Abstract
In the Grinnellian niche concept, the realized niche and potential distribution is characterized as an interplay among the fundamental niche, biotic interactions and geographic accessibility. Climate is one of the main drivers for this concept and is essential to predict a taxon’s distribution. Mechanistic approaches can be useful tools, which use fitness-related aspects like locomotor performance and critical thermal limits to predict the potential distribution of an organism. These mechanistic approaches allow the inclusion key ecological processes like local adaptation and can account for thermal performance traits of different life-history stages. The African Clawed Frog, Xenopus laevis, is a highly invasive species occurring on five continents. The French population is of special interest due to an ongoing expansion for 40 years and a broad base of knowledge. We hypothesize that (1) the French population exhibits increased activity time in the invasive European range that could be devoted to fitness-relevant activity and (2) tadpoles may have less activity time available than adult frogs from the same range. We investigate how thermal performance traits translate into activity time budgets and how local adaptation and differences in the thermal responses of life-history stages may boost the European Xenopus invasion. We use a mechanistic approach based on generalized additive mixed models, where thermal performance curves were used to predict the hours of activity and to compare the potential activity time budgets for two life-history stages of native and invasive populations. Our results show that adult French frogs have more activity time available in Europe compared to South African frogs, which might be an advantage in searching for prey or escaping from predators. However, French tadpoles do not have more activity time in Europe compared to the native South African populations suggesting that tadpoles do not suffer the same strong selective pressure as adult frogs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Anthropocene is characterized by profound environmental changes that have been suggested to be drivers of the sixth mass extinction (Steffen et al. 2011; Ceballos et al. 2017; Maslin and Lewis 2018). Anthropogenic activities, including the introduction of invasive species, are widely recognized to be among the main drivers of biodiversity loss (Early et al. 2016; IPBES 2019). The ecological impacts of invasive species to native taxa can be various and include mechanisms such as predation, herbivory, poisoning, competition, disease transmission, trophic subsidy and hybridization (Blackburn et al. 2014). Particularly for IUCN Critically Endangered species, those invasive aliens constitute a major threat (Duenas et al. 2021). Concurrently, the global emergence of invasive taxa represents unintended experiments providing potentially important insights in range dynamics under changing environmental conditions. The concept of the Grinnellian niche has become a key framework when assessing the impacts of climate change on species. In this concept, the niche and resulting (potential) geographic distribution is described as the interplay of abiotic (fundamental) and biotic factors (interactions among taxa) and geographic climate accessibility (Soberón and Peterson 2005). The fundamental climatic niche is defined as comprising all abiotic environmental conditions that are essential for a species’ persistence and reproduction, irrespective of whether these conditions are available in geographic space, while the realized climatic niche considers those conditions that are actually available (Grinnell 1917; Hutchinson 1978; Soberón and Peterson 2005; Soberón 2007).
Climate is one of the main drivers of the ecological niche and is therefore predictive of the potential distribution of species on broad geographic scales (Thuiller et al. 2004; Soberón 2007). Historical range shifts towards and from refugia (e.g. quantified in phylogeographic studies) suggest that species tend to track their Grinnellian niche instead of persisting and adapting to novel environmental conditions (Cordellier and Pfenninger 2009; Barros et al. 2020). Therefore, it is often assumed that a species’ climatic niche is conserved across space and time (niche conservatism, Wiens et al. 2010; Strubbe et al. 2013; Dorey et al. 2020).
Biological invasions, however, demonstrate numerous reports of ‘niche shifts’, as shown by mismatches between the potential distributions of native and non-native ranges (Broennimann et al. 2007; da Mata et al. 2010; Orsted and Orsted 2019; Christina et al. 2020). In most studies, it remains unclear if these ‘niche shifts’ are associated with shifts in the fundamental niche or in the realized niche only (Petitpierre et al. 2012; Schulte et al. 2012; Strubbe et al. 2013; Poursanidis et al. 2020). Distinguishing between these possibilities is impossible based on correlative models that make use of distribution patterns, since in both native and invaded ranges, species may occupy only a subset of their fundamental niche. Hence, niche shifts may represent a better exploitation of the niche due to a novel accessibility of climate space.
To overcome this limitation, a mechanistic understanding of the climate dependence of physiological processes is necessary. Two different approaches are available for the characterization of the relationship between (potential) distributions and environmental data: (1) the redundant correlative models, which statistically link geographic occurrence data of species with environmental data, and (2) process-based or mechanistic approaches, which determine mechanistic interactions between aspects of the performance as surrogates of the fitness of organisms, like performance and critical thermal limits, and their environment (Kearney and Porter 2009; Ceia-Hasse et al. 2014; Krehenwinkel et al. 2015). Mechanistic SDMs are constrained by availability of data and the identification of essential key processes limiting species' distributions (Elith et al. 2010). While correlative SDMs can capture the realized niche of a species, mechanistic models emphasize the processes involved in determining range constraints (Morin and Thuiller 2009).
Physiological performance traits, like locomotor performance or endurance capacity, are critical to amphibian life-history, and may provide an indicator for potential energy use and balance (Wygoda 1984). Additionally, performance traits that represent those key processes can be translated into hours of activity, the proportion of the day when temperatures allow a specific level of performance (Taylor et al. 2021). For ectotherms, the inclusion of both estimates of performance and activity times in modelling frameworks allows for better predictions of a species' distribution than the sole inclusion of pure environmental variables (Caetano et al. 2020). As such, mechanistic or hybrid SDMs, which combine mechanistic and correlative approaches, are more robust in terms of transferability across space and time (Elith et al. 2010).
Mechanistic models allow the inclusion of key ecological processes like hard thermo-ecological limits [i.e. critical thermal minimum (CTmin) and maximum (CTmax), which are the thresholds to death or represent the inability to move] or shifts in performance traits among life-history stages, which can have rather soft and different thermal dependent responses affecting fitness (Wilson et al. 2000; Bodensteiner et al. 2021). In amphibians, ontogeny is complex by comprising four distinct life-history stages (i.e. egg, larva/tadpole, metamoph, adult). However, in the past, all studies on amphibians using SDMs have focused on the adult stage, while other life-history stages have been neglected. Despite this, the larval/tadpole stage might be of special interest for an amphibians’ distribution as it can last for several weeks, months or even years and it is very sensitive to environmental changes such as pond drying (Sinai et al. 2022). Furthermore, tadpoles represent an important life-history stage because they provide greater opportunity for selection than adults. Due to large clutch sizes in many amphibian species accompanied by often high mortality rates of larvae, they provide ideal model organisms to study selection processes in different life-history stages as genetic and phenotypic variation of tadpoles may be weeded out before selection acts on adults. Furthermore, tadpoles as a life-history stage may have greater capacity for phenotypic plasticity than adults i.e. due to predation pressure (Buskirk and Relyea 1998; Middlemis Maher et al. 2013; Mori et al. 2017), environmental stress (Denver 1997; Bókony et al. 2021), increasing larval density (Gouchie et al. 2008), or different diet (Katayama et al. 2021). In addition, due to different physiological responses of different life-history stages, one life-history stage of a species might be more limited by physiological constraints than other stages and might be a “weak link” for the species in the context of biological invasions.
