Abstract
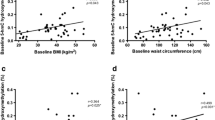
In children, teenagers, and young adults, environmental factors and genetic modifications have contributed to the development of obesity. There is a close relationship between obesity and circadian rhythm. To understand the role of CLOCK and BMAL1 in obesity, we analyzed the methylation status of CLOCK and BMAL1 in obese and control subjects. In this paper, we analyzed the methylation status of the CLOCK and BMAL1 genes by using MS-HRM in a total of 55 obese and 54 control subjects. In our study, we demonstrated that the level of fasting glucose and the level of HDL-cholesterol were associated with CLOCK methylation in obesity. We also showed a significant association between BMAL1 gene methylation and waist and hip circumference in obese subjects. This is the first study that shows the methylation of BMAL1 is associated with the obese phenotype. However, we could not show a direct association between CLOCK methylation and the obese phenotype. In this paper, a novel epigenetic interaction between circadian clock genes and obesity was demonstrated.


Similar content being viewed by others
Data Availability
The genetics data that support the findings of this study are available on request from the corresponding author (RK).
References
Azevedo PG, Miranda LR, Nicolau ES, Alves RB, Bicalho MAC, Couto PP, Ramos AV, Souza RP, Longhi R, Friedman E, Marco L (2021) Bastos-Rodrigues L. Genetic association of the PERIOD3 (PER3) Clock gene with extreme obesity. Obes Res Clin Pract 15(4):334–338
Barnea M, Chapnik N, Genzer Y, Froy O (2015) The circadian clock machinery controls adiponectin expression. Mol Cell Endocrinol 399:284–287
Baron KG, Reid KJ, Kim T et al (2017) Circadian timing and alignment in healthy adults: associations with BMI, body fat, caloric intake and physical activity. Int J Obes (lond) 41(2):203–209
Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ (2005) Circadian rhythms from multiple oscillators: lessons from divers organisms. Nat Rev Genet 6:544–556
Boutari C, Mantzoros CS (2022) A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 133:155217. https://doi.org/10.1016/j.metabol.2022.155217
Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA (2000) Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103:1009–1017
Dyar KA, Ciciliot S, Wright LE, Biensø RS, Tagliazucchi GM, Patel VR, Forcato M, Paz MIP, Gudiksen A, Solagna F et al (2014) Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab 3:29–41
Eide EJ, Woolf MF, Kang H et al (2005) Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation. Mol Cell Biol 25:2795–2807
Garaulet M, Lee YC, Shen J et al (2009) CLOCK genetic variation and metabolic syndrome risk: modulation by monounsaturated fatty acids. Am J Clin Nutr 90:1466–1475
Garaulet M, Corbalán MD, Madrid JA, Morales E, Baraza JC, Lee YC, Ordovas JM (2010) CLOCK gene is implicated in weight reduction in obese patients participating in a dietary programme based on the Mediterranean diet. Int J Obes 34(3):516–523
Gibson EM, Wang C, Tjho S, Khattar N, Kriegsfeld LJ (2010) Experimental “jet lag” inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PLoS ONE 5(12):e15267. https://doi.org/10.1371/journal.pone.0015267
Godinho SI, Maywood ES, Shaw L et al (2007) The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science 316:897–900
Gooley JJ (2016) Circadian regulation of lipid metabolism. Proc Nutr Soc 75:440–450
Grosjean E, Simonneaux V, Challet E (2023) Reciprocal interactions between circadian clocks, food intake, and energy metabolism. Biology 12(4):539. https://doi.org/10.3390/biology12040539
Honma S, Kawamoto T, Takagi Y, Fujimoto K, Sato F, Noshiro M, Kato Y, Honma K (2002) Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature 419:841–844
Kalkan R, Becer E (2019) RANK/RANKL/OPG pathway is an important for the epigenetic regulation of obesity. Mol Biol Rep 46(5):5425–5432. https://doi.org/10.1007/s11033-019-04997-z
Kettner NM, Mayo SA, Hua J, Lee C, Moore DD, Fu L (2015) Circadian dysfunction induces leptin resistance in mice. Cell Metab 22(3):448–459. https://doi.org/10.1016/j.cmet.2015.06.005
Lamia KA, Storch KF, Weitz CJ (2008) Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA 105:15172–15177
Li Y, Ma J, Yao K, Su W, Tan B, Wu X, Huang X, Li T, Yin Y, Tosini G, Yin J (2020) Circadian rhythms and obesity: timekeeping governs lipid metabolism. J Pineal Res 69(3):e12682. https://doi.org/10.1111/jpi.12682
Liu X, Zheng C, Cheng X, Qian L, Peng Z (2017) Nighttime snacking is associated with risk of obesity and hyperglycemia in adults: a cross-sectional survey from Chinese adult teachers. J Biomed Res 31(6):541
Loos RJF, Yeo GSH (2022) The genetics of obesity: from discovery to biology. Nat Rev Genet 23(2):120–133. https://doi.org/10.1038/s41576-021-00414-z
Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH et al (2010) Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466:627–631
Matthews DR, Hosker JP, Rudenski AS et al (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia 28:412–419
Milagro FI, Gómez-Abellán P, Campión J, Martínez JA, Ordovás JM, Garaulet M (2012) CLOCK, PER2 and BMAL1 DNA methylation: association with obesity and metabolic syndrome characteristics and monounsaturated fat intake. Chronobiol Int 29(9):1180–1194. https://doi.org/10.3109/07420528.2012.719967. (Epub 2012 Sep 24 PMID: 23003921)
Nguyen KD et al (2013) Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 341:1483–1488
Pan X, Jiang XC, Hussain MM (2013) Impaired cholesterol metabolism and enhanced atherosclerosis in clock mutant mice. Circulation 128(16):1758–1769
