Abstract
Thrips of the subtropical genus Scirtothrips are emerging as important pests in several crops. Scirtothrips dorsalis has been increasingly invading new areas outside of its native region of South and East Asia causing economic damage to several crops. Scirtothrips inermis is another polyphagous species with worldwide distribution. Both species are polyphagous, and in recent years have emerged as key pests in strawberry. In this study, we first evaluated the predation and oviposition rate of commercially available phytoseiid predatory mites Amblyseius swirskii, Amblydromalus limonicus, Transeius montdorensis, and Neoseiulus cucumeris on larval stages of both Scirtothrips species, and oviposition rates of predatory mites on the supplementary food source Artemia franciscana cysts were also assessed. Predatory mites equally accepted both thrips species as prey and showed stable oviposition rates on these diets. Amblyseius swirskii and A. limonicus were the most voracious, also exhibiting the highest oviposition rate of the predators tested. We further evaluated the biological control potential of predatory mites and anthocorid predators Orius laevigatus and Orius limbatus in a greenhouse experiment. Predators were released preventively and supported with Artemia cysts before the introduction of S. inermis. Both Orius predators achieved good control of the pest, with O. limbatus developing higher numbers than O. laevigatus. Regarding phytoseiids, A. swirskii and A. limonicus both controlled the pest and built higher populations than T. montdorensis and N. cucumeris. Our results show that a preventive strategy based on phytoseiid or anthocorid predators in strawberry can be effective in suppressing S. inermis.
Similar content being viewed by others
Introduction
Thrips are important pests of many crops around the world, partly due to their cryptic lifestyle, short life cycle, and polyphagy, making them also very successful when invading new regions (Morse and Hoddle 2005; Mound 2005). Most pestiferous thrips species are found in the Thripidae family, and these can be further distinguished according to their primary feeding preferences to true flower feeders (i.e., feeding on pollen and flower tissues), mature and senescing foliage feeders, and finally immature foliage feeders (Morse and Hoddle 2005). In the latter feeding guild, the tropical genus Scirtothrips Shull is found, with more than 100 species known from the tropics and subtropics (Mound and Palmer 1981; Hoddle and Mound 2003; Mound and Stiller 2011). Scirtothrips are typically yellow in color and minute in size, rarely exceeding 1 mm as adults. As mentioned above, Scirtothrips prefer feeding on developing plant tissue, such as young leaves, buds, and fruits. Feeding damage from Scirtothrips leads to leaf distortion, dark bronze coloration of leaves, stunt growth, and defoliation, while feeding on developing fruit leads to scarring. Due to the severity of leaf-distortion Scirtothrips feeding causes, the injection of toxic saliva in the plant tissue has been suggested (Mound and Palmer 1981). Many species in this genus are economically important pests, and while most species are thought to be monophagous damaging specific crops, such as Scirtothrips perseae Nakahara on avocado in the Americas (Hoddle et al. 2003), others have wider host range and cause significant damage to an array of crops. Arguably the most important pest in this group is the Scirtothrips dorsalis Hood species complex, comprised of several cryptic and two morphologically distinguishable species (Dickey et al. 2015). One of the species native to India (South Asia 1 sensu Dickey et al. 2015) is extremely polyphagous and has successfully invaded many parts of the world attacking a plethora of crops, including tea, mango, strawberries, kiwi, onion, persimmon, beans, peach, peppers, citrus, mango, roses, cotton, groundnuts, soybean, and tobacco, while also vectoring plant viruses in many crops (for an extensive list see Hoddle 2022). Scirtothrips aurantii is another important invasive species, predominantly damaging citrus in its native South Africa, and recently berry crops in the Iberian Peninsula (EPPO RS 2022/084). Finally, Scirtothrips inermis Priesner, a species initially described from the Canary Islands but found worldwide, is increasingly causing economic damages in crops such as strawberry, mango, citrus, and persimmon (Lacasa et al. 1996; Modesto-Henández et al. 2010; Marrero et al. 2023).
