Abstract
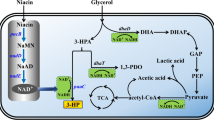
The limiting step for biosynthesis of 3-hydroxypropionic acid (3-HP) in Klebsiella pneumoniae is the conversion of 3-hydroxypropionaldehyde (3-HPA) to 3-HP. This reaction is catalyzed by aldehyde dehydrogenase (ALDH) with NAD+ as a cofactor. Although NAD+-dependent ALDH overexpression facilitates 3-HP biosynthesis, ALDH activity decreases and 3-HP stops accumulation when NAD+ is exhausted. Here, we show that an NAD+-independent aldehyde oxidase (AOX) from Pseudomonas sp. AIU 362 holds promise for cofactor-balanced 3-HP production in K. pneumoniae. The AOX coding gene, alod, was heterologously expressed in E. coli and K. pneumoniae, and their respective crude cell extracts showed 38.1 U/mg and 16.6 U/mg activities toward propionaldehyde. The recombinant K. pneumoniae expressing alod showed 13.7 U/mg activity toward 3-HPA; K m and V max were 6.7 mM and 42 μM/min/mg, respectively. In shake-flask cultures, the recombinant K. pneumoniae strain produced 0.89 g 3-HP/l, twice that of the control. Moreover, it produced 3 g 3-HP/l during 24 h fed-batch cultivation in a 5 l bioreactor. The results indicate that AOX can efficiently convert 3-HPA into 3-HP.



Similar content being viewed by others
References
Ashok S, Raj S, Ko Y, Sankaranarayanan M, Zhou S, Kumar V, Park S (2012) Effect of puuC overexpression and nitrate addition on glycerol metabolism and anaerobic 3-hydroxypropionic acid production in recombinant Klebsiella pneumoniae ΔglpK ΔdhaT. Metab Eng 15:1–24
Calzi M, Raviolo C, Ghibaudi E, Gioia L, Salmona M, Cazzaniga G, Kurosaki M, Terao M, Garattini E (1995) Purification, cDNA cloning, and tissue distribution of bovine liver aldehyde oxidase. J Biol Chem 270:31037–31045
Felsted R, Chu A, Chaykin S (1973) Purification and properties of the aldehyde oxidases from hogand rabbit livers. J Biol Chem 248:2580–2587
Forage RG, Foster MA (1982) Glycerol fermentation in Klebsiella pneumoniae: functions of the coenzyme B12-dependent glycerol and diol dehydratases. J Bacteriol 149:413–419
Forage RG, Lin EC (1982) DHA system mediating aerobic and anaerobic dissimilation of glycerol in Klebsiella pneumoniae NCIB 418. J Bacteriol 151:591–599
Huang D, Ichikawa Y (1994) Two different enzymes are primarily responsible for retinoic acid synthesis in rabbit liver cytosol. Biochem Biophys Res Commun 205:1278–1283
Huang Y, Li Z, Shimizu K, Ye Q (2012) Simultaneous production of 3-hydroxypropionic acid and 1,3-propanediol from glycerol by a recombinant strain of Klebsiella pneumoniae. Bioresour Technol 103:351–359
Huang Y, Li Z, Shimizu K, Ye Q (2013) Co-production of 3-hydroxypropionic acid and 1,3-propanediol by Klebseilla pneumoniae expressing aldH under microaerobic conditions. Bioresour Technol 128:505–512
Kumar V, Sankaranarayanan M, Jae K, Durgapal M, Ashok S, Ko Y, Sarkar R, Park S (2012) Co-production of 3-hydroxypropionic acid and 1,3-propanediol from glycerol using resting cells of recombinant Klebsiella pneumoniae J2B strain overexpressing aldehyde dehydrogenase. Appl Microbiol Biotechnol 96:373–383
Li Y, Su MY, Ge XZ, Tian PF (2013) Enhanced aldehyde dehydrogenase activity by regenerating NAD+ in Klebsiella pneumoniae and implications for the glycerol dissimilation pathways. Biotechnol Lett 35:1609–1615
Luo LH, Seo J, Oh B, Seo P, Heo S, Hong W, Kim D, Kim CH (2011) Stimulation of reductive glycerol metabolism by overexpression of an aldehyde dehydrogenase in a recombinant Klebsiella pneumoniae strain defective in the oxidative pathway. J Ind Microbiol Biotechnol 38:991–999
Sasaki Y, Isobe K, Kataoka M, Ogawa J, Iwasaki A, Hasegawa J, Shimizu S (2008) Purification and characterization of a new aldehyde oxidase from Pseudomonas sp. AIU 362. J Biosci Bioeng 106:297–302
Sasaki Y, Urano N, Kataoka M, Ogawa J, Iwasaki A, Hasegawa J, Isobe K, Shimizu S (2012) Cloning and sequencing of a gene encoding of aldehyde oxidase in Pseudomonas sp. AIU 362. J Biosci Bioeng 114:28–32
Sekimoto H, Seo M, Kawakami N, Komano T, Desloire S, Liotenberg S, Marion A, Caboche M, Kamiya Y, Koshiba T (1998) Molecular cloning and characterization of aldehyde oxidases in Arabidopsis thaliana. Plant Cell Physiol 39:433–442
Werpy T, Petersen G (2004) Top value added chemicals from biomass. U.S. DOE, Washington, DC
Yasuhara A, Akiba M, Fujishiro K, Uchida H, Uwajima T, Aisaka K (2002) Production of aldehyde oxidases by microorganisms and their enzymatic properties. J Biosci Bioeng 94:124–129
Acknowledgments
This work was supported by Grants from National Natural Science Foundation of China (No. 21276014) and National Basic Research Program of China (973 Program) (2012CB725200).
Author information
Authors and Affiliations
Corresponding author
Additional information
Ying Li and Luo Liu have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Y., Liu, L. & Tian, P. NAD+-independent aldehyde oxidase catalyzes cofactor balanced 3-hydroxypropionic acid production in Klebsiella pneumoniae . Biotechnol Lett 36, 2215–2221 (2014). https://doi.org/10.1007/s10529-014-1590-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1590-6