 and the southernmost of our survey sites, Mucka in January 2011 (Fig. 1).
We commenced formal boat-based spotlight surveys for toads in 2008. In 2008, cane toads were detected at all waterholes except Rifle, No Name and Mucka. Toads were first detected at Rifle and No Name in 2009 and at Mucka in 2011 (Fig. 1).
Variation in cane toad abundance was best explained (R2(adjusted) = 0.27) by a model testing the effect of calendar year (ω = 0.93; Table S1a). At each waterhole, toad abundance increased rapidly for the first 3 years post-invasion and then decreased (Fig. 3a). Other models that tested the effects of year post toad arrival, crocodile abundance, annual rainfall and the null model were poorly supported (AICc > 2) and explained little variation in cane toad abundance.
Line plots for a Cane toad abundance vs calendar year, b the number of fresh dead crocodiles observed vs years since toad invasion, c the number of crocodile skeletal remains observed vs years since toad invasion and d crocodile abundance vs years since toad invasion. In each panel the values for each waterhole are presented by coloured dots/lines. The fitted values for the most parsimonious GAMM model (grey) and its 95% confidence region (grey) are included in each plot
Interactions between cane toads and crocodiles
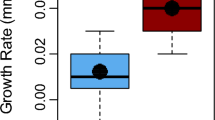
Out of a total of 5788 observations of crocodiles, we observed 37 crocodiles holding cane toads in their jaws (Fig. 2a). The crocodiles holding toads ranged in size from 45 to 240 cm TL (Fig. 4a). The average total length of crocodiles holding toads was 100 cm (st dev ± 0.38 cm). Of these crocodiles, we were able to estimate the size of the toad that they had captured on 33 occasions. The toads captured by crocodiles ranged in size from 40 to 130 mm SUL (mean = 88.0 mm, std dev = 23.9 mm). Bigger crocodiles tended to catch bigger toads (Fig. 5a; regression; toad SUL (mm) = 0.24 croc TL (cm) + 63.5, df = 1,32, F = 5.7, P = 0.02, R2 = 0.16).
Frequency histograms showing a the total length (cm) of live crocodiles holding toads (alive) and fresh dead crocodiles (dead) with toads in their stomachs observed on the Victoria River and b the snout urostyle length (SUL) of cane toads observed captured by live crocodiles (alive) and removed from the stomachs of dead crocodiles (dead)
a Scatterplot of the relationship between the total length of crocodiles observed feeding on cane toads and the size of cane toads on which they were feeding. b Scatterplot of the relationship between the snout urostyle length (SUL) of toads removed from the stomachs of dead crocodiles and the total length of the dead crocodiles from which they were removed
Of these crocodile-toad interactions, in most instances we observed crocodiles holding toads in their jaws. On several occasions we observed that the toads captured by crocodiles were visibly exuding poison from their parotid glands (Fig. 2a). On five occasions, we observed the crocodile to hold a toad in its jaws for approximately 10 min. We observed crocodiles to swallow toads on 4 occasions. The crocodiles that swallowed toads were > 90 cm TL. Once we observed a small (60 cm TL) crocodile attack a large (120 mm SUL) toad, and then begin twisting the toad so that it dismembered the toad’s hind leg. However, we lost sight of the crocodile when it submerged so we were unable to determine if it swallowed the toad’s leg. On two other occasions we found large (> 100 mm SUL) toads on the river’s edge with missing hind legs and puncture marks consistent with the teeth of crocodiles. The frequency of observations of crocodiles attacking toads increased during the first 3 years post-invasion and then fluctuated thereafter (Fig. 6). Crocodiles were observed to attack toads at 14 years post-invasion (Fig. 6). Other prey that we observed crocodiles attacking or capturing were shrimp (Macrobrachium spinipes; 2 occasions), frogs (Litoria inermis; 2 occasions) and fish (1 occasion).