Control of Scirtothrips in field crops and orchards has been mostly relying on the use of insecticides (Hoddle et al. 2003; Seal and Kumar 2010), while monitoring of populations is usually carried out with yellow sticky traps, proven to be the most attractive color for multiple species of this genus and around the world (Bara and Laing 2020; Carrillo-Arámbula et al. 2022). However, due to the development of insecticide resistance in Scirtothrips (Kaur et al. 2023), and cases of pesticide-induced hormoligosis (Morse and Zareh 1991), rotation of chemical applications has been proposed (Kumar et al. 2017), and further research has focused on biological control solutions. Soil applications with either chemical or biocontrol agents have also been suggested, as species of this genus are thought to pupate in the soil (Grout et al. 1986; Shibao et al. 1991; Yee et al. 2001; Gilbert and Samways 2018). Many beneficial arthropods are known to attack Scirtothrips in natural ecosystems, and these have been evaluated for use in agriculture. Several generalist predators are associated with Scirtothrips in their native range and are known to limit their populations, such as predatory thrips (Thysanoptera: Aeolothripidae) (Yee et al. 2001), and generalist predatory mites of the genus Euseius Wainstein (Acari: Phytoseiidae) (Grout and Richards 1992; Grafton-Cardwell et al. 1999; Tsuchida and Masui 2023). Many studies have explored the efficacy of conserving or augmenting the population of generalist phytoseiid predators in citrus (McMurtry et al. 1992; Grafton-Cardwell and Ouyang 1995), grape (Tsuchida and Masui 2023), strawberry (Lahiri and Yambisa 2021), pepper (Arthurs et al. 2009; Doĝramaci et al. 2011), and roses (Schoeller et al. 2022). Furthermore, commercially available generalist predators have been evaluated against Scirtothrips, and while generalist predatory chrysopids (Neuroptera: Chrysopidae) have failed to provide significant pest suppression (Hoddle and Robinson 2004), anthocorid predators have been applied with success in flowering crops (Doĝramaci et al. 2011).
Advances in the field of biological control using beneficial arthropods in horticulture have led to the wide adoption of the predator-in-first or standing army approach (Messelink et al. 2014; Pijnakker et al. 2020). This strategy is based on the preventive release of generalist predators before the primary pest arrives in the crops. Generalist predators employed are in many cases omnivores and can feed on either plant-provided foods such as nectar and pollen, or alternative arthropod prey found in the crop (Coll and Guershon 2002; Symondson et al. 2002). Biological control practitioners may encourage the establishment of beneficial arthropods on banker plants that may provide food and shelter (Huang et al. 2011), or provide supplementary food sources to support a population build-up in the crop before the arrival of the pest. Such food sources may be pollen, irradiated Lepidoptera eggs, or cysts of the brine shrimp Artemia franciscana Kellog (Anostraca: Artemiidae) (Pijnakker et al. 2020). However, caution is needed when applying these food sources, as omnivorous pests such as the Western Flower thrips Frankliniella occidentalis (Pergrande) may also feed on these resources (Leman and Messelink 2015; Vangansbeke et al. 2016b), thus application needs to be limited in space and time so that it can be monopolized by natural enemies. In Scirtothrips species, omnivory has only been reported for Scirtothrips citri (Moulton) feeding on eggs and immatures of tetranychid phytophagous mites (Acari: Tetranychidae) in cotton (Mollet and Sevacherian 1985), but no other cases have been reported since then (Wang et al. 2022).
While many studies have evaluated the biological control potential of generalist predators released in various horticultural crops for the suppression of Scirtothrips species (Arthurs et al. 2009; Doĝramaci et al. 2011; Lahiri and Yambisa 2021), few have demonstrated successful control with preventive predator releases (but see: Schoeller et al. 2022). In this study, we evaluated the efficacy of both phytoseiid and anthocorid generalist predators against Scirtothrips infesting strawberry cultivation in the Canary Islands (Spain). In recent years, S. inermis has been increasingly causing significant crop damage in mango and strawberry in the archipelago (Modesto-Henández et al. 2010; Marrero et al. 2023). Furthermore, the invasive species S. dorsalis South Asia 1 has been recently detected in mango on the island of Tenerife (Mouratidis et al. 2023), and this species is known to be a pest of strawberry in Florida (USA) (Panthi et al. 2021). Here, we first evaluated the predation rate and reproductive potential of four commercially available predatory mites: Amblyseius swirskii Athias-Henriot, Amblydromalus limonicus (Garman and McGregor), Transeius montdorensis (Schicha) and Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae), feeding on larvae of either S. dorsalis, S. inermis or Artemia cysts. Then, we compared their pest control efficacy in a greenhouse experiment where predators were released on strawberry plants and supplemented with Artemia cysts before the introduction of S. inermis. In the greenhouse experiment, we further included the commercially available Orius laevigatus (Fieber) and the endemic to the Canary Islands Orius limbatus Wagner due to its importance as it spontaneously colonizes many horticultural crops in its native range (Carnero et al. 1993).
