Abstract
Objective
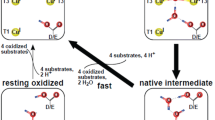
Laccase is one of the best known biocatalysts which degrade wide varieties of complex molecules that are both non-cyclic and cyclic in structure. The study focused on enzyme kinetics of a purified laccase from Trametes hirsuta L. fungus and its application on biotransformation of a carcinogenic molecule 1,4-dioxane.
Results
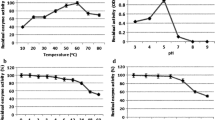
Laccase was purified from white-rot fungus T. hirsuta L. which showed specific activity of 978.34 U/mg after the purification fold of 54.08. The stable laccase activity (up to 16 h) is shown at 4–6 pH and 20–40 °C temperature range. The purified enzyme exhibited significant stability for 10 metal ions up to 10 mM concentration, except for Fe2+ and Hg2+. The Cu2+ ion induced laccase activity up to 142% higher than the control at 10 mM concentration. The laccase enzyme kinetic parameters Km was 20 ± 5 µM and 400 ± 60 µM, whereas Kcat was 198.29 ± 0.18/s and 80.20 ± 1.59/s for 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) and guaiacol respectively. The cyclic ether 1,4-dioxane (100 ppm) was completely degraded in presence of purified laccase within 2 h of incubation and it was confirmed by HPLC and GC analysis. The oxidation reaction was accelerated by 25, 22, 6 and 19% in presence of 1 mM syringaldehyde, vanillin, ABTS and guaiacol mediators respectively.
Conclusions
In this study, fungal laccase (a natural biocatalyst) based degradation of synthetic chemical 1,4-dioxane was reported for the first time. This method has added advantages over the multiple methods reported earlier being a natural remedy.






Similar content being viewed by others
References
Balcázar-López E, Méndez-Lorenzo LH, Batista-García RA et al (2016) Xenobiotic compounds degradation by heterologous expression of a Trametes sanguineus laccase in Trichoderma atroviride. PLoS ONE 11:1–13. https://doi.org/10.1371/journal.pone.0147997
Baldrian P (2006) Fungal laccases-occurrence and properties. FEMS Microbiol Rev 30:215–241. https://doi.org/10.1111/j.1574-4976.2005.00010.x
Battersby NS, Wilson V (1989) Survey of the anaerobic biodegradation potential of organic chemicals in digesting sludge. Appl Environ Microbiol 55:433–439. https://doi.org/10.1007/s00381-013-2309-z
Bernhardt D, Diekmann H (2004) Degradation of dioxane, tetrahydrofuran and other cyclic ethers by an environmental Rhodococcus strain. Appl Microbiol Biotechnol 36:120–123. https://doi.org/10.1007/BF00164711
Bourbonnais R, Paice MG (1990) Oxidation of non-phenolic substrates: an expanded role for laccase in lignin biodegradation. FEBS Lett 267:99–102
Burback BL, Perry JJ (1993) Biodegradation and biotransformation of groundwater pollutant mixtures by Mycobacterium vaccae. Appl Environ Microbiol 59:1025–1029
Campos PA, Levin LN, Wirth SA (2016) Heterologous production, characterization and dye decolorization ability of a novel thermostable laccase isoenzyme from Trametes trogii BAFC 463. Process Biochem 51:895–903. https://doi.org/10.1016/j.procbio.2016.03.015
Chakroun H, Mechichi T, Martinez MJ et al (2010) Purification and characterization of a novel laccase from the ascomycete Trichoderma atroviride: application on bioremediation of phenolic compounds. Process Biochem 45:507–513. https://doi.org/10.1016/j.procbio.2009.11.009
Chandra R, Chowdhary P (2015) Properties of bacterial laccases and their application in bioremediation of industrial wastes. Environ Sci Process Impacts 17:326–342. https://doi.org/10.1039/c4em00627e