Previous studies, irrespective of whether they use correlative or mechanistic SDMs, focus on only a few key ecological processes, such as species’ dispersal ability and biotic interactions, while mechanisms such as phenotypic plasticity and local adaptation have not been addressed (Garzon et al. 2019). Both mechanisms may occur in natural populations and vary across a species’ range. Indeed, performance traits may strongly differ among populations (Savolainen et al. 2013; Valladares et al. 2014; Des Roches et al. 2018). Phenotypic plasticity involves the capacity of a single genotype to express different phenotypes across environmental conditions (Nicotra et al. 2010). In contrast, local adaptation involves selection acting on heritable traits (Savolainen et al. 2013). Recently, a few SDM approaches have addressed local adaptation and phenotypic plasticity (Valladares et al. 2014; Hällfors et al. 2016; Des Roches et al. 2018, as reviewed in Garzon et al. 2019). However, these trait-based SDMs have mainly been applied to plant species (Garzon et al. 2019, reviewed in Peterson et al. 2019). thus, there is still a need to integrate evolutionary processes in predictive modelling of animal invasions.
The African Clawed Frog, Xenopus laevis (Daudin, 1802), is one of the world’s most widely distributed amphibian species occurring on five continents with an ongoing expansion of its invasive range in many areas (Measey et al. 2012; Ihlow et al. 2016; Wang et al. 2019). The species shows broad tolerance to a variety of environmental conditions (Tinsley and Kobel 1996). Historically, X. laevis was exported to be used for human pregnancy testing, laboratory research (Gurdon and Hopwood 2003; van Sittert and Measey 20162016) and the pet trade (Herrel and van der Meijden 2014; Measey 2017). In Europe, the species has established populations in Portugal (Rebelo et al. 2010), Italy (Lillo et al. 2005) and France (Fouquet 2001) with a predicted potential expansion of up to 2 million km2 for Europe (Ginal et al. 2021), such that it is now listed as an invasive species of Union concern (EU Regulation no. 2022/1203). Among the invasive European populations, Western France is of particular concern due to its ongoing expansion for 40 years, now occupying an area of 4000 km2 (Vimercati et al. 2020). Furthermore, a broad knowledge base of its physiology and morphology (Louppe et al. 2018; Herrel et al. 2019; Padilla et al. 2019, 2020; Kruger et al. 2022), locomotor performance (Louppe et al. 2017; Araspin et al. 2020), reproduction (Courant et al. 2017), behaviour (Kruger et al. 2019), and genetics (De Busschere et al. 2016) has been established. Rödder et al. (2017) showed that the oceanic temperate climate experienced by the French population is different from the native South African climate, which was interpreted as a shift in the realized climatic niche. Recent laboratory performance trials, of adult frogs from two native South African (Western Cape and KwaZulu-Natal) populations and one European (France) population, revealed a shift in the locomotor performance, represented by the proxies endurance time and distance, of the French population, suggesting local adaptation to the cooler European climate (Araspin et al. 2020). This shift may represent a change in the species´ fundamental climate niche. However, these data are restricted to adult specimens and similar data is lacking for other life-history stages like tadpoles. Moreover, it is currently unknown how the local adaptation of X. laevis relates to its potential geographic distribution.
Consequently, we tested the following hypotheses: (1) considering the shift in temperature dependent performance of adult frogs from France, we hypothesize that the French population exhibit increased in activity time in the invasive European range that could be devoted to fitness-relevant activity, and (2) tadpoles may have less activity time available than adult frogs from the same range because larval stages may be more sensitive to environmental conditions like temperature variation. We assess how temperature-dependent performance traits translate into activity time budgets and to what degree local adaptation and differences in the thermal responses of life-history stages may foster invasion success. We used performance data in a mechanistic framework to compute performance curves which were selected based on generalized additive mixed models (GAMMs) ranked by information criteria. Best fitting performance curves were used to predict the hours of activity and to compare the potential activity time budgets for two life-history stages (adults and tadpoles) of two native and one invasive population.
Methods
Selection of sampling sites, specimens and traits
Numerous studies have shown that the most likely origin for European X. laevis populations is the south-western Cape of South Africa’s winter rainfall region (De Busschere et al. 2016; van Sittert and Measey 2016; Rödder et al. 2017; Measey et al. 2020), while the origin of the French population is likely a mixture of two clades from South Africa’s winter and summer rainfall region (De Busschere et al. 2016). Under laboratory conditions, we assessed fitness-relevant locomotor performance because it has been shown to have important ecological relevance and is also correlated with individual fitness (Burggren and Warburton 2007; Gvoždík and Van Damme 2008). Herein, we used performance datasets from two previous studies. For adult frogs, Araspin et al. (2020) measured endurance distance (EndDist), which is relevant in the context of mate-searching or dispersal and they found rapid shifts in the temperature dependence of locomotor performance. For tadpoles, maximum velocity (MaxVel) was recorded by Kruger (2020) and Wagener et al. (2021), which is relevant in the context of predator escape. They used common garden experiments to determine whether tadpoles with varying parental origins show adaptive phenotypic variation related to different temperatures experienced in parental environments. For thermal performance trials and breeding of adults, we used specimens collected in the native region from KwaZulu-Natal (summer rainfall region, data from Wagener et al. 2021) and Stellenbosch (Western Cape province, winter rainfall region, Kruger 2020), South Africa and the French invasive area from the Département Deux-Sèvres, France (data from Kruger 2020; Araspin et al. 2020). For climatic information of the three regions see Supplementary Material S1.
Performance of adults
Performance was measured for frogs of both sexes from KwaZulu-Natal (N = 13, 6 males, 7 females), from the Western Cape (N = 13, 4 males, 9 females) and from France (N = 40, 21 males, 19 females; for details see Araspin et al. 2020). EndDist, the distance travelled until exhaustion, was recorded by chasing adult X. laevis individually across a 3 m long circular track with a humified cork substrate (Herrel and Bonneaud 2012; Louppe et al. 2017). Frogs were considered exhausted when a specimen was no longer able to right itself. Performance was measured at 19 °C, 23 °C, 27 °C and 31 °C. After each trial, the frogs were returned to their aquarium, fed and left to rest for at least two days. Trials were repeated twice per specimen and the maximal distance was used for further analyses (for details see Araspin et al. 2020). Critical thermal limits were determined by placing the same individuals, which were already used for the performance trials, in containers within an incubator where temperature was increased or decreased slowly (as detailed in Araspin et al. 2020). Animals were inspected regularly for a lack of righting response. Once animals were unable to turn over after being placed on their backs, we considered that temperature to be the animal’s critical temperature (Araspin et al. 2020).
Performance of tadpoles
Wild-caught Xenopus laevis adults from South Africa (n = 7) and France (n = 4) were bred to produce F1-tadpoles used in all performance experiments. We followed the breeding and husbandry guidelines by Schultz and Dawson (2003) and the OECD (2008) described in Wagener et al. (2021). Tadpoles from each breeding pair were raised at a water temperature of 20.0 °C. The preferred rearing and holding temperatures are between 18 and 24 °C, this aligns with the daily average temperature in their native region during the active period for tadpoles (Schultz and Dawson 2003; OECD 2008; Wagener et al. 2021). Developing eggs and tadpoles were kept in 12:12 day:night cycle, fed Frog Brittle® for tadpoles (NASCO), and kept at a high density of 15 individuals L−1. Tadpoles were kept at high densities to slow development rates that would otherwise increase variation of tadpole size tested on subsequent days (Tejedo and Reques 1994).
We followed an adapted version of the protocol to measure velocity of tadpoles in response to acute temperature designed by Wilson et al. (2000) (see Wagener et al. 2021 for details). Xenopus laevis tadpoles were lab-reared until NF stage 45–47 (Nieuwkoop and Faber 1994). Tadpoles were tested for burst swimming speed at five temperatures: 5 °C, 10 °C, 20 °C, 30 °C, and 35 °C. Temperatures were verified with a thermocouple (to the nearest 0.01 °C). Each tadpole was only tested once at one test temperature and then euthanized by an overdose of tricaine methanesulfonate (ms-222). Performance was measured for 20 tadpoles from the Western Cape, 30 tadpoles from KwaZulu-Natal and 50 tadpoles from France for each temperature level (see Kruger 2020).