Materials and methods
Plants and insects
Strawberry plants (Fragaria × ananassa, cv. Portola, University of California, USA) were received from a commercial propagator (Viveros Campiñas SCA., Segovia, Spain). This day-neutral everbearing variety immediately starts blooming after transplant. Plants were transplanted in groups of four in plastic pots (28 cm Ø × 35 cm, Murgiplast SL, Almería, Spain) containing cocopeat (Pelemix SL, Murcia, Spain) and following standard agricultural practices. These plants were used for insect rearings, laboratory, and greenhouse experiments.
All phytoseiid predators (A. swirskii, A. limonicus, N. cucumeris, and T. montdorensis) and O. laevigatus were supplied by a biological control company (Koppert BV, Berkel en Rodenrijs, The Netherlands), and an O. limbatus rearing was initiated from a population collected from Plocama pendula Aiton plants in Guía de Isora (Tenerife, Spain), and identified through preparation of male genitalia (Péricart 1972). All insects were reared in the laboratory for at least two generations before being used in the experiments. Predatory mites were reared on plastic arenas placed on water-saturated floral foam (18 × 12 cm) that were further placed in plastic containers containing water (25 × 18 cm) in laboratory conditions (23 ± 3 °C, 60 ± 10% RH). Eggs of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) (Koppert BV) and hydrated decapsulated cysts of Artemia franciscana Kellogg (Anostraca: Artemiidae) (BioBee Biological Systems, Sde Eliyahu, Israel) (hereafter Artemia cysts) were added twice per week in the mite rearings ad libitum. Orius predators were reared in plastic jars (Ø 11 cm × 13 cm) with lids covered with a mesh gauze (size 80 μm) for ventilation. Flat green bean pods (Phaseolus vulgaris L.) purchased from a local market were washed rigorously, dried, and offered to the predators as an oviposition substrate and water source. Buckwheat husks were added to jars to provide a walking substrate for the predators. A 50:50 mix of E. kuehniella eggs and A. franciscana cysts were added in the jars twice per week ad libitum, decaying pods were replenished, and bean pods carrying predator eggs were removed to start a new synchronized rearing unit.
Scirtothrips colonies were maintained on flat green bean pods (P. vulgaris) in plastic jars (Ø 11 cm × 13 cm) with their bottom layer covered with vermiculite to provide a pupation surface for the thrips. Colonies of Scirtothrips were initiated from individuals collected from mango (Mangifera indica L.) in Guía de Isora (Tenerife, Spain) for S. dorsalis, and a strawberry crop in Güímar (Tenerife, Spain) for S. inermis. Bean pods were replaced weekly and new synchronized units were initiated. Laboratory rearings of Scirtothrips and Orius predators were maintained in separate climatic cabinets (MLR—350H®, Sanyo, Japan) at 25 ± 1 °C, 70 ± 10% RH, and a L:D 16:8 photoperiod. A second colony of S. inermis was initiated on strawberry plants in a greenhouse compartment in the Instituto Canario de Investigaciones Agrarias (ICIA, Valle Guerra, Tenerife, Spain), and thrips were reared for at least one generation before release in the greenhouse experiment.