Crocodile mortality
We indexed crocodile mortality using two indices, counts of fresh dead crocodiles and counts of skeletal crocodile remains. Neither fresh dead crocodiles nor skeletal crocodile remains were observed at any waterhole prior to the invasion of cane toads, but were located at all waterholes following the invasion of toads (Table 1; Fig. 3b,c). The best supported GAMM models (ω’s = 0.84–1.00, Table S1b,c) for fresh dead crocodiles and skeletal crocodile remains indicated that variation (R2(adjusted) = 0.49 – 0.72) in the number of dead crocodiles was most influenced by the number of years since toad invasion. Plots of the GAMM models (Table S1b,c) showed a humped relationship between the number of dead crocodiles and years since toad invasion (Fig. 3b,c). The number of fresh dead crocodiles and crocodile skeletal remains that we located increased rapidly following toad invasion, peaked at 2 years post-toad invasion and then declined (Fig. 3b, c). Although the number of fresh dead crocodiles that we observed declined considerably after their peak, dead crocodiles with toad remains in their stomachs were observed 8, 9 and 12 years after toad invasion (Fig. 3b). The frequency of occurrence of crocodile skeletal remains showed a similar temporal response to fresh dead crocodiles (Fig. 3c). GAMM models showed that the frequency of skeletal remains peaked at 2 years post toad invasion and then declined (Fig. 3c).
The mean total length (TL) of fresh dead crocodiles that we measured was 153 cm (n = 87, range: 73–221 cm, std ± 33 cm; Fig. S1). The mean total length (TL) of fresh dead crocodiles with toads in their stomachs was 153 cm (n = 44, range 96–220 cm, std ± 35 cm; Fig. 5A) and did not differ that from that of dead crocodiles without toads in their stomachs (mean = 160 cm, n = 27, range 100–221 cm, std ± 33 cm: ANOVA F = 0.980, df 1,69, P = 0.326). On average, fresh dead crocodiles with toads in their stomachs were 50 cm longer than live crocodiles that were observed to have caught toads (Fig. 5A; ANOVA, F = 31.1, df 1,66, P = 0.001). The mean estimated total length of crocodiles for which we found skeletal remains was 155 cm (n = 37; range: 61–232 cm, std ± 39 cm; Fig. S1). On average, there was no difference in the estimated total length of fresh dead crocodiles and skeletal crocodile remains (Fig. S1; ANOVA, F = 0.096, df 1, 123, P = 0.757).
We dissected 71 dead crocodiles, of which 44 (62%) had the remains of cane toads in their stomachs. In many cases, the cane toad remains were substantially decomposed and all that remained of the toads were the distinctive leg bones. Of crocodiles containing toad remains, all had just one toad in the stomach except one crocodile which contained two cane toads. Of the dissected crocodiles that did not contain toads, 25 contained no food items. Food items other than toads found in crocodile stomachs were wallaby bones (n = 1), flying fox (n = 1), lizard (n = 1), beetles (n = 2), grasshoppers (n = 2), turtle (n = 1), fish (n = 1), freshwater shrimp (n = 1), unidentified insect (1) and unidentified mammal (1).
The mean size of cane toads removed from the stomachs of dead crocodiles was 103 mm SUL (n = 34, range: 72–130 mm, std ± 13 mm; Fig. 5b). Toads removed from dead crocodiles were on average larger than the toads observed to have been captured by live crocodiles (Fig. 5B; ANOVA F = 9.8, df1,62, P = 0.003). Of the toads found in the stomachs of dead crocodiles, only three individuals were smaller than 90 mm SUL which is approximately the size at which toads become adults. There was a positive relationship between the SUL of toads found in crocodile stomachs and the length of the dead crocodiles (Toad SUL (mm) = 0.157*crocodile TL (cm) + 79.1, F = 6.4, df 1,32, P = 0.02, R2 = 0.17).
Crocodile abundance
Time series plots showed that crocodile numbers declined following the invasion of cane toads at all the waterholes (Fig. 3d). These declines were particularly sharp at the northernmost waterholes, Victoria River Gorge and Wickham. Across all waterholes, the decline in crocodile numbers stabilised about 4–5 years after toad invasion. The best model predicting crocodile numbers was that containing the term years post-toad invasion (Fig. 3d; Table S1d). This model received substantial support (ω = 1) and explained considerable variation (R2(adjusted) = 0.76) in crocodile numbers.
Discussion
Consistent with our predictions, the arrival of cane toads at each of the 7 waterholes coincided with both mortality of crocodiles and a decline in the number of crocodiles observed in our surveys. There was a humped relationship between time since toad invasion and crocodile mortalities whereby the peak in the number of dead crocodiles that we detected declined markedly 3 years after the invasion of toads (Fig. 3b,c). Crocodile numbers in each waterhole showed a marked decline soon after the arrival of cane toads but then stabilised approximately 4 years post-toad invasion (Fig. 3d). However, we found that crocodiles still attacked toads 8–14 years after toads invaded the study sites and we found dead crocodiles with toads in their stomachs at 12 years post-invasion. Taken together, our findings suggest that crocodiles are adapting to the presence of toads, but their populations have shown no sign of numerical recovery in the 8–14 years post toad invasion.