Tadpoles were acclimated for the trials by changing the tank water temperature at a rate of 0.2 °C min−1 until the target temperature was reached (verified with a thermocouple to the nearest 0.01 °C). The initial burst swimming sequence was filmed with a camera (Olympus, TG-4) at 120 frames s−1). Tadpoles were touched with a fine wire at the tip of their tail to initiate movement. We used a 0.42 m × 0.08 m × 0.17 m clear plastic tank filled with 1 L aged tap water for performance trials. For better visualization of movement by tadpoles in the lateral plane we used a mirror attached to the side of the tank at an angle of 45°. The bottom of the tank was covered with a false bottom where water could circulate through custom-built copper pipes. A water bath (Julabo F12, 4.5 L, max flow 15 L min−1, pressure = 0.35 bar) maintained the desired water temperature (for figures see Supplementary Material S2 and S3).
High-speed videos were filmed of the bottom of the tank and the image in the mirror. All burst swimming sequences were visually proofed and only recordings perpendicular to the camera were retained. We used the motion analysis software Tracker (Open Source Physics; Brown 2012) to obtain X and Y coordinates from the high-speed videos. Coordinates were exported to Excel (Microsoft) and the displacement (cm) of the specimen was computed. We chose a fourth order zero-phase shift low-pass Butterworth filter (Winter 2004) with 12 Hz filter frequency to filter the displacement data. Cut-off frequency was chosen as a 10th of the recording frame rate. From the filtered data, maximum velocity and distance moved in 200 ms were extracted.
Critical thermal limits of tadpoles were tested by placing tadpoles individually in a two-compartment cooling/heating chamber (described in Wagener et al. 2021). Tadpoles were placed in the inner chamber which exchanged heat with the outer chamber through which water was pumped at an adjustable rate. Prior to heating (CTmax) or cooling (CTmin), tadpoles were allowed 10 min to acclimate. Individuals were exposed to a constant heating/cooling rate of 0.3 °C.min−1 until an endpoint was attained. The endpoint was determined when a tadpole was unable to right itself after 30 s. CTmax and CTmin was then recorded as the water temperature beside the tadpole, verified with a thermocouple (to the nearest 0.01 °C). Critical thermal limits were measured for 8 tadpoles from the Western Cape, 48 tadpoles from KwaZulu-Natal and 8 tadpoles from France for each critical thermal limit.
Mechanistic modelling
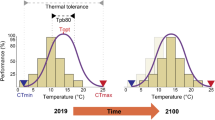
The two thermal performance datasets (i.e., adults and tadpoles) were tested for significant differences among the three populations using the pairwise.wilcox.test() in R ver. 3.6.2 (R Core Team 2019). Based on laboratory performance trials, we computed temperature dependent performance functions for adults and tadpoles using the PerfGAMM()-function of the Mapinguari package (Caetano et al. 2019; Sinervo, unpubl.) for R. The function uses generalized additive mixed models (GAMMs), selected by Akaike Information Criterion (AIC), with temperature as a smooth predictor. The performance functions were scaled to population specific measures of critical thermal minimum (CTmin) and maximum (CTmax), wherein at both extremes a performance of zero is assumed. Furthermore, the function was projected on an array of temperatures ranging between CTmin and CTmax with 0.1 °C increments to calculate the optimum temperature (Topt); i.e. the temperature where the highest performance value is reached. Afterwards, we computed the minimum and maximum temperatures at the 80% maximum performance breadth interval (Tpb80) for each population and life-history stage. Even if critical thermal limits among different populations or life-history stages may be similar, the Tpb80 may differ strongly (Taylor et al. 2021).
Monthly temperature minimum, maximum, and averages for South Africa and Europe with a spatial resolution of 2.5 arc min were obtained from www.worldclim.org (Hijmans et al. 2005) as environmental layers. Daily hours of activity were estimated applying the model of Sinervo et al. (2010) in the Mapinguari package (sin_h()-function, upper and lower limits of Tpb80 with monthly minimum and maximum temperatures as covariables and a temporal resolution of 15 min; Caetano et al. 2019). The function estimates the activity time budget of a species while simulating daily variation in temperature for each grid cell by applying a sine function between daily thermal limits and counting the time above a specific threshold (Tpb80 limits herein). Total available activity times were computed by subtracting time above the Tpb80 upper limit from time above the lower limit. We used the biovars()-function of the dismo package (Hijmans et al. 2017) to create activity layers for annual activity, activity during the warmest quarter and activity during the coldest quarter. As these bioclimatic variables cover different time spans, we rescaled the activity time budgets to 0–24 h, allowing to compare differences between population or life-history stages as fractions on a daily basis. To obtain the activity time for each population and life-history stage, we extracted the respective values from the activity layers by using occurrence records from the native (616 records) and invasive populations (425 records). The activity time budgets were classified into the categories low (0–6 h/day), moderate (6–12 h/day) and high (more than 12 h/day). Finally, we subtracted the activity budgets based on performance of the European population in Europe to obtain the energetic differences for each population and life-history stage as follows: France—KwaZulu-Natal, France—Western Cape, KwaZulu-Natal—Western Cape. Boxplots are used to visualise the differences between the respective medians, minima and maxima for activity time.
Results
For adult frogs, the pairwise Wilcoxon test reveals that thermal performance breadth intervals (Tpb80) are significantly different between the invasive and the two native populations (p < 0.05), but not significant for the other groups (Tables 1 and 2, Fig. 1). The lower limit for Tpb80 is lowest for the French population, followed by the Western Cape population and then KwaZulu-Natal individuals. The upper limit is also lowest for the French population, followed by Western Cape and then KwaZulu-Natal animals. Optimum performance temperature (Topt) is lowest for the French frogs, followed by the Western Cape and then KwaZulu-Natal individuals.
For tadpoles, the lower limit for Tpb80 is lowest for the Western Cape population, followed by the French population and the KwaZulu-Natal tadpoles. The upper limit is lowest for the French population, followed by Western Cape and then KwaZulu-Natal tadpoles. Tadpoles show a similar pattern for Topt as adult frogs, which is lowest for the French population, followed by Western Cape and then KwaZulu-Natal tadpoles. The average performance values of adults and tadpoles at the respective tested temperatures are provided in the supplementary material (Supplementary Material S4).
Differences among adult frogs
The annual activity of adult frogs from the invasive French population shows a moderate to high potential for activity across most parts of Europe (excluding cold mountain ranges like the Alps and Pyrenees) with highest activity time budgets in the Mediterranean and oceanic regions (Fig. 2). In comparison, adult frogs from the Western Cape population show lower potential for activity with mainly moderate values across Europe. Adult frogs from the KwaZulu-Natal population show the lowest potential for activity across Europe with low to moderate values.
Activity time budgets for annual activity, activity during the warmest quarter of the year and activity during the coldest quarter of the year of adult and tadpole Xenopus laevis for the invasive European range of one invasive (France- FR) and two native (Western Cape—WC and KwaZulu-Natal—KZN) populations. Activity time budgets are scaled to daily activity (0–24 h)
For the activity during the warmest quarter, our results suggest that adult frogs from the invasive French population show a low to moderate potential for activity across most parts of Europe with highest potential for activity in the Mediterranean and oceanic regions (Fig. 2). Compared to the French specimens, adult frogs from the Western Cape population show a lower potential for activity with low values across most parts of Europe and only a moderate potential for activity in some coastal regions. Adult frogs from the KwaZulu-Natal population show the lowest activity across Europe with low values across the whole of Europe.
