Abstract
Objective
To improve the production of trans-10,cis-12-conjugated linoleic acid (t10,c12-CLA) from linoleic acid in recombinant Yarrowia lipolytica.
Results
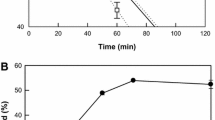
Cells of the yeast were permeabilized by freeze/thawing. The optimal conditions for t10,c12-CLA production by the permeabilized cells were at 28 °C, pH 7, 200 rpm with 1.5 g sodium acetate l−1, 100 g wet cells l−1, and 25 g LA l−1. Under these conditions, the permeabilized cells produced 15.6 g t10,c12-CLA l−1 after 40 h, with a conversion yield of 62 %. The permeabilized cells could be used repeatedly for three cycles, with the t10,c12-CLA extracellular production remaining above 10 g l−1.
Conclusion
Synthesis of t10,c12-CLA was achieved using a novel method, and the production reported in this work is the highest value reported to date.





Similar content being viewed by others
References
Aguedo M, Waché Y, Coste F, Husson F, Belin JM (2004) Impact of surfactants on the biotransformation of methyl ricinoleate into γ-decalactone by Yarrowia lipolytica. J Mol Catal B 29:31–36
Churruca I, Fernández-Quintela A, Portillo MP (2009) Conjugated linoleic acid isomers: differences in metabolism and biological effects. Biofactors 35:105–111
Crumb DJ, Vattem DA (2011) Conjugated linoleic acid (CLA)-an overview. Int J Appl Res Nat Prod 4:12–18
de Carvalho CCRC (2011) Enzymatic and whole cell catalysis: finding new strategies for old processes. Biotechnol Adv 29:75–83
Deng MD, Grund AD, Schneider KJ, Langley KM, Wassink SL, Peng SS, Rosson RA (2007) Linoleic acid isomerase from Propionibacterium acnes: purification, characterization, molecular cloning, and heterologous expression. Appl Biochem Biotechnol 143:199–211
He XH, Shang JL, Li F, Liu H (2015) Yeast cell surface display of linoleic acid isomerase from Propionibacterium acnes and its application for the production of trans10, cis12 conjugated linoleic acid. Biotechnol Appl Biochem 62:1–8
Rosberg-Cody E, Johnson MC, Fitzgerald GF, Ross PR, Stanton C (2007) Heterologous expression of linoleic acid isomerase from Propionibacterium acnes and anti-proliferative activity of recombinant trans-10, cis-12 conjugated linoleic acid. Microbiology 153:2483–2490
Ruan ZH, Hollinshead W, Isaguirre C, Tang TJ, Liao W, Liu Y (2015) Effects of inhibitory compounds in lignocellulosic hydrolysates on Mortierella isabellina growth and carbon utilization. Bioresour Technol 183:18–24
Sehat N, Yurawecz MP, Roach JA, Mossoba MM, Kramer JK, Ku Y (1998) Silver-ion high-performance liquid chromatographic separation and identification of conjugated linoleic acid isomers. Lipids 33:217–221
Thevenieau F, Beopoulos A, Desfougeres T, Sabirova J, Albertin K, Zinjarde S, Nicaud JM (2010) Uptake and assimilation of hydrophobic substrates by the oleaginous yeast Yarrowia lipolytica. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin, pp 1513–1527
Zhang BX, Chen HQ, Li M, Gu ZN, Song YD, Ratledge C, Chen YQ, Zhang H, Chen W (2013) Genetic engineering of Yarrowia lipolytica for enhanced production of trans-10, cis-12 conjugated linoleic acid. Microb Cell Fact 12:70
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31501457), the Natural Science Foundation of Jiangsu Province (No. BK20150144) and the “Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province”.
Supporting information
Supplementary Figure 1—Effects of pH (A) and phosphate buffer concentration (B) on t10,c12-CLA production.
Supplementary Figure 2—Effects of substrate concentration on t10,c12-CLA production and conversion yield (A) and lipid-free biomass and cell viability (B).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10529_2016_2175_MOESM1_ESM.tif
Supplementary material 1 (TIFF 1993 kb) Effects of pH on t10,c12-CLA production. Reactions were performed in phosphate buffer containing 100 g wet cells l−1 and 25 g LA l−1 at 28 ºC for 24 h at an agitation speed of 200 rpm. Data are the mean ± SD of three replicates, and different letters represent significant differences (p < 0.05)
10529_2016_2175_MOESM2_ESM.tif
Supplementary material 2 (TIFF 1939 kb) Effects of phosphate buffer concentration on t10,c12-CLA production. Reactions were performed in phosphate buffer containing 100 g wet cells l−1 and 25 g LA l−1 at 28 ºC for 24 h at an agitation speed of 200 rpm. Data are the mean ± SD of three replicates, and different letters represent significant differences (p < 0.05)
10529_2016_2175_MOESM3_ESM.tif
Supplementary material 3 (TIFF 1974 kb) Effects of substrate concentration on t10,c12-CLA production and conversion yield. Reactions were performed in 50 mM phosphate buffer (pH 6.8) containing 100 g wet cells l−1 and different concentrations of LA at 28 ºC for 24 h at an agitation speed of 200 rpm. Data are the mean ± SD of three replicates, and different letters represent significant differences (p < 0.05)
10529_2016_2175_MOESM4_ESM.tif
Supplementary material 4 (TIFF 1953 kb) lipid-free biomass and cell viability. Reactions were performed in 50 mM phosphate buffer (pH 6.8) containing 100 g wet cells l−1 and different concentrations of LA at 28 ºC for 24 h at an agitation speed of 200 rpm. Data are the mean ± SD of three replicates, and different letters represent significant differences (p < 0.05)
Rights and permissions
About this article
Cite this article
Zhang, B., Song, Y., Chen, H. et al. Production of trans-10,cis-12-conjugated linoleic acid using permeabilized whole-cell biocatalyst of Yarrowia lipolytica . Biotechnol Lett 38, 1917–1922 (2016). https://doi.org/10.1007/s10529-016-2175-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2175-3