Although our results provide a compelling link between cane toad invasion, crocodile mortality and the decline of crocodile populations, we cannot rule out the possibility that crocodile deaths were due to another factor. It also possible that we may have underestimated the mortality of small crocodiles (< 1 m TL) because their carcasses were more difficult to locate than those of larger crocodiles. However, we think it is unlikely that the deaths of crocodiles and decline of crocodile populations that we report was due to a factor other than the invasion of toads for two reasons.
First, previous studies have provided evidence that ingestion of cane toads can be fatal to freshwater crocodiles (Letnic and Ward 2005; Smith and Phillips 2006) and 62% of the dead crocodiles that we dissected had ingested cane toads. That many of these dead crocodiles did not have toads in their stomachs could have been due to crocodiles ingesting fatal quantities of poison without ingesting a toad or due to crocodiles regurgitating cane toads after ingesting them. This may particularly have been the case for dead crocodiles < 1 m TL. Crocodiles below this size were frequently observed to have adult toads in their jaws (Fig. 4a) but may have difficulty in swallowing them due to their relatively small gape. As evidence of this, only two out of seven of the dead crocodiles < 1 m TL that we dissected had toads in their stomachs and both of these individuals were > 90 cm TL (Fig. 4b). Evidence for the above explanation is also provided by our observations that crocodiles holding live toads could have ingested toad toxin without consuming toads because the toads that they held in their jaws were visibly exuding poisonous parotid secretions (Fig. 2a), and a study showing that crocodiles can display an emetic response after ingesting toxic substances (Andrews et al. 2000).
Second, that crocodile mortality and declines were linked to the arrival of toads is evidenced by our results showing that the seven waterholes which we monitored were progressively invaded by cane toads over a 5-year period and that crocodile mortalities and declines of crocodile populations occurred sequentially across the catchment coincident with arrival of toads. Importantly, we did not observe dead crocodiles at any of the waterholes prior to the invasion of toads. This finding suggests that the rate of mortality of crocodiles due to causes such as senescence, infectious disease, predation and intra-specific conflict was so low that we were unable to detect mortalities by conducting boat-based surveys until the rate of mortality increased following the invasion of cane toads. This is consistent with previous studies which have estimated that annual mortality rates for crocodiles are ~ 15% for individuals aged between one and 10 years before decreasing to < 1% in individuals aged over 11 years (Smith and Webb 1985).
Results of our GAMM models showed that time since toad invasion was a better predictor of the abundance of dead and living crocodiles than the abundance of toads. This finding suggests that toad density, a presumed correlate for the encounter rate between crocodiles and toads, was not the key factor driving cane toad impact on crocodiles. Rather, the humped relationship between crocodile mortality and time since toad-invasion and a tailing-off of the rate of crocodile population decline with time since toad invasion suggest that the strength of the interaction between cane toads and crocodiles has diminished with time since invasion.
Freshwater crocodiles have a relatively slow rate of population increase because females do not reach sexual maturity until they are approximately 10 years old and have a relatively small clutch size in comparison to other crocodilians (Tucker 2001). Assuming an intrinsic rate of population increase of 1.5%, modelling of freshwater crocodile population responses to population reduction (harvesting) suggest that would it take 44 years for a population to recover to pre-reduction invasion levels following a 50% population decline if the initial factor driving mortalities was no longer a source of mortality (Smith and Webb 1985). Under this scenario, we would expect that crocodile numbers in our study waterholes would have displayed increases of 11–21% from their post-toad lows during the 8–14 year period since the invasion of toads. However, our data suggest that crocodile numbers have stabilized but shown no sign of recovery since the reduction in their numbers following the invasion of toads. A plausible explanation for this pattern is that the impact of toads on the crocodile population has attenuated over time but numerical recovery of the crocodile population has been constrained by a “trickle” of mortality of crocodiles that die after consuming toads well after the initial invasion.