Chandra R, Singh R (2012) Decolourisation and detoxification of rayon grade pulp paper mill effluent by mixed bacterial culture isolated from pulp paper mill effluent polluted site. Biochem Eng J 61:49–58. https://doi.org/10.1016/j.bej.2011.12.004
Chen DZ, Jin XJ, Chen J et al (2016) Intermediates and substrate interaction of 1,4-dioxane degradation by the effective metabolizer Xanthobacter flavus DT8. Int Biodeterior Biodegrad 106:133–140. https://doi.org/10.1016/j.ibiod.2015.09.018
Coleman HM, Vimonses V, Leslie G, Amal R (2007) Degradation of 1,4-dioxane in water using TiO2 based photocatalytic and H2O2/UV processes. J Hazard Mater 146:496–501. https://doi.org/10.1016/j.jhazmat.2007.04.049
D’Souza DT, Tiwari R, Sah AK, Raghukumar C (2006) Enhanced production of laccase by a marine fungus during treatment of colored effluents and synthetic dyes. Enzyme Microb Technol 38:504–511. https://doi.org/10.1016/j.enzmictec.2005.07.005
Dong JL, Zhang YZ (2004) Purification and characterization of two laccase isoenzymes from a ligninolytic fungus Trametes gallica. Prep Biochem Biotechnol 34:179–194. https://doi.org/10.1081/PB-120030876
Feng Y, Li H, Lin L et al (2018) Degradation of 1,4-dioxane via controlled generation of radicals by pyrite-activated oxidants: synergistic effects, role of disulfides, and activation sites. Chem Eng J. 336:416–426. https://doi.org/10.1016/j.cej.2017.12.011
Fincher EEL, Payne WJ (1962) Bacterial utilization of ether glycols. Appl Envir Microbiol 10:542–547
Frasconi M, Favero G, Boer H et al (2010) Kinetic and biochemical properties of high and low redox potential laccases from fungal and plant origin. Biochim Biophys Acta 1804:899–908. https://doi.org/10.1016/j.bbapap.2009.12.018
Hadibarata T, Yusoff ARM, Kristanti RA (2012) Decolorization and metabolism of anthraquionone-type dye by laccase of white-rot fungi Polyporus sp. S133. Water Air Soil Pollut 223:933–941. https://doi.org/10.1007/s11270-011-0914-6
Haibo Z, Yinglong Z, Feng H et al (2009) Purification and characterization of a thermostable laccase with unique oxidative characteristics from Trametes hirsuta. Biotechnol Lett 31:837–843. https://doi.org/10.1007/s10529-009-9945-0
Han MJ, Choi HT, Song HG (2005) Purification and characterization of laccase from the white rot fungus Trametes versicolor. J Microbiol 43:555–560. https://doi.org/10.1016/0922-338X(95)98183-L
Hand S, Wang B, Chu KH (2015) Biodegradation of 1,4-dioxane: effects of enzyme inducers and trichloroethylene. Sci Total Environ 520:154–159. https://doi.org/10.1016/j.scitotenv.2015.03.031
Patel H, Gupte S, Mayur Gahlout Gupte A (2014) Purification and characterization of an extracellular laccase from solid-state culture of Pleurotus ostreatus HP-1. Biotech 3:77–84. https://doi.org/10.1007/s13205-013-0129-1
Heinzkill M, Bech L, Halkier T et al (1998) Characterization of laccases and peroxidases from wood-rotting fungi (family Coprinaceae). Appl Environ Microbiol 64:1601–1606. https://doi.org/10.1016/0038-0717(90)90187-5
Inoue D, Tsunoda T, Sawada K et al (2016) 1,4-Dioxane degradation potential of members of the genera Pseudonocardia and Rhodococcus. Biodegradation 27:277–286. https://doi.org/10.1007/s10532-016-9772-7
Inoue D, Tsunoda T, Yamamoto N et al (2018) 1,4-Dioxane degradation characteristics of Rhodococcus aetherivorans JCM 14343. Biodegradation 29:301–310. https://doi.org/10.1007/s10532-018-9832-2
Iyer G, Chattoo BB (2003) Purification and characterization of laccase from the rice blast fungus Magnaporthe grise. FEMS Microbiol Lett 227:121–126. https://doi.org/10.1016/S0378-1097(03)00658-X