For the activity during the coldest quarter, adult frogs from the invasive French population show a moderate to high potential for activity across most parts of Europe (excluding cold mountain ranges like Alps or Pyrenees) with highest potential for activity in the Mediterranean and oceanic regions (Fig. 2). Compared to the French frogs, adult frogs from the Western Cape population show a lower potential activity with moderate values across Europe. Adult frogs from the KwaZulu-Natal population show the lowest potential activity across Europe with low to moderate values.
Adult French frogs appear to have more time available for activity in Europe (Fig. 3). Annually, an average difference of 5 h more per day (compared to animals from KwaZulu-Natal) or respectively 1.5 h more per day (compared to animals from the Western Cape) is available for activity to the French frogs. While during the coldest quarter, the French frogs have 3 h more per day (compared to animals from KwaZulu-Natal) or respectively 1 h more per day (compared to animals from the Western Cape), no differences in the activity time budget were found during the warmest quarter.
Differences in activity time budgets for annual activity and activity during the coldest quarter of the year of adult and tadpole Xenopus laevis for the invasive European range of one invasive (France—FR) and two native (Western Cape—WC and KwaZulu-Natal—KZN) populations. Activity time budgets are scaled to daily activity (0–24 h)
Differences among tadpoles
Our results indicate that for annual activity, tadpoles from the native Western Cape population show moderate potential for activity across most parts of Europe (excluding cold mountain ranges like the Alps or Pyrenees) with highest potential activity in the Mediterranean and oceanic regions (Fig. 2). French tadpoles perform similarly well but show a less contrasting pattern than the Western Cape tadpoles with mainly moderate potential activity in continental Europe. Tadpoles from the KwaZulu-Natal population show the lowest potential for activity across Europe with low to moderate values.
For activity during the warmest quarter, the map indicates that tadpoles from the native Western Cape population may have low to moderate activities across most parts of Europe with highest potential for activity in the South-western Iberian Peninsula (Fig. 2). French tadpoles show similar activity patterns, but with moderate values only at the South-Western coastal regions of the Iberian Peninsula. Tadpoles from the KwaZulu-Natal population show the lowest potential for activity across Europe with low values across all areas.
For activity during the coldest quarter, tadpoles from the native Western Cape population show moderate to high potential activity across most parts of Europe (excluding cold mountain ranges like Alps or Pyrenees) with highest potential for activity in the Mediterranean and oceanic regions (Fig. 2). French tadpoles perform with a similar activity budget, but mainly show a moderate potential activity in continental Europe. Tadpoles from the KwaZulu-Natal population show the lowest activity budgets across Europe with low to moderate values.
Our results suggest that compared to adult frogs, French tadpoles do not necessarily have more time available for activity in Europe (Fig. 3). While annually, an average difference of 4.5 h more per day (compared to KwaZulu-Natal) is available for activity time of the French tadpoles. The activity time of the French tadpoles compared to Western Cape animals is 2 h less per day. As for adult frogs, during the warmest quarter of the year, the activity time among the populations were identical for tadpoles. During the coldest quarter, the French tadpoles have 4 h more available for activity per day (compared to KwaZulu-Natal) or 1.5 h less per day (compared to Western Cape).
Discussion
For adult frogs and tadpoles, we found that the differences in daily activity time in Europe are highest between the French and the KwaZulu-Natal individuals regardless of life-history stage, followed by the difference between the French and the Western Cape population, and then between the two native South African populations. Our results suggest that for the European range, annual activity time budgets, as well as activity time budgets during the warmest and coldest quarter are highest for the French population, followed by activity time of the Western Cape and then KwaZulu-Natal animals. Furthermore, in adults Tpb80 was distinctly lower among the two native and invasive populations with a left-shift in temperature-dependence of performance for the French population, while for tadpoles no significant differences were found.
Differences among native and invasive populations
Physiological processes of ectotherms such as muscle contraction are strongly dependent on environmental conditions (i.e. temperature; James et al. 2012; Padilla et al. 2020) and affect performance traits like locomotion (Feder and Burggren 1992; Herrel and Bonneaud 2012; Wilson et al. 2000; Padilla et al. 2020). The higher activity time budget of French adult frogs compared to the South African populations should allow the French animals to spend more time on dispersal, foraging or mating (Measey 2016; Courant et al. 2019a, b). Further, escape from predators may be hampered as well (see Eggert and Fouquet 2006). While during the warmest quarter of the year, no differences in the activity time budgets among the populations were found, during the coldest quarter, the French adult frogs have 3 h (compared to KwaZulu-Natal) or 1 h (compared to Western Cape) more available for activity per day. This may be crucial in a period when activity time is minimal due to suboptimal climatic conditions and allows adult frogs to spend more time on foraging and feeding on prey.
Compared to adult frogs and tadpoles of KwaZulu-Natal, the differences in activity time are lower between the French and Western Cape animals. This is not surprising, considering that most of the European populations originate from Western Cape animals (De Busschere et al. 2016). However, the French population consists of animals of two genetic lineages, from the Western Cape and northern South Africa, including the KwaZulu-Natal area that we tested (De Busschere et al. 2016). The admixture of several genetic lines can boost the expansion of invasive species (Sakai et al. 2001; Frankham 2005; Krehenwinkel et al. 2015), and our results suggest that this has been the case in the invasion in Western France. The increased activity time might be especially beneficial during the tadpole stage. The tadpoles of X. laevis are pelagic filter feeders, increased activity time for swimming and feeding may promote growth and fecundity of juvenile frogs (Gouchie et al. 2008), which might be a contributing factor to the success of the French invasion. It is known that within their native range, populations differ in their adaptive and plastic responses with respect to development, morphology and survival (Kruger et al. 2022).
Our study supports the findings of Araspin et al. (2020), finding a left-shift of the thermal optimum in locomotor performance of adult frogs due to the cooler French climate, either caused by local adaptation or phenotypic plasticity. It is known that X. laevis shows high phenotypic plasticity among native populations (Du Preez et al. 2009), including plasticity in physiological traits (Wagener et al. 2021; Kruger et al. 2022). However, we suggest that selection caused by novel climatic conditions may have driven local adaptation in the French population rather than plasticity; but this assertion requires further experimentation to determine the potential for different traits to respond in an adaptive and/or plastic nature. This result supports the hypothesis of a shift in the fundamental climatic niche. However, the real differences in activity time budgets among the populations might differ from our calculations as mechanisms such as adaptive behaviour (e.g. by searching climatically suitable microhabitats) might to some degree reduce differences in activity time among populations.