Attenuation of the interaction strength between crocodiles and toads with time since invasion is a scenario consistent with the ideas that exposure to toads has induced learning or imposed selection on crocodiles to reduce the likelihood of a fatal encounter (Aiyer et al. 2022). Presumably, the intial invasion of toads removed the crocodiles which were most vulnerable to the toxin, resulting in natural selection for increased physiological resistance to toad toxins (Phillips and Shine 2006) or behavioural avoidance of toads (Aiyer et al. 2022). Previous studies have reported that predators such as blacksnakes and quolls from toad-exposed locations ignore toads as prey, while increases in physiological resistance to toad toxins have been reported in blacksnakes from toad-exposed populations. However, these studies did not report mortality from toad ingestion in populations that had been exposed to toads for several decades (Kelly and Phillips 2017; Phillips and Shine 2006). Thus, the fact that mature crocodiles continue to ingest, and die from toad poisoning more than 10 years after the invasion of toads in the Victoria River is intriguing.
One explanation for the low levels of toad-induced mortality of crododiles that occurred following the initial invasion is that toad ingestion induces strong toad aversions that subsequently wane over time. Crocodiles that survive initial encounters with toads may subsequently avoid consuming toads as a result of conditioned taste aversion (Webb et al. 2008). This hypothesis is supported by a study showing that captive hatchlings which survived consuming metamorph toads subsequently learnt to avoid toads (Somaweera et al. 2011). Similarly, a comparison of freshwater crocodile interactions with toads in toad invaded and non-toad invaded environments found that crocodiles were less likley to consume toad baits in toad invaded areas (Aiyer et al. 2022). This study also found crocodiles in toad invaded areas were less likley to consume toad-baits that were deployed on land than water. Aiyer et al. (2022) hypothesized, that crocodiles may avoid taking toad baits on land because they could not dilute the poison contained in toads in terrestrial environments. However, like associative learning, conditioned taste aversions can become extinct over time (Rosas and Bouton 1998), which would explain our field-observations of crocodiles attacking and ingesting toads well after the initial invasion of cane toads. Likewise, observations that cane toads were reported in the diets of crocodiles at a site in Queensland > 50 years post toad invasion provide little support for the theory that cane toads’ impacts on crocodiles diminish over time because crocodiles learn to avoid eating them (Tucker et al. 1996).
An explanation for the apparent absence of crocodiles avoiding cane toads as a prey item is that consuming toads (and other anurans) confers an advantage that outweighs the advantage gained by avoiding them. Such a situation could arise if the risk of dying after attacking a cane toad was outweighed by the nutrition that consuming toads provides. Support for this “toad gamble” hypothesis comes from our observations that toads were the prey species that we most frequently observed crocodiles attacking. Furthermore, we found no evidence of a reduction in the frequency of crocodiles attacking toads with time since toad invasion (Fig. 6). We speculate that small crocodiles (< 0.9 m TL) may generally benefit from consuming juvenile toads (< 900 mm SUL) which contain much less toxin than adult toads (Shine 2010). Small crocodiles may also obtain nutritional benefits by dismembering and consuming the limbs of toads that are too large to swallow whole but contain relatively little toxin because the toxin is most concentrated in the trunk of toads (Britton et al. 2013; Clarke et al. 2020). Following this line of thinking, crocodiles could benefit from consuming toads until they reach a size large enough to swallow an adult toad whole, at which point the crocodiles die or perhaps develop an aversive response and learn to avoid toads (Somaweera et al. 2013). Such a scenario could also explain the continued low level or ‘trickle’ mortality of large (> 1 m) crocodiles with toads in their stomachs that we observed many years after the invasion of toads.
Based on our results showing: (1) that crocodiles feed on toads; (2) crocodiles are occasionally killed by toads; (3) the impact of toads on crocodiles has diminished through time; and (4) the crocodile population has shown little sign of a numeric recovery, we hypothesise that crocodiles have adapted imperfectly to the presence of toads and that cane toads place an upper limit on the crocodile population because some crocodiles are still susceptile to be poisoned by toads. This theory is consistent with the findings of Doody et al. (2017) who found no evidence that goanna populations had recovered in the 10 years following a 90% population knockdown following toad invasion. Taken together, the results of our study and that of Doody et al. (2017) suggest that the invasion of cane toads may have shifted toad-impacted predator populations to new equilibria that are considerably lower than those which existed before toads. Such a scenario could occur, if toads are an ongoing source of mortality for predators because there has been selection on predators not to just avoid toads but also to obtain the nutrition that toads provide.