Laboratory assessments of predatory mite predation and oviposition rates
Predation and oviposition rates of A. swirskii, A. limonicus, N. cucumeris, and T. montdorensis offered a diet of Scirtothrips larvae were assessed under laboratory conditions. In addition, the quality of Artemia cysts as a supplemental food source for the predatory mites was assessed by recording the daily oviposition rates when provided with this food source. The experiment had a total of 14 treatments, where each predatory mite was provided with either second-stage larvae (L2) of S. dorsalis, S. inermis, or Artemia cysts as a food source. Controls of S. dorsalis or S. inermis larvae in the absence of predators were also included to assess the natural mortality of thrips in the experimental set-up. The experiment was conducted in plastic medicine cups (Ø 3.5 cm × 3 cm high) (DEPA Disposables BV, Beuningen, The Netherlands) with a strawberry leaf disc (Ø 3.5 cm) placed with the abaxial side up in water agar 1.5%. Ventilation was ensured via a hole drilled in the lid and covered with a fine mesh (80 μm mesh size). In each leaf disc ten L2 S. dorsalis, ten L2 S. inermis, or an ad libitum quantity of A. franciscana cysts were added. Thrips were collected from the synchronized laboratory culture on bean pods, and added in the arenas with a minute camel brush. Then, a gravid female predatory mite (11 ± 1 days old) was added to the arena. Predatory mites were transferred to a new cup containing the same food source every day and for three consecutive days. Predation rates were assessed directly after the mites were transferred, by counting the number of thrips cadavers. Oviposited eggs of predatory mites were counted only on the second and third day of the experiment, to exclude effects of previous food sources on the predatory mites (Sabelis 1990; van Houten et al. 1995). Each predatory mite-food source combination was replicated between 8 and 12 times.
Greenhouse experiment
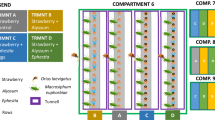
The greenhouse experiment was conducted in spring 2022 in a 60 m2 glass greenhouse compartment in the facilities of ICIA, Spain. Strawberry plants were transplanted on the 25th of April (week 1) in pots containing four plants and were individually placed inside cages (47.5 × 47.5 × 93 cm, 160 μm mesh size, BugDorm-4E4590, MegaView Science Co., Taichung, Taiwan), and provided with a standard nutrient solution through a dripping irrigation system. During the first week after transplant, flower buds were removed every other day to encourage foliage growth of young plants. In week 2, this practice stopped, to ensure the presence of flowers and thus pollen for the natural enemies upon release. In week 3 and for three consecutive weeks, twenty gravid female predatory mites were released on the plants. Furthermore, two couples of one-week-old Orius laevigatus or O. limbatus adults from the rearings were released twice in two consecutive weeks (weeks 3 and 4). All predators were provided with 0.05 g of Artemia cysts in each cage equally distributed over the four plants every two weeks as supplementary food, starting with the first predator introduction (week 3). Finally, 20 randomly selected S. inermis adults from the strawberry rearing were released twice in all cages in weeks 5 and 6. Populations of predators and thrips were monitored weekly starting one week after the last thrips introduction (week 7). From each cage, one young trifoliate, one old trifoliate, and two flowers along with their petiole were sampled and immediately assessed under a stereo microscope in the greenhouse. The number of predatory mite eggs and mobile stages, thrips larvae and adults, and predatory bug eggs, nymphs, and adults were scored. Then, the plant material along with the arthropods were returned to their corresponding cage. This experimental approach ensured that we would not disturb the population dynamics of predators and prey in our experiment. Between sampling of predator treatments, all material and the observer were disinfected to prevent cross-contamination. The following seven treatments were performed: (1) control (no predator release), (2) O. laevigatus, (3) O. limbatus, (4) N. cucumeris, (5) T. montdorensis, (6) A. limonicus, and (7) A. swirskii. Treatments were randomly assigned in five blocks consisting of seven cages each in a randomized complete block design for a total of 35 cages and five replicates per treatment. Throughout the experiment, temperature and RH were registered every 30 min with a datalogger (OM-92, Omega Engineering, Norwalk, USA). The average measured temperature was 23.6 °C (range 15.8–41.5 °C) and average RH was 65.1% (range 30.3–89.2%).