Johannes C, Majcherczyk A (2000) Laccase activity tests and laccase inhibitors. J Biotechnol 78:193–199
Kawasaki M (1980) Experiences with the test scheme under the chemical control law of Japan: an approach to structure-activity correlations. Ecotoxicol Environ Saf 4:444–454. https://doi.org/10.1016/0147-6513(80)90046-9
Koroleva OV, Gavrilova VP, Stepanova EV et al (2002) Production of lignin modifying enzymes by co-cultivated white-rot fungi Cerrena maxima and Coriolus hirsutus and characterization of laccase from Cerrena maxima. Enzyme Microb Technol 30:573–580. https://doi.org/10.1016/S0141-0229(02)00021-2
Koroleva OV, Stepanova EV, Gavrilova VP et al (2002) Lactase and Mn-peroxidase production by Coriolus hirsutus strain 075 in a jar fermentor. J Biosci Bioeng 3:449–455
Kumar A, Chandra R (2020) Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 6:e03170. https://doi.org/10.1016/j.heliyon.2020.e03170
Lettera V, Pezzella C, Cicatiello P et al (2015) Efficient immobilization of a fungal laccase and its exploitation in fruit juice clarification. Food Chem 196:1272–1278. https://doi.org/10.1016/j.foodchem.2015.10.074
Levin L, Papinutti L, Forchiassin F (2004) Evaluation of Argentinean white rot fungi for their ability to produce lignin-modifying enzymes and decolorize industrial dyes. Bioresourc Technol 94:169–176. https://doi.org/10.1016/j.biortech.2003.12.002
Li M, Mathieu J, Liu Y et al (2013) The abundance of tetrahydrofuran/dioxane monooxygenase genes (thmA/dxmA) and 1,4-dioxane degradation activity are significantly correlated at various impacted aquifers. Environ Sci Technol Lett 1:122–127. https://doi.org/10.1021/ez400176h
Maciel MJM et al (2010) Industrial and biotechnological applications of ligninolytic enzymes of the basidiomycota: a review. Electron J Biotechnol 13:1–13. https://doi.org/10.2225/vol13-issue6-fulltext-2
Marques De Souza CG, Peralta RM (2003) Purification and characterization of the main laccase produced by the white-rot fungus Pleurotus pulmonarius on wheat bran solid state medium. J Basic Microbiol 43:278–286. https://doi.org/10.1002/jobm.200390031
Michniewicz A, Ullrich R, Ledakowicz S, Hofrichter M (2006) The white-rot fungus Cerrena unicolor strain 137 produces two laccase isoforms with different physico-chemical and catalytic properties. Appl Microbiol Biotechnol 69:682–688. https://doi.org/10.1007/s00253-005-0015-9
Mishra A, Kumar S (2009) Kinetic studies of laccase enzyme of Coriolus versicolor MTCC 138 in an inexpensive culture medium. Biochem Eng J 46:252–256. https://doi.org/10.1016/j.bej.2009.02.016
More SS et al (2011) Isolation, purification, and characterization of fungal laccase from Pleurotus sp. Enzyme Res 2011:1–7. https://doi.org/10.4061/2011/248735
Murugesan K, Kim Y, Jeon J, Chang Y (2009) Effect of metal ions on reactive dye decolorization by laccase from Ganoderma lucidum. J Hazard Mater 168:523–529. https://doi.org/10.1016/j.jhazmat.2009.02.075
Nakamiya K, Hashimoto S, Ito H, Edmonds JS (2005) Degradation of 1,4-dioxane and cyclic ethers by an isolated fungus. Appl Environ Microbiol 71:1254–1258. https://doi.org/10.1128/AEM.71.3.1254
Navada KK, Kulal A (2019) Enzymatic degradation of chloramphenicol by laccase from Trametes hirsuta and comparison among mediators. Int Biodeterior Biodegrad 138:63–69. https://doi.org/10.1016/j.ibiod.2018.12.012
Navada KK, Sanjeev G, Kulal A (2018) Enhanced biodegradation and kinetics of anthraquinone dye by laccase from an electron beam irradiated endophytic fungus. Int Biodeterior Biodegrad 132:241–250. https://doi.org/10.1016/j.ibiod.2018.04.012