By following the impacts of an invasive species before and after invasion, our study provides insights into the time course of the impacts of biological invasions and has implications for the management of invasive species’ impacts. The pulsed nature of crocodile mortality suggests that invaders can prompt rapid evolution or learning, which can enable coexistence between the invader and impacted native species. However, that crocodile populations have shown little evidence of recovery post-toad invasion and that mortality linked to toad ingestion was still occurring 12 years post-invasion highlights that adaptation by native species to the presence of invaders may be imperfect and thus does not necessarily entail a numerical recovery, but instead shifts to new equilibria due to ongoing interactions with the invader (Dominguez Almela et al. 2021).
Data availability
The data will be made available on reasonable request to ML.
Code availability
Code will be uploaded to Dryad or available from RAB upon publication of this manuscript.
References
Aiyer A, Shine R, Somaweera R et al (2022) Shifts in the foraging tactics of crocodiles following invasion by toxic prey. Sci Rep 12:1–9
Alves JM, Carneiro M, Cheng JY et al (2019) Parallel adaptation of rabbit populations to myxoma virus. Science 363:1319–1326
Andrews P, Axelsson M, Franklin C et al (2000) The emetic reflex in a reptile (Crocodylus porosus). J Exp Biol 203:1625–1632
Bayliss P (1987) Survey methods and monitoring within crocodile management programmes. Wildlife management: crocodiles and alligators. pp 157–175
Britton AR, Britton EK, McMahon CR (2013) Impact of a toxic invasive species on freshwater crocodile (Crocodylus johnstoni) populations in upstream escarpments. Wildl Res 40:312–317
Brodie EDI, Brodie EDJ (1999) Costs of exploiting poisonous prey: evolutionary trade-offs in a predator–prey arms race. Evolution 53:626–631
Burnham KP, Anderson DR (2003) Model selection and multimodel inference: a practical information-theoretic approach. Springer, Berlin
Carlsson NOL, Sarnelle O, Strayer DL (2009) Native predators and exotic prey – an acquired taste? Front Ecol Environ 7:525–532
Clarke GS, Hudson CM, Shine R (2020) Encounters between freshwater crocodiles and invasive cane toads in north-western Australia: does context determine impact. Aust Zool 41(1):94–101
Covacevich J, Archer M (1975) The distribution of the cane toad, Bufo marinus, in Australia and its effects on indigenous vertebrates. Memoirs Qld Mus 17:305–310
Dominguez Almela V, South J, Britton JR (2021) Predicting the competitive interactions and trophic niche consequences of a globally invasive fish with threatened native species. J Anim Ecol 90(11):2651–2662
Doody JS, Green B, Rhind D et al (2009) Population-level declines in Australian predators caused by an invasive species. Anim Conserv 12:46–53
Doody JS, Rhind D, Green B et al (2017) Chronic effects of an invasive species on an animal community. Ecology 98:2093–2101
Fukuda Y, Tingley R, Crase B et al (2016) Long-term monitoring reveals declines in an endemic predator following invasion by an exotic prey species. Anim Conserv 19:75–87
Hastie TJ, Tibshirani RJ (1990) Generalized additive models, volume 43 of Monographs on Statistics and Applied Probability. Chapman & Hall, London
Jolly CJ, Kelly E, Gillespie GR et al (2018) Out of the frying pan: reintroduction of toad-smart northern quolls to southern Kakadu National Park. Austral Ecol 43:139–149
Kelly E, Phillips BL (2017) Get smart: native mammal develops toad-smart behavior in response to a toxic invader. Behav Ecol 28:854–858
Letnic M, Ward S (2005) Observation of freshwater crocodiles (Crocodylus johnstoni) preying upon cane toads (Bufo marinus) in the Northern Territory. Herpetfauna 35:98
Letnic M, Webb JK, Shine R (2008) Invasive cane toads (Bufo marinus) cause mass mortality of freshwater crocodiles (Crocodylus johnstoni) in tropical Australia. Biol Cons 141:1773–1782
Letnic M, Webb JK, Jessop TS et al (2014) Artificial water points facilitate the spread of an invasive vertebrate in arid Australia. J Appl Ecol 51:795–803
Letnic M, Webb JK, Jessop TS et al (2015) Restricting access to invasion hubs enables sustained control of an invasive vertebrate. J Appl Ecol 52:341–347
Mack RN, Simberloff D, Mark Lonsdale W et al (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Mooney HA, Cleland EE (2001) The evolutionary impact of invasive species. Proc Natl Acad Sci USA 98:5446–5451