After the last counting performed in week 11, plants were destructively sampled to assess the total number of mobile stages (nymphs and adults) of Orius found in the cages. Furthermore, a damage rating on each leaf (= trifoliate) was scored to assess thrips' damage at the end of the experiment. As S. inermis damages the plant in a visually identical way to S. dorsalis, we adopted a damage scale (0 to 4, with 4 being the highest damage) developed for the latter in previous studies for assigning damage rating (Lahiri and Yambisa 2021; Panthi et al. 2021) (Supplementary Fig. S1).
Statistical analysis
Differences in predation and oviposition rates of predatory mites among the different treatments were analysed with linear mixed effect models (LME) with log(x + 1) transformed data to meet the assumptions of homoscedasticity and normality, as none of the available error distribution of generalized linear mixed models provided a good fit (assessed through residual plot diagnostics). LME models included predator and prey species and their interaction as fixed effects, and experiment individual and observation day as random effects. The analysis excluded natural thrips mortality, as it was negligible (less than 5%, data not shown). Generalized linear mixed models (GLMM) were fitted for the greenhouse experiment, with predator treatment as the fixed factor, and replicate (cage) and time added as random factors to the model to account for temporal pseudoreplication. Data were analysed with a negative binomial distribution and a log link function, as it provided the best fit based on visual diagnostic plots (Hartig 2022). The total number of Orius predators found at the end of the greenhouse experiment were compared with a Generalized Linear Model (GLM) with a Poisson error distribution and a log link function, accounting for overdispersion by including a dispersion parameter equal to Pearson’s χ2-based dispersion divided by the residual df (Hardin and Hilbe 2018). Data on leaf damage ratings were analysed with an ANOVA after the data were checked for normality and homoscedasticity through diagnostic plots. Contrasts among significant fixed factors for GLMM and LME were assessed through Tukey’s HSD with estimated marginal means (Lenth 2021). All analyses were performed using the statistical software R 4.2.2 (R Core Team 2021).
Results
Predation and oviposition rates of predatory mites on Scirtothrips larvae and Artemia
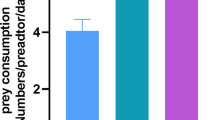
All predatory mites consumed larvae of S. dorsalis and S. inermis. No significant interaction was found between predator and thrips prey (χ2 = 1.08, df = 3, P = 0.781), and predators killed a similar number of prey in 24 h, regardless of the prey species offered (χ2 = 0.06, df = 1, P = 0.804). However, significant differences were found in the predation rates of the different predator species (χ2 = 99.45, df = 3, P < 0.001), with A. limonicus and A. swirskii being the most voracious (Fig. 1a).
Predation (a) and oviposition rate (b) of gravid female Amblydromalus limonicus, Amblyseius swirskii, Transeius montdorensis, and Neoseiulus cucumeris provided with ten second-stage larvae of either S. dorsalis, S. inermis, or feeding ad libitum on Artemia cysts, on strawberry leaf discs. Shown are the daily mean predation rates per predator (days 1–3) and daily mean oviposition rates (days 2–3) per female predator (± SE). Different letters above grouped bars indicate significant differences among predatory mite species (Tukey’s HSD, P < 0.05)
Predatory mites successfully laid eggs feeding on thrips larvae (S. dorsalis or S. inermis) or Artemia cysts. Interaction between predator species and prey was not significant (χ2 = 10.53, df = 6, P = 0.104), and predator oviposition was comparable among the different diets (χ2 = 3.11, df = 2, P = 0.211). Oviposition rate however did differ significantly among the different predators (χ2 = 31.39, df = 3, P < 0.001), with A. limonicus laying the highest number of eggs, followed by A. swirskii (Fig. 1b).
Greenhouse experiment
Densities of adult and juvenile thrips followed similar patterns and thus were pooled together (data not shown). Predator treatment had a significant effect on S. inermis populations (χ2 = 162.4, df = 6, P < 0.001). All predators significantly reduced thrips populations, with A. swirskii and A. limonicus along with both Orius predators achieving the strongest suppression compared to the control (Fig. 2). Furthermore, predators significantly reduced plant damage compared to the control treatment (F6, 28 = 46.31, P < 0.001), and plants with good control of thrips showed little to no damage symptoms (Supplementary Fig. S2).
Population dynamics of Scirtothrips inermis (larvae and adults). Mites were released