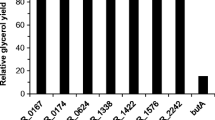
Abstract
Objectives
To investigate the efficiency of a cofactor regeneration enzyme co-expressed with a glycerol dehydrogenase for the production of 1,3-dihydroxyacetone (DHA).
Results
In vitro biotransformation of glycerol was achieved with the cell-free extracts containing recombinant GlyDH (glycerol dehydrogenase from Escherichia coli), LDH (lactate dehydrogenase form Bacillus subtilis) or LpNox1 (NADH oxidase from Lactobacillus pentosus), giving DHA at 1.3 g l−1 (GlyDH/LDH) and 2.2 g l−1 (GlyDH/LpNox1) with total turnover number (TTN) of NAD+ recycling of 6039 and 11100, respectively. Whole cells of E. coli (GlyDH–LpNox1) co-expressing both GlyDH and LpNox1 were constructed and converted 10 g glycerol l−1 to DHA at 0.2–0.5 g l−1 in the presence of zero to 2 mM exogenous NAD+. The cell free extract of E. coli (GlyDH–LpNox) converted glycerol (2–50 g l−1) to DHA from 0.5 to 4.0 g l−1 (8–25 % conversion) without exogenous NAD+.
Conclusions
The disadvantage of the expensive consumption of NAD+ for the production of DHA has been overcome.





Similar content being viewed by others
References
Beauchamp J, Gross PG, Vieille C (2014) Characterization of Thermotoga maritime glycerol dehydrogenase for the enzymatic production of dihydroxyacetone. Appl Microbiol Biotechnol 98:7039–7050
Enders D, Voith M, Lenzen A (2005) The dihydroxyacetone unit—a versatile C3 building block in organic synthesis. Angew Chem Int Edit 44:1304–1325
Hu ZC, Zheng YG, Shen YC (2011) Use of glycerol for producing 1,3-dihydroxyacetone by Gluconobacter oxydans in an airlift bioreactor. Bioresour Technol 102:7177–7182
Katryniok B, Kimura H, Skrzynska E, Girardon JS, Fongarland P, Capron M, Ducoulombier R, Mimura N, Paul S, Dumeignil F (2011) Selective catalytic oxidation of glycerol: perspectives for high value chemicals. Green Chem 13:1960–1979
Li C, Lesnik KL, Liu H (2013) Microbial conversion of waste glycerol from biodiesel production into value-added products. Energies 6:4739–4768
Liu ZQ, Hu ZC, Zheng YG, Shen YC (2008) Optimization of cultivation conditions for the production of 1,3-dihydroxyacetone by Pichia membranifaciens using response surface methodology. Biochem Eng J 38:285–291
Pagliaro M, Ciriminna R, Kimura H, Rossi M, Della Pina C (2007) From glycerol to value-added products. Angew Chem Int Edit 46:4434–4440
Rahmat N, Abdullah AZ, Mohamed AR (2010) Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: a critical review. Renew Sust Energy Rev 14:987–1000
Rocha-Martín J, de las Rivas B, Muñoz R, Guisán JM, López-Gallego F (2012) Rational co-immobilization of bi-enzyme cascades on porous supports and their applications in bio-redox reactions with in situ recycling of soluble cofactors. Chem Cat Chem 4:1279–1288
Suga Y, Ikejima A, Matsuba S, Ogawa H (2002) Medical pearl: DHA application for camouflaging segmental vitiligo and piebald lesions. J Am Acad Dermatol 47:436–438
Wang Z, Zhuge J, Fang H, Prior BA (2001) Glycerol production by microbial fermentation: a review. Biotechnol Adv 19:201–223
Weiner M, Albermann C, Gottlieb K, Sprenger GA, Weuster-Botz D (2014) Fed batch production of l-phenylalanine from glycerol and ammonia with recombinant Escherichia coli. Biochem Eng J 83:62–69
Zhang W, O’Connor K, Wang DI, Li Z (2009) Bioreduction with efficient recycling of NADPH by coupled permeabilized microorganisms. Appl Environ Microbiol 75:687–694
Zhang JD, Li AT, Yu HL (2010) Synthesis of optically pure S-sulfoxide by Escherichia coli transformant cells coexpressing the P450 monooxygenase and glucose dehydrogenase genes. J Ind Microbiol Biotechnol 38:633–641
Zhang JD, Wu SK, Wu JC, Li Z (2015) Enantioselective cascade biocatalysis via epoxide hydrolysis and alcohol oxidation: one-pot synthesis of (R)-α-hydroxy ketones from Meso- or racemic epoxides. ACS Catal 5:51–58
Zhang JD, Cui ZM, Fan XJ, Wu HL, Chang HH (2016) Cloning and characterization of two distinct water-forming NADH oxidases from Lactobacillus pentosus for the regeneration of NAD. Bioproc Biosyst Eng 39:603–611
Zhou YJ, Wang L, Yang F, Lin XP, Zhang SF, Zhao ZBK (2011) Determining the extremes of the cellular NAD(H) level by using an Escherichia coli NAD+-auxotrophic mutant. Appl Environ Microbiol 77:6133–6140
Acknowledgements
This work was financially supported by the Qualified Personnel Foundation of Taiyuan University of Technology (Grant No. tyut-rc201484a) and the Youth Foundation of Taiyuan University of Technology (Grant No. 1205-0020202) and the Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (STIP) (Grant No. 2015132).
Supporting information
Supplementary Table 1—The recombinant plasmids and E. coli strains used in this study.
Supplementary Table 2—The primers used for PCR amplification of DNA sequences.
Supplementary Fig. 1—SDS-PAGE analysis of cell free extract of E. coli (LDH), E. coli (LpNox1) and E. coli (GlyDH).
Supplementary Fig. 2—SDS-PAGE analysis of the cell free extract of E. coli (GlyDH–LpNox1) after induction.
Supplementary Fig. 3—Effect of IPTG concentration (a) and induction temperature (b) on GlyDH and LpNox1 expression in E. coli (GlyDH–LpNox1).
Supplementary Fig. 4—Time profiles of cell density (g cdw l−1) and the activity of GlyDH and LpNox1 during fermentation process.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, J., Cui, Z., Chang, H. et al. Conversion of glycerol to 1,3-dihydroxyacetone by glycerol dehydrogenase co-expressed with an NADH oxidase for cofactor regeneration. Biotechnol Lett 38, 1559–1564 (2016). https://doi.org/10.1007/s10529-016-2130-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2130-3