O’Donnell S, Webb JK, Shine R (2010) Conditioned taste aversion enhances the survival of an endangered predator imperilled by a toxic invader. J Appl Ecol 47:558–565
Phillips BL, Shine R (2006) An invasive species induces rapid adaptive change in a native predator: cane toads and black snakes in Australia. Proc R Soc London B Biol Sci 273:1545–1550
Phillips BL, Brown GP, Travis JM et al (2008) Reid’s paradox revisited: the evolution of dispersal kernels during range expansion. Am Nat 172:S34–S48
Rosas JM, Bouton ME (1998) Context change and retention interval can have additive, rather than interactive, effects after taste aversion extinction. Psychon Bull Rev 5:79–83
Savidge JA (1987) Extinction of an island forest avifauna by an introduced snake. Ecology 68:660–668
Shine R (2010) The ecological impact of invasive cane toads (Bufo marinus) in Australia. Q Rev Biol 85:253–291
Smith JG, Phillips BL (2006) Toxic tucker: the potential impact of cane toads on Australian reptiles. Pac Conserv Biol 12:40–49
Smith A, Webb G (1985) Crocodylus johnstoni in the McKinlay Area, NT VIII. A Popul Simul Model Wildl Res 12:541–554
Somaweera R, Webb JK, Brown GP et al (2011) Hatchling Australian freshwater crocodiles rapidly learn to avoid toxic invasive cane toads. Behaviour 148:501–517
Somaweera R, Shine R, Webb J et al (2013) Why does vulnerability to toxic invasive cane toads vary among populations of Australian freshwater crocodiles? Anim Conserv 16:86–96
Strayer DL, Eviner VT, Jeschke JM et al (2006) Understanding the long-term effects of species invasions. Trends Ecol Evol 21:645–651
Suarez AV, Tsutsui ND (2008) The evolutionary consequences of biological invasions. Mol Ecol 17:351–360
Tucker A (2001) Sensitivity analysis of stage-structured demographic models for freshwater crocodiles. Matrix 4:F5
Tucker AD, Limpus CJ, McCallum HI et al (1996) Ontogenetic dietary partitioning by Crocodylus johnstoni during the dry season. Copeia 1996:978–988
Ujvari B, Madsen T (2009) Increased mortality of naive varanid lizards after the invasion of non-native cane toads (Bufo marinus). Herpetol Conserv Biol 4:248–251
Ujvari B, Hc M, Conigrave AD et al (2013a) Isolation breeds naivety: island living robs Australian varanid lizards of toad-toxin immunity via four-base-pair mutation. Evolut Int J Org Evolut 67:289–294
Ujvari B, Oakwood M, Madsen T (2013b) Queensland northern quolls are not immune to cane toad toxin. Wildl Res 40:228–231
Vitousek PM, D’Antonio CM, Loope LL et al (1996) Biological invasions as global environmental change. Am Sci 84:218–228
Ward-Fear G, Shine R, Brown G (2020) Within-population variation in dietary traits: implications for vulnerability and impact of imperiled keystone predators. Ecosphere 11:e03136
Webb G, Manolis S, Buckworth R (1983a) Crocodylus johnstoni in the McKinlay River area N. T, III.* Growth, movement and the population age structure. Wildl Res 10:383–401
Webb GJ, Manolis S, Buckworth R (1983b) Crocodylus johnstoni in the McKinlay River Area N. T, VI.* Nesting biology. Wildl Res 10:607–637
Webb JK, Brown GP, Child T et al (2008) A native dasyurid predator (common planigale, Planigale maculata) rapidly learns to avoid a toxic invader. Austral Ecol 33:821–829
Woinarski JCZ, Armstrong M, Brennan K et al (2010) Monitoring indicates rapid and severe decline of native small mammals in Kakadu National Park, northern Australia. Wildl Res 37:116–126
Wood S, Wood MS (2015) Package ‘mgcv.’ R Package Version 1:29
Acknowledgements
Graeme Sawyer, Tom Nichols and the croc team. Managers of stations, in particular Camfield and the Underwood family.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research wa supported by the Hermon Slade Foundation (ML, TD) and Australian Research Council (JKW).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design. TJ and ML led the analyses. All authors contributed to the writing and approved submission of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have no financial interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Letnic, M., Dempster, T., Jessop, T.S. et al. Imperfect adaptation by freshwater crocodiles to the invasion of a toxic prey species. Biol Invasions (2024). https://doi.org/10.1007/s10530-024-03273-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10530-024-03273-x