Parales RE, Adamus JE, White N, May HD (1994) Degradation of 1, 4-dioxane by an actinomycete in pure culture. Appl environ Microbiol 60:4527–4530
Park YM, Pyo H, Park SJ, Park SK (2005) Development of the analytical method for 1,4-dioxane in water by liquid-liquid extraction. Anal Chim Acta 548:109–115. https://doi.org/10.1016/j.aca.2005.05.057
Patil PD, Yadav GD (2018) Application of microwave assisted three phase partitioning method for purification of laccase from Trametes hirsuta. Process Biochem 65:220–227. https://doi.org/10.1016/j.procbio.2017.10.006
Zapata-Castillo P (2012) Purification and characterization of laccase from Trametes hirsuta Bm-2 and its contribution to dye and effluent decolorization. Afr J Biotechnol 11:3603–3611. https://doi.org/10.5897/AJB11.2050
Polak J, Jarosz-Wilkolazka A, Szuster-Ciesielska A et al (2016) Toxicity and dyeing properties of dyes obtained through laccase-mediated synthesis. J Clean Prod 112:4265–4272. https://doi.org/10.1016/j.jclepro.2015.07.044
Prinz A, Hönig J, Schüttmann I et al (2014) Separation and purification of laccases from two different fungi using aqueous two-phase extraction. Process Biochem 49:335–346. https://doi.org/10.1016/j.procbio.2013.11.006
Raseda N, Hong S, Yul Kwon O, Ryu K (2014) Kinetic evidence for the interactive inhibition of laccase from Trametes versicolor by pH and chloride. J Microbiol Biotechnol 24:1673–1678. https://doi.org/10.4014/jmb.1408.08012
Rebrikov DN, Stepanova EV, Koroleva OV et al (2006) Laccase of the lignolytic fungus Trametes hirsuta: purification and characterization of the enzyme, and cloning and primary structure of the gene. Appl Biochem Microbiol 42:564–572. https://doi.org/10.1134/S0003683806060068
Robles A, Lucas R, Martínez-Cañamero M et al (2002) Characterisation of laccase activity produced by the hyphomycete Chalara (syn. Thielaviopsis) paradoxa CH32. Enzyme Microb Technol 31:516–522. https://doi.org/10.1016/S0141-0229(02)00142-4
Ryan S, Schnitzhofer W, Tzanov T et al (2003) An acid-stable laccase from Sclerotium rolfsii with potential for wool dye decolourization. Enzyme Microb Technol 33:766–774. https://doi.org/10.1016/S0141-0229(03)00162-5
Sadeghi S, Fooladi E, Malekaneh M (2014) A new amperometric biosensor based on Fe3O4/polyaniline/laccase/chitosan biocomposite-modified carbon paste electrode for determination of catechol in tea leaves. Appl Biochem Biotechnol 175:1603–1616. https://doi.org/10.1007/s12010-014-1380-6
Sadhasivam S, Savitha S, Swaminathan K, Lin FH (2008) Production, purification and characterization of mid-redox potential laccase from a newly isolated Trichoderma harzianum WL1. Process Biochem 43:736–742. https://doi.org/10.1016/j.procbio.2008.02.017
Scalia S (1990) Reversed-phase high-performance liquid chromatographic method for the assay of 1,4-dioxane in sulphated polyoxyethylene alcohol surfactants. J Pharm Biomed Anal 8:867–870. https://doi.org/10.1016/0731-7085(90)80134-B
Sei K, Miyagaki K, Kakinoki T et al (2013) Isolation and characterization of bacterial strains that have high ability to degrade 1,4-dioxane as a sole carbon and energy source. Biodegradation 24:665–674. https://doi.org/10.1007/s10532-012-9614-1
Sekretaryova AN, Volkov AV, Zozoulenko IV et al (2016) Total phenol analysis of weakly supported water using a laccase-based microband biosensor. Anal Chim Acta 907:45–53. https://doi.org/10.1016/j.aca.2015.12.006
Shin KS, Lee YJ (2000) Purification and characterization of a new member of the laccase family from the white-rot basidiomycete Coriolus hirsutus. Arch Biochem Biophys 384:109–115. https://doi.org/10.1006/abbi.2000.2083