Differences between life-history stages
Different life-history stages of an organism may have different responses in their temperature dependent performance and activity time (Wilson et al. 2000; Bodensteiner et al. 2021). However, in contrast to adult amphibians (Orille et al. 2020), the knowledge about thermal tolerance or temperature-dependent activity of tadpoles is limited (Wilson et al. 2000; Ruthsatz et al. 2018). Although, it was already assumed that thermal limits among different life-history stages of X. laevis may differ (Balinsky 1969; Nelson et al. 1982; Wu and Gerhart 1991). Comparing the results obtained for adults and tadpoles, our results suggest that activity time is consistently higher in French compared to Western Cape adult frogs, while lower for tadpoles, which reveals that French tadpoles have less time available for activity in Europe than Western Cape tadpoles. This suggests that tadpoles may be more constrained by physiological limits compared to adult frogs, which could indicate a possible weak link in the European Xenopus invasion. In France, breeding was observed between April and June from late spring to early summer (Courant 2017), and metamorphosis duration can be variable depending on food intake and temperature but metamorphosis can be achieved within two months after fertilisation (Nieuwkoop and Faber 1994; Kruger et al. 2022). Therefore, the tadpole stage is mostly active during the summer months where the models suggest no differences among populations. Only adults are likely experiencing thermal selection pressures in the winter, when tadpoles have already metamorphosed. Adults in France have been found to experience selection pressures during spatial sorting which results in the enhancement of their dispersal ability at the periphery (Louppe et al. 2017, 2018; Courant et al. 2017; Courant et al. 2019a, b; Padilla et al. 2019). Tadpoles of X. laevis do not experience the same selection pressures as adults with no suggestion that spatial sorting occurs (Kruger et al. 2021). This can be due to decoupling of traits whereby variation of a trait takes place at a particular life-history stage without affecting the expression of traits in other life-history stages (Moran 1994; Sherrat et al. 2017). Therefore, each stage can experience a unique set of constraints and selection pressures (e.g. Bonnet and Blair 2017; Kruger et al. 2021).
We found that the Tpb80 of tadpoles is much broader than that of adult frogs. It is known that even if critical thermal limits of ectotherms, like amphibians, have little inter- or intra-specific variation, their Tpb80 might be very different. Moreover, thermal responses among life-history stages may differ (for a recent review see Taylor et al. 2021). However, it should be noted that locomotor performance traits (endurance distance for adult frogs and maximum velocity for tadpoles) respond differently to temperature. While endurance is a proxy for sustained locomotion, maximum velocity represents burst performance. Although more unlikely, it could be possible that the broader performance breadth of tadpoles results from a broader thermal tolerance, which shifts during metamorphosis (Cavieres et al. 2016; Wilson et al. 2000).
We did observe a left-shift in the optimum for locomotor performance of French tadpoles, but the Wilcoxon test indicated no significant differences among the three populations (Table 1, Fig. 1), which may suggest that tadpoles did not go through such a strong thermal dependent selection as adult frogs. Our results revealed that the lower limit of Tpb80 for the Western Cape tadpoles is closer to that of the French animals. The upper limit for Western Cape tadpoles is closer to those of the tadpoles from KwaZulu-Natal, but both adaptive and plastic differences may still be present (see Kruger et al. 2022). The French tadpoles may thus also show local adaptation to the cooler European climate (compared to climate charts according to Zepner et al. 2020; Supplementary Material S1), indicating a starting shift in the fundamental climatic niche of tadpoles.
This shift seems not to be as strong as for the adult frogs but might increase their chances to survive, successfully escape from predators and/or search for food, which in turn allows faster growth. Further, Western Cape tadpoles adapted to the broadest temperature range, while KwaZulu-Natal tadpoles are more restricted to higher temperatures. This might be due to breeding times. In the summer rainfall region, breeding takes place from September to mid-March from spring to summer (Balinsky 1969). Whereas, in the winter rainfall region breeding takes place from July to September from winter to the onset of spring (Kalk 1960). The performance curves and activity maps of adults and tadpoles suggest that KwaZulu-Natal animals would have much lower chances to establish populations in Europe compared to Western Cape animals. It would be of particular interest to know how the intense selection during this French invasion has acted on genes from the great diversity in the available gene-pool (see de Busschere et al. 2016).
Comparison with previous correlative and mechanistic SDM approaches for Europe
In the past, several correlative approaches tried to predict the invasion risk of X. laevis for Europe (i.e. Measey et al. 2012; Ihlow et al. 2016; Rödder et al. 2017). However, correlative approaches all demonstrate the caveat that their predictions can reflect the realized niche of a species only, while the physiology of a species and its critical thermal thresholds as well as local adaptation or different life-history stages are neglected. A recent ecophysiological approach from Ginal et al. (2021), which was also the precursor for this study, provided the first mechanistic SDMs for Europe. According to our knowledge, this was also the first time different life-history stages of an amphibian (tadpoles and adults) were included for modelling purposes. The mechanistic predictions clearly showed a much higher potential invasive risk of X. laevis with c. 2 Million km2 of predicted suitable area in Europe, which is an area up to twice as large as that predicted by the worst-case scenario of the correlative approaches. The authors presented three different models, one including adult frogs only, the other including tadpoles only and the third one including adults and tadpoles together. However, the models’ predictions for the native South African range differed from each other, the predictions for Europe revealed the same size of predicted area, which might indicate that tadpoles as a life-history stage might have no impact on the size of potentially colonisable area. In contrast, our findings of this study clearly demonstrate that French tadpoles have less time for activity in Europe compared to their native South African progenitors and are constrained by physiological limits, while adult French frogs already adapted to the much cooler European climate.
Conclusion
The results of our novel approach suggest that invasive populations are able to adapt to novel environments by going through a shift in their fundamental Grinnellian niche, which results in more time available for activity. This gain in the activity time budget might allow individuals to survive and reproduce better, as a result of enhanced foraging or predator evasion, and might allow populations to expand in novel environments. Furthermore, the investigation of temperature-dependent responses of different life-history stages gives deeper insights into the physiology and invasion biology of a species and allows us to identify the life-history stage limiting the geographical expansion of a population. Both types of information, local adaptation and stage limited expansion, are crucial for the risk assessment of alien invasive species. Our study suggests that accounting for local adaptation in the different life-history stages of invasive populations might greatly improve the robustness or reliability of SDM predictions, even those using mechanistic approaches.
Data availability
Data are available on request to the corresponding author.