Parsons MJ et al (2015) Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int J Obes (lond) 39:842–848
Paschos GK et al (2012) Obesity in mice with adipocyte-specific deletion of clock component BMAL1. Nat Med 18:1768–1777
Peng H, Zhu Y, Goldberg J, Vaccarino V, Zhao J (2019) DNA methylation of five core circadian genes jointly contributes to glucose metabolism: a gene-set analysis in monozygotic twins. Front Genet 10:329. https://doi.org/10.3389/fgene.2019.00329
Qian J, Scheer FA (2016) Circadian system and glucose metabolism: implications for physiology and disease. Trends Endocrinol Metab 27(5):282–293
Schabler S, Amatobi KM, Horn M et al (2020) Loss of function in the Drosophila clock gene period results in altered intermediary lipid metabolism and increased susceptibility to starvation. Cell Mol Life Sci. https://doi.org/10.1007/s00018-019-03441-6
Scott EM, Carter AM, Grant PJ (2008) Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (lond) 32:658–662
Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, Reppert SM (2000) Interacting molecular loops in the mammalian circadian clock. Science 288(5468):1013–1019. https://doi.org/10.1126/science.288.5468.1013
Shen L, Cui A, Xue Y, Cui Y, Dong X, Gao Y, Yang H, Fang F, Chang Y (2014) Hepatic differentiated embryo-chondrocyte-expressed gene 1 (Dec1) inhibits sterol regulatory element-binding protein-1c (Srebp-1c) expression and alleviates fatty liver phenotype. J Biol Chem 289:23332–23342
Shimba S (2013) The roles of clock genes in obesity. Nihon rinsho. Jpn J Clin Med 71(2):244–248
Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, HayashiM WT, Aoyagi T, Tezuka M (2005) Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci USA 102:12071–12076
Shimba S, Ogawa T, Hitosugi S, Ichihashi Y, Nakadaira Y, Kobayashi M, Tezuka M, Kosuge Y, Ishige K, Ito Y et al (2011) Deficient of a clock gene, brain and muscle arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS ONE 6:e25231
Shirogane T, Jin J, Ang XL et al (2005) SCFbeta-TRCP controls clock-dependent transcription via casein kinase 1-dependent degradation of the mammalian period-1 (Per1) protein. J Biol Chem 280:26863–26872
Siepka SM, Yoo SH, Park J et al (2007) Circadian mutant overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell 129:1011–1023
Sookoian S, Gemma C, Gianotti TF et al (2008) Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am J Clin Nutr 87:1606–1615
Touitou Y, Reinberg A, Touitou D (2017) Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: health impacts and mechanisms of circadian disruption. Life Sci 173:94–106
Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E et al (2005) Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308(5724):1043–1045
Uemura H, Katsuura-Kamano S, Yamaguchi M et al. (2015) A variant of the CLOCK gene and related haplotypes are associated with the prevalence of type 2 diabetes in the Japanese population. J Diab (Epublication ahead of print version)
Villanueva JE, Livelo C, Trujillo AS et al (2019) Time-restricted feeding restores muscle function in Drosophila models of obesity and circadian-rhythm disruption. Nat Commun 10(1):2700
Wang S, Lin Y, Gao L, Yang Z, Lin J, Ren S et al (2022) PPAR-γ integrates obesity and adipocyte clock through epigenetic regulation of Bmal1. Theranostics 12(4):1589
WHO European Regional Obesity Report 2022, ISBN: 978–92–890–5773–8.
WHO (2016) Word Health Organization (WHO). Obesity and overweight. Geneva: WHO;2016. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/
Woon PY, Kaisaki PJ, Braganc AJ, Bihoreau MT, Levy JC, Farrall M, Gauguier D (2007) Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci USA 104:14412–14417
Yagawa M, Nagatomo Y, Izumi Y, Mahara K, Tomoike H, Shiraishi Y, Kohno T, Mizuno A, Goda A, Kohsaka S, Yoshikawa T, West Tokyo Heart Failure (WET-HF) Registry Collaborative Group (2017) Effect of obesity on the prognostic impact of atrial fibrillation in heart failure with preserved ejection fraction. Circ J 81(7):966–973. https://doi.org/10.1253/circj.CJ-16-1130
Yoo SH, Mohawk JA, Siepka SM et al (2013) Competing E3 ubiquitin ligases govern circadian periodicity by degradation of CRY in nucleus and cytoplasm. Cell 152:1091–1105
Zhang R, Lahens NF, Balance HI, Hughes ME, Hogenesch JB (2014) A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA 111(45):16219–16224
Funding
The authors received no specific funding for this work. We would like to thank all the participants involved in this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: RK; Methodology: RK and TGJ; Software: RK and TGJ; Validation: RK and TGJ; Formal analysis: RK, TGJ, and EB; Investigation: RK and TGJ; Resources: RK, TGJ, and EB; Data curation: RK, TGJ, and EB; Writing—original draft preparation: RK, TGJ, and EB; Writing—review and editing: RK, TGJ, and EB; Visualization: RK, TGJ, and EB; Supervision: R.K.; Project administration: RK; Funding acquisition: RK.
Corresponding author
Ethics declarations
Conflict of Interest
The authors affirm that they do not have any competing interests.
Ethical Approval
All subjects signed written consent forms after being fully informed. The study protocol was approved by the Research Ethics Committee of the Near East University and performed in accordance with the Declaration of Helsinki (YDU-2021/96–1425).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jesse, T.G., Becer, E. & Kalkan, R. Identification of the Relationship Between DNA Methylation of Circadian Rhythm Genes and Obesity. Biochem Genet 62, 281–293 (2024). https://doi.org/10.1007/s10528-023-10415-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-023-10415-8