Shleev SV, Morozova OV, Nikitina OV et al (2004) Comparison of physico-chemical characteristics of four laccases from different basidiomycetes. Biochimie 86:693–703. https://doi.org/10.1016/j.biochi.2004.08.005
Sun B, Ko K, Ramsay JA (2011) Biodegradation of 1,4-dioxane by a Flavobacterium. Biodegradation 22:651–659. https://doi.org/10.1007/s10532-010-9438-9
Wang L, Nie Y, Tang YQ et al (2016) Diverse bacteria with lignin degrading potentials isolated from two ranks of coal. Front Microbiol 7:1–14. https://doi.org/10.3389/fmicb.2016.01428
Yamamoto N, Saito Y, Inoue D et al (2018) Characterization of newly isolated Pseudonocardia sp. N23 with high 1,4-dioxane-degrading ability. J Biosci Bioeng 125:552–558. https://doi.org/10.1016/j.jbiosc.2017.12.005
Yang Y, Ding Y, Liao X, Cai Y (2013) Purification and characterization of a new laccase from Shiraia sp.SUPER-H168. Process Biochem 48:351–357. https://doi.org/10.1016/j.procbio.2012.12.011
Zapata-Castillo P, Villalonga-Santana L, Islas-Flores I et al (2015) Synergistic action of laccases from Trametes hirsuta Bm2 improves decolourization of indigo carmine. Lett Appl Microbiol 61:252–258. https://doi.org/10.1111/lam.12451
Zenker MJ, Borden RC, Barlaz MA (2003) Occurrence and treatment of 1,4-dioxane in aqueous environments. Environ Eng Sci 20:423–432. https://doi.org/10.3906/zoo-1705-3
Zou YJ, Wang HX, Ng TB et al (2012) Purification and characterization of a novel laccase from the edible mushroom Hericium coralloides. J Microbiol 50:72–78. https://doi.org/10.1007/s12275-012-1372-6
Zouari-Mechichi H, Mechichi T, Dhouib A et al (2006) Laccase purification and characterization from Trametes trogii isolated in Tunisia: decolorization of textile dyes by the purified enzyme. Enzyme Microb Technol 39:141–148. https://doi.org/10.1016/j.enzmictec.2005.11.027
Acknowledgements
This work was supported by BRNS, DAE, Govt. of India, Project No. 34(1)/14/01/2014. Authors thank Vision Group on Science and Technology, Govt. of Karnataka, India and Admar Mutt Education Foundation (AMEF), Udupi, India for the infrastructure facility. KKN would like to thank Mr.Nagendra Kulal and his guide Dr. Ganapati V Shanbhag from Materials Science division of PPISR for helping in GC analysis.
Funding
Funding was supported by Board of Research in Nuclear Sciences [Grant No. 34(1)/14/01/2014].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Supplementary information
Supplementary Fig. 1—Kinetics of laccase inhibition represented through Lineweaver-Burke plot and secondary plot for Ki determination. (a) LB plot depicting laccase inhibition by NaN3, (b) Secondary plot for laccase inhibition by NaN3 and (c) LB plot depicting laccase inhibition by EDTA and (d) Secondary plot for laccase inhibition by EDTA.
Supplementary Fig. 2—HPLC chromatograms for laccase mediated 1,4-dioxane degradation. Degradation of 1,4-dioxane run in a RP C18 column using 95% acetonitrile as mobile phase and chromatogram represented (a) before degradation, peaks at RT 3.43, 4.12 min-un-identified peaks and at RT 5.53 min represented for 1,4-dioxane (b) after 2 h of laccase mediated degradation, peak at RT 3.35 min-un-identified peak.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Navada, K.K., Kulal, A. Kinetic characterization of purified laccase from Trametes hirsuta: a study on laccase catalyzed biotransformation of 1,4-dioxane. Biotechnol Lett 43, 613–626 (2021). https://doi.org/10.1007/s10529-020-03038-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-03038-1