References
Araspin L, Martinez AS, Wagener C, Courant J, Louppe V, Padilla P, Measey J, Herrel A (2020) Rapid shifts in the temperature dependence of locomotor performance in an invasive frog, Xenopus laevis, implications for conservations. Integr Comp Biol 60(2):456–466. https://doi.org/10.1093/icb/icaa010
Balinsky BI (1969) The reproductive ecology of amphibians of the Transvaal highveld. Zoologica Africana 4(1):37–93
Barros MJ, Silva-Arias GA, Segatto ALA, Reck-Kortmann M, Fregonezi JN, Diniz-Filho JAF, Freitas LB (2020) Phylogenetic niche conservatism and plant diversification in South American subtropical grasslands along multiple climatic dimensions. Genet Mol Biol. https://doi.org/10.1590/1678-4685-gmb-2018-0291
Blackburn TM, Essl F, Evans T, Hulme PE, Jeschke JM, Kühn I, Kumschick S, Mrugala A, Nentwig W, Pergl J, Pysek P, Rabitsch W, Ricciardi A, Richardson DM, Sendek A, Vila M, Wilson JRU, Winter M, Genovesi P, Bacher S (2014) A unified classification of alien species based on the magnitude of their environmental impacts. PLoS Biol 12(5):e1001850
Bodensteiner BL, Agudelo-Cantero GA, Arietta AA, Gunderson AR, Muñoz MM, Refsnider JM, Gangloff EJ (2021) Thermal adaptation revisited: How conserved are thermal traits of reptiles and amphibians? J Exp Zool Part A Ecol Integr Physiol 335:173–194
Bokony V, Ujhegyi N, Hamow KÁ, Bosch J, Thumsova B, Vöros J, Aspbury AS, Gabor CR (2021) Stressed tadpoles mount more efficient glucocorticoid negative feedback in anthropogenic habitats due to phenotypic plasticity. Sci Total Environ 753:141896
Bonett RM, Blair A (2017) Evidence for complex life cycle constraints on salamander body form diversification. Proc Natl Acad Sci USA 114:9936–9941
Broennimann O, Treier UA, Muller-Scharer H, Thuiller W, Peterson AT, Guisan A (2007) Evidence of climatic niche shift during biological invasion. Ecol Lett 10(8):701–709
Brown D (2012) Tracker free video analysis and modelling tool for physics education. http://www.cabrillo.edu/~dbrown/tracker/
Burggren WW, Warburton S (2007) Amphibians as animal models for laboratory research in physiology. ILAR J 48(3):260–269
Buskirk J, Relyea RA (1998) Selection for phenotypic plasticity in Rana sylvatica tadpoles. Biol J Lin Soc 65(3):301–328
Caetano G, Santos J, Sinervo B (2019) Mapinguari: Process-based biogeographical analysis. R package version 1.0.0. https://CRAN.R-project.org/package=Mapinguari
Caetano G, Santos JC, Godinho LB, Cavalcante VHGL, Diele-Viegas LM, Campelo PH, Martins LF, Oliveira AFS, Alvarenga JM, Wiederhecker HC, de Silva V, Werneck FP, Miles DB, Colli GR, Sinervo BR (2020) Time of activity is a better predictor of the distribution of a tropical lizard than pure environmental temperatures. Oikos 129(7):953–963
Cavieres G, Bogdanovich JM, Bozinovic F (2016) Ontogenetic thermal tolerance and performance of ectotherms at variable temperatures. J Evol Biol 29:1462–1468. https://doi.org/10.1111/jeb.12886
Ceballos G, Ehrlich PR, Dirzo R (2017) Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc Natl Acad Sci USA 114(30):e6089–e6096
Ceia-Hasse A, Sinervo B, Vicente L, Pereira HM (2014) Integrating ecophysiological models into species distribution projections of European reptile range shifts in response to climate change. Ecography 37(7):679–688
Christina M, Limbada F, Atlan A (2020) Climatic niche shift of an invasive shrub (Ulex europaeus): a global scale comparison in native and introduced regions. J Plant Ecol 13(1):42–50
Cordellier M, Pfenninger M (2009) Inferring the past to predict the future: Climate modelling predictions and phylogeography for the freshwater gastropod Radix balthica (Pulmonata, Basommatophora). Mol Ecol 18(3):534–544
Courant J (2017) Invasive biology of Xenopus laevis in Europe: ecological effects and physiological adaptations (Doctoral dissertation, Paris, Muséum national d'histoire naturelle).
Courant J, Secondi J, Bereiziat V, Herrel A (2017) Resources allocated to reproduction decrease at the range edge of an expanding population of an invasive amphibian. Biol J Lin Soc 122(1):157–165
Courant J, Adil L, De Kegel B, Adriaens D, Herrel A (2019a) Conserved growth rate and age structure of Xenopus laevis in the edge and core of an expanding population. Biol J Lin Soc 128(1):122–128
Courant J, Secondi J, Guillemet L, Vollette E, Herrel A (2019b) Rapid changes in dispersal on a small spatial scale at the range edge of an expanding position. Evol Ecol 33(4):599–612
Da Mata RA, Tidon R, Cortes LG, De Marco P, Diniz JAF (2010) Invasive and flexible: niche shift in the drosophilid Zaprionus indianus (Insecta, Diptera). Biol Invasions 12(5):1231–1241
De Busschere C, Courant J, Herrel A, Rebelo R, Rödder D, Measey J, Backeljau T (2016) Unequal contribution of native South African phylogeographic lineages to the invasion of the African clawed frog, Xenopus laevis, in Europe. PeerJ 4:e1659
Denver RJ (1997) Environmental stress as a developmental cue: corticotropin-releasing hormone is a proximate mediator of adaptive phenotypic plasticity in amphibian metamorphosis. Horm Behav 31(2):169–179
Des Roches S, Post DM, Turley NE, Bailey JK, Hendry AP, Kinnison MT, Schweitzer JA, Palkovacs EP (2018) The ecological importance of intraspecific variation. Nat Ecol Evol 2(1):57–64
Dorey JB, Groom SVC, Freedman EH, Matthews CS, Davies OK, Deans EJ, Rebola C, Stevens MI, Lee MSY, Schwarz MP (2020) Radiation of tropical island bees and the role of phylogenetic niche conservatism as an important driver of biodiversity. Proc R Soc B Biol Sci 287:1925
Duenas MA, Hemming DJ, Roberts A, Diaz-Soltero H (2021) The threat of invasive species to IUCN-listed critically endangered species: a systematic review. Global Ecol Conserv e01476
Du Preez LH, Kunene N, Hanner R, Giesy JP, Solomon KR, Hosmer A, Van Der Kraak GJ (2009) Population-specific incidence of testicular ovarian follicles in Xenopus laevis from South Africa: A potential issue in endocrine testing. Aquat Toxicol 95(1):10–16
Early R, Bradley BA, Dukes JS, Lawler JJ, Olden JD, Blumenthal DM, Gonzalez P, Grosholz ED, Ibanez I, Miller LP, Sorte CJB, Tatem AJ (2016) Global threats from invasive alien species in the twenty-first century and national response capacities. Nat Commun 7:12485
Eggert C, Fouquet A (2006) A preliminary biotelemetric study of a feral invasive Xenopus laevis population in France. Alytes 23(3/4):144
Elith J, Kearney M, Phillips S (2010) The art of modelling range-shifting species. Methods Ecol Evol 1(4):330–342
Feder ME, Burggren WW (1992) Environmental physiology of the amphibians. University of Chicago Press, Chicago
Fouquet A (2001) Des Clandestins Aquatiques. Zamenis 6:10–11
Frankham R (2005) Resolving the genetic paradox in invasive species. Heredity 94(4):385–385
Garzon MB, Robson TM, Hampe A (2019) Delta TraitSDMs: Species distribution models that account for local adaptation and phenotypic plasticity. New Phytol 222(4):1757–1765
Ginal P, Mokhatla M, Kruger N, Secondi J, Herrel A, Measey J, Rödder D (2021) Ecophysiological models for global invaders: Is Europe a big playground for the African clawed frog? J Exp Zool Part A Ecol Integr Physiol 335:158–172. https://doi.org/10.1002/jez.2432
Gouchie GM, Roberts LF, Wassersug RJ (2008) The effect of mirrors on African clawed frog (Xenopus laevis) larval growth, development, and behavior. Behav Ecol Sociobiol 62(11):1821–1829
Gurdon JB, Hopwood N (2003) The introduction of Xenopus laevis into developmental biology: Of empire, pregnancy testing and ribosomal genes. Int J Dev Biol 44(1):43–50
Gvozdik L, Van Damme R (2008) The evolution of thermal performance curves in semi-aquatic newts: thermal specialists on land and thermal generalists in water? J Therm Biol 33(7):395–403
Grinnell J (1917) The niche-relationships of the California Thrasher. Auk 34:427–433
Hällfors MH, Liao J, Dzurisin J, Grundel R, Hyvarinen M, Towle K, Wu GC, Hellmann JJ (2016) Addressing potential local adaptation in species distribution models: implications for conservation under climate change. Ecol Appl 26(4):1154–1169
Herrel A, Bonneaud C (2012) Temperature dependence of locomotor performance in the tropical clawed frog, Xenopus tropicalis. J Exp Biol 215(14):2465–2470
Herrel A, Van Der Meijden A (2014) An analysis of the live reptile and amphibian trade in the USA compared to the global trade in endangered species. Herpetol J 24(2):103–110
Herrel A, Orpel J, Padilla P, Courant J, Rebelo R (2019) Do invasive populations of Xenopus laevis living in different environments differ in morphology? Integr Comp Biol 59:e98–e98
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25(15):1965–1978
Hijmans RJ, Phillips S, Leathwick J, Elith J (2017) dismo: Species distribution modeling. R package version 1.1–4. https://CRAN.R-project.org/package=dismo.
