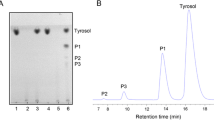
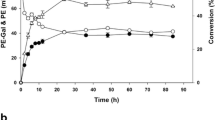
Abstract
Objective
To improve the stability of β-galactosidase from Bacillus megaterium YZ08 (BMG) in aqueous hydrophilic solvents and promote its application in the galactosylation of natural products.
Results
The addition of 5 mM Mg2+ significantly enhanced the stability of BMG in aqueous hydrophilic solvents, and the half-lives of BMG in these solutions reached 56 min to 208 h, while they were only 7 min to 5.9 h without addition of Mg2+. Studies on the kinetic parameters in buffer solution and 30% dimethyl sulfoxide (DMSO) indicated that the affinity of BMG to 2-nitrophenyl-β-d-galactopyranoside and its catalytic efficiency (κ cat/K m) increased with the addition of Mg2+. Furthermore, the addition of Mg2+ facilitated galactosylation reactions in 30% DMSO and increased product conversions by 24–41% due to the reversal of the thermodynamic equilibrium of hydrolysis.
Conclusion
A convenient approach was established to improve the stability of BMG in aqueous hydrophilic solvents.



Similar content being viewed by others
References
Bohm G, Muhr R, Jaenicke R (1992) Quantitative-analysis of protein far uv circular-dichroism spectra by neural networks. Protein Eng 5:191–195
Chu J, Wu X, Li B, He B (2014) Efficient glucosylation of flavonoids by organic solvent-tolerant Staphylococcus saprophyticus CQ16 in aqueous hydrophilic media. J Mol Catal B 99:8–13
Devalapallya H, Navath RS, Yenamandra V, Akkinepally RR, Devarakonda RK (2007) β-Galactoside pro-drugs of doxorubicin for application in antibody directed enzyme pro-drug therapy/pro-drug monotherapy. Arch Pharmacol Res 30:723–732
Dunn IS, Jennings PA (1992) Mutant forms of β-galactosidase with an altered requirement for magnesium ions. Protein Eng 5:441–446
Heidecke CD, Parsons TB, Fairbanks AJ (2009) Endohexosaminidase-catalysed glycosylation with oxazoline donors: effects of organic co-solvent and pH on reactions catalysed by Endo A and Endo M. Carbohydr Res 344:2433–2438
Juers DH, Matthews BW, Huber RE (2012) LacZ β-galactosidase: structure and function of an enzyme of historical and molecular biological importance. Protein Sci 21:1792–1807
Lousa D, Cianci M, Helliwell JR, Halling PJ, Baptista AM, Soares CM (2012) Interaction of counterions with subtilisin in acetonitrile: insights from molecular dynamics simulations. J Phys Chem B 116:5838–5848
Melisi D, Curcio A, Luongo E, Morelli E, Rimoli MG (2011) d-Galactose as a vector for pro-drug design. Curr Top Med Chem 11:2288–2298
Micaelo NM, Teixeira VH, Baptista AM, Soares CM (2005) Water dependent properties of cutinase in nonaqueous solvents: a computational study of enantioselectivity. Biophys J 89:999–1008
Page MJ, Di Cera E (2006) Role of Na+ and K+ in enzyme function. Physiol Rev 86:1049–1092
Sun H, He B, Xu J, Wu B, Ouyang P (2011) Efficient chemo-enzymatic synthesis of endomorphin-1 using organic solvent stable proteases to green the synthesis of the peptide. Green Chem 13:1680–1685
Wu X, Chu J, Wu B, Zhang S, He B (2013) An efficient novel glycosylation of flavonoid by β-fructosidase resistant to hydrophilic organic solvents. Bioresour Technol 129:659–662
Xu J, Zhuang Y, Wu B, Su L, He B (2013) Calcium-ion-induced stabilization of the protease from Bacillus cereus WQ9-2 in aqueous hydrophilic solvents: effect of calcium ion binding on the hydration shell and intramolecular interactions. J Biol Inorg Chem 18:211–221
Zhou Y, Chu J, Zhang J, Liu K, He B (2016) Precisely regulated galactosylation of nucleoside analogues in aqueous hydrophilic solvents catalyzed by solvent-stable β-galactosidase. Rsc Adv 6:64841–64846
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81673321, 21376119, 21506099), and Natural Science Foundation of the Jiangsu Higher Education Institution of China (15KJB530008), and Colleges and Universities in Jiangsu Province plans to graduate research and innovation (KYZZ16_0241).
Supporting information
Supplementary Fig. 1—Thermostability of BMG. For determination of thermostability, the residual activity of BMG was measured according to the standard assay after a 2 h pre-incubation at different temperatures.
Supplementary Fig. 2—HPLC analysis of the mixture of enzymatic galactosylation of aesculin in buffer and 30 % (v/v) DMSO.
Supplementary Fig. 3—HPLC analysis of the mixture of enzymatic galactosylation of naringin in buffer and 30 % (v/v) DMSO.
Supplementary Fig. 4—HPLC analysis of the mixture of enzymatic galactosylation of polydatin in buffer and 30 % (v/v) DMSO.
Supplementary Fig. 5—HPLC analysis of the mixture of enzymatic galactosylation of bergenin in buffer and 30 % (v/v) DMSO.
Supplementary Fig. 6—Mass spectrometry for β-galactosyl polydatin. The MS was operated in the positive ion mode.
Supplementary Fig. 7—Mass spectrometry for β-galactosyl naringin. The MS was operated in the negative ion mode.
Supplementary Fig. 8—Mass spectrometry for β-galactosyl aesculin. The MS was operated in the negative ion mode.
Supplementary Fig. 9—Mass spectrometry for β-galactosyl bergenin. The MS was operated in the negative ion mode.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, Y., Liu, K., Zhang, J. et al. Mg2+-induced stabilization of β-galactosidase from Bacillus megaterium and its application in the galactosylation of natural products. Biotechnol Lett 39, 1175–1181 (2017). https://doi.org/10.1007/s10529-017-2344-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2344-z