Hutchinson GE (1978) An introduction to population ecology. Yale University Press, New Haven
Ihlow F, Courant J, Secondi J, Herrel A, Regelo R, Measey J, Lillo F, De Villiers FA, Vogt S, De Busschere C, Backeljau T, Rödder D (2016) Impacts of climate change on the global invasion potential of the African clawed frog Xenopus laevis. PLoS ONE 11(6):e0154869
IPBES (2019) Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Bonn, Germany
James RS, Tallis J, Herrel A, Bonneaud C (2012) Warmer is better: Thermal sensitivity of both maximal and sustained power output in the iliotibialis muscle isolated from adult Xenopus tropicalis. J Exp Biol 215(3):552–558. https://doi.org/10.1242/jeb.063396
Kalk M (1960) Climate and breeding in Xenopus laevis. S Afr J Sci 56(11):271–276
Katayama N, Okamura K, Tanimura K (2021) Phenotypic plasticity of salamander hatchlings in the pre-feeding stage in response to future prey. Zool Sci 38(5):397–404
Kearney M, Porter W (2009) Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol Lett 12(4):334–350
Krehenwinkel H, Rödder D, Tautz D (2015) Eco-genomic analysis of the poleward range expansion of the wasp spider Argiope bruennichi shows rapid adaptation and genomic admixture. Glob Change Biol 21(12):4320–4332
Kruger N (2020) Do vulnerable life-history stages of Xenopus laevis reduce its invasion potential? Ph.D. Thesis (Stellenbosch University). http://hdl.handle.net/10019.1/109402
Kruger N, Measey J, Herrel A, Secondi J (2019) Anti-predator strategies of the invasive African clawed frog, Xenopus laevis, to native and invasive predators in western France. Aquat Invasions 14(3):433–443
Kruger N, Measey J, Vimercati G, Herrel A, Secondi J (2021) Does the spatial sorting of dispersal traits affect the phenotype of the non-dispersing stages of the invasive frog Xenopus laevis through coupling? Biol J Lin Soc 132:257–269. https://doi.org/10.1093/biolinnean/blaa191
Kruger, N, Secondi, J, Du Preez, L, Herrel, A, Measey, J (2022) Phenotypic variation in Xenopus laevis tadpoles from contrasting climatic regimes is the result of adaptation and plasticity. Oecologia.
Lillo F, Marrone F, Sicilia A, Castelli G, Zava B (2005) An invasive population of Xenopus laevis (Daudin, 1802) in Italy. Herpetozoa 18:63–64
Louppe V, Courant J, Herrel A (2017) Differences in mobility at the range edge of an expanding invasive population of Xenopus laevis in the west of France. J Exp Biol 220(2):278–283
Louppe V, Courant J, Videlier M, Herrel A (2018) Differences in standard metabolic rate at the range edge versus the center of an expanding invasive population of Xenopus laevis in the West of France. J Zool 305(3):163–172
Maslin M, Lewis S (2018) Anthropocene now. New Scientist 239(3188):24–25
Measey J (2016) Overland movement in African clawed frogs (Xenopus laevis): a systematic review. PeerJ 4:e2474
Measey J (2017) Where do African clawed frogs come from? An analysis of trade in live Xenopus laevis imported into the USA. Salamandra 53(3):398–404
Measey J, Rödder D, Green SL, Kobayashi R, Lillo F, Lobos G, Rebelo R, Thirion JM (2012) Ongoing invasions of the African clawed frog, Xenopus laevis: a global review. Biol Invasions 14(11):2255–2270
Measey J, Hui C, Somers MJ (2020) Terrestrial vertebrate invasions in South Africa. In: Van Wilgen B, Measey J, Richardson D, Wilson J, Zengeya T (eds) Biological invasions in South Africa. Invading Nature—Springer Series in Invasion Ecology 14. Springer, Cham
Middlemis Maher J, Werner EE, Denver RJ (2013) Stress hormones mediate predator-induced phenotypic plasticity in amphibian tadpoles. Proc R Soc B Biol Sci 280(1758):20123075
Moran NA (1994) Adaptation and constraint in the complex life cycles of animals. Annu Rev Ecol Evol Syst 25:573–600
Mori T, Yanagisawa Y, Kitani Y, Yamamoto G, Goto-Inoue N, Kimura T, Kashiwagi K, Kashiwagi A (2017) The constant threat from a non-native predator increases tail muscle and fast-start swimming performance in Xenopus tadpoles. Biol Open 6(11):1726–1733
Morin X, Thuiller W (2009) Comparing niche- and process-based models to reduce prediction uncertainty in species range shifts under climate change. Ecology 90(5):1301–1313
Nelson L, Mild KH, Lovtrup S (1982) Changes in temperature tolerance during the development of Xenopus laevis embryos. J Exp Zool 222(1):103–104
Nicotra AB, Atkin OK, Bonser SP, Davidson AM, Finnegan EJ, Mathesius U, Poot P, Purugganan MD, Richards CL, Valladares F, Van Kleunen M (2010) Plant phenotypic plasticity in a changing climate. Trends Plant Sci 15(12):684–692
Nieuwkoop PD, Faber J (1994) Normal table of Xenopus laevis (Daudin): A systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. Garland Publishing Inc, New York
OECD (Organisation for Economic Co–operation and Development) (2008) Final report of the validation of the amphibian metamorphosis assay: Phase 2—Multi– chemical interlaboratory study. Environment, Health, and Safety Publications Series on Testing and Assessments, 77, 96
Orille AC, Mcwhinnie RB, Brady SP, Raffel TR (2020) Positive effects of acclimation temperature on the critical thermal maxima of Ambystoma mexicanum and Xenopus laevis. J Herpetol 54(3):289–292
Orsted IV, Orsted M (2019) Species distribution models of the Spotted Wing Drosophila (Drosophila suzukii, Diptera: Drosophilidae) in its native and invasive range reveal an ecological niche shift. J Appl Ecol 56(2):423–435
Padilla P, Courant J, Herrel A (2019) Allocation trade-offs impact organ size and muscle architecture in an invasive population of Xenopus laevis in Western France. J Anat 235(6):1057–1064
Padilla P, Tallis J, Hurst J, Courant J, James RS, Herrel A (2020) Do muscle contractile properties drive differences in locomotor performance in invasive populations of Xenopus laevis in France? J Comp Physiol B 190(6):771–778. https://doi.org/10.1007/s00360-020-01310-4
Peterson ML, Doak DF, Morris WF (2019) Incorporating local adaptation into forecasts of species’ distribution and abundance under climate change. Glob Change Biol 25(3):775–793
Petitpierre B, Kueffer C, Broennimann O, Randin C, Daehler C, Guisan A (2012) Climatic niche shifts are rare among terrestrial plant invaders. Science 335(6074):1344–1348
Poursanidis D, Kalogirou S, Azzurro E, Parravicini V, Bariche M, Dohna HZ (2020) Habitat suitability, niche unfilling and the potential spread of Pterois miles in the Mediterranean Sea. Mar Pollut Bull 154:111054
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Rebelo R, Amaral P, Bernardes M, Oliveira J, Pinheiro P, Leitao D (2010) Xenopus laevis (Daudin, 1802), a new exotic amphibian in Portugal. Biol Invasions 12(10):3383–3387. https://doi.org/10.1007/s10530-010-9757-0
Rödder D, Ihlow F, Courant J, Secondi J, Herrel A, Rebelo R, Measey J, Lillo F, De Villiers FA, De Busschere D, Backeljau T (2017) Global realized niche divergence in the African clawed frog Xenopus laevis. Ecol Evol 7(11):4044–4058
Ruthsatz K, Dausmann KH, Peck MA, Drees C, Sabatino NM, Becker LI, Reese J, Hartmann L, GLOS J, (2018) Thyroid hormone levels and temperature during development alter thermal tolerance and energetics of Xenopus laevis larvae. Conserv Physiol 6(1):coy059
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, Mccauley DE, O’neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Annu Rev Ecol Syst 32(1):305–332
Savolainen O, Lascoux M, Merila J (2013) Ecological genomics of local adaptation. Nat Rev Genet 14(11):807–820
Schultz TW, Dawson DA (2003) Housing and husbandry of Xenopus for oocyte production. Lab Anim 32:34–39
Schulte U, Hochkirch A, Lötters S, Rödder D, Schweiger S, Weimann T, Veith M (2012) Cryptic niche conservatism among evolutionary lineages of an invasive lizard. Glob Ecol Biogeogr 21(2):198–211
Sherrat E, Vidal-Garcia M, Anstis M, Keogh JS (2017) Adult frogs and tadpoles have different macroevolutionary patterns across the Australian continent. Nat Ecol Evolut 1:1385–1391
Sinai N, Glos J, Mohan AV, Lyra ML, Riepe M, Thöle E, Zummach C, Ruthsatz K (2022) Developmental plasticity in amphibian larvae across the world: Investigating the roles of temperature and latitude. J Therm Biol 106:103233
Sinervo B, Mendez-De-La-Cruz F, Miles DB, Heulin B, Bastiaans E, Villagrán-Santa Cruz M, Lara-Resendiz R, Martínez-Méndez N, Calderón-Espinosa ML, Meza-Lázaro RN, Gadsden H, Avila LJ, Morando M, De La Riva IJ, Sepulveda PV, Rocha CFD, Ibargüengoytía N, Puntriano CA, Massot M, Lepetz V, Oksanen TA, Chapple DG, Bauer AM, Branch WR, Clobert J, Sites JRJW (2010) Erosion of lizard diversity by climate change and altered thermal niches. Science 328(5980):894–899
Soberón J (2007) Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett 10(12):1115–1123
Soberón J, Peterson AT (2005) Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers Inform 2:1–10
Steffen W, Persson A, Deutsch L, Zalasiewicz J, Williams M, Richardson K, Crumley C, Crutzen P, Folke C, Gordon L, Molina M, Ramanathan V, Rockstrom J, Scheffer M, Schellnhuber HJ, Svedin U (2011) The Anthropocene: from global change to planetary stewardship. Ambio 40(7):739–761
Strubbe D, Broennimann O, Chiron F, Matthysen E (2013) Niche conservatism in non-native birds in Europe: niche unfilling rather than niche expansion. Glob Ecol Biogeogr 22(8):962–970
Taylor EN, Diele Viegas LM, Gangloff EJ, Hall JM, Halpern B, Massey MD, Rödder D, Rollinson N, Spears S, Sun B-J, Telemeco RS (2021) The thermal ecology and physiology of reptiles and amphibians: a user’s guide. J Exp Zool Part A Ecol Integr Physiol 335:13–44. https://doi.org/10.1002/jez.2396
Tejedo M, Reques R (1994) Plasticity in metamorphic traits of natterjack tadpoles: the interactive effects of density and pond duration. Oikos 71:295–304
Thuiller W, Araujo MB, Lavorel S (2004) Do we need land-cover data to model species distributions in Europe? J Biogeogr 31(3):353–361
Tinsley RC, Kobel HR (1996) The biology of Xenopus. Zoological Society of London, London
Valladares F, Matesanz S, Guilhaumon F, Araujo MB, Balaguer L, Benito-Garzon M, Cornwell W, Gianoli E, Van Kleunen M, Naya DE, Nicotra AB, Poorter H, Zavala MA (2014) The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol Lett 17(11):1351–1364
Van Sittert L, Measey J (2016) Historical perspectives on global exports and research on African clawed frogs (Xenopus laevis). Trans R Soc South Africa 71(2):157–166. https://doi.org/10.1080/0035919X.2016.1158747
Vimercati G, Labadesse M, Dejean T, Secondi J (2020) Assessing the effect of landscape features on pond colonisation by an elusive amphibian invader using environmental DNA. Freshw Biol 65(3):502–513
Wang SP, Hong YF, Measey J (2019) An established population of African clawed frogs, Xenopus laevis (Daudin, 1802), in mainland China. Bioinvasions Rec 8(2):457–464
Wagener C, Kruger N, Measey J (2021) Progeny of Xenopus laevis from altitudinal extremes display adaptive physiological performance. J Exp Biol 224(7):jeb233031
Wiens JJ, Ackerly DD, Allen AP, Anacker BL, Buckley LB, Cornell HV, Damschen EI, Davies TJ, Grytnes JA, Harrison SP, Hawkins BA, Holt RD, Mccain CM, Stephens PR (2010) Niche conservatism as an emerging principle in ecology and conservation biology. Ecol Lett 13(10):1310–1324
Wilson RS, James RS, Johnston IA (2000) Thermal acclimation of locomotor performance in tadpoles and adults of the aquatic frog Xenopus laevis. J Comp Physiol B 170(2):117–124
Winter D (2004) Biomechanics and motor control of human movement. Wiley, New York
Wu M, Gerhart J (1991) Raising Xenopus in the Laboratory. Methods Cell Biol 36:3–18
Wygoda ML (1984) Low cutaneous evaporative water-loss in arboreal frogs. Physiol Zool 57(3):329–337
Zepner L, Karrasch P, Wiemann F, Bernard L (2020) ClimateCharts.net—an interactive climate analysis web platform. Int J Digital Earth. https://doi.org/10.1080/17538947.2020.1829112
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the ERANET BiodivERsA grant INVAXEN, with the national funders Agence Nationale de la Recherche (ANR), Deutsche Forschungsgemeinschaft (DFG), Belgian Federal Science Policy Office (BELSPO), and Fundaçao para a Ciencia e a Tecnologia (FCT), as part of the 2013 BiodivERsA call for research proposals. INVAXEN “Invasive biology of Xenopus laevis in Europe: ecology, impact and predictive models”. CW, NK, MM and JM thank the DSI-NRF Centre of Excellence for Invasion Biology (South Africa) and the National Research Foundation of South Africa (NRF Grant No. 87759 to JM). NK would like to acknowledge the Ambassade de France en Afrique du Sud (France). This study was part of the project Life Control Strategies of Alien Invasive Amphibians (CROAA)—LIFE15 NAT/FR/000864 funded by the Life program of the European Commission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethics approval
We thank the Communauté de Communes du Thouarsais and Communauté de Communes du Bressuirais for allowing access to sampling sites and for their technical assistance. Further, we acknowledge that in South Africa collections in KZN occurred under permission from KZN Wildlife (collection permit: OP 3169/2018, import permit: CN28-31–5262), while frogs in WC were collected under permission of CapeNature (CN15-31–5189).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ginal, P., Kruger, N., Wagener, C. et al. More time for aliens? Performance shifts lead to increased activity time budgets propelling invasion success. Biol Invasions 25, 267–283 (2023). https://doi.org/10.1007/s10530-022-02903-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-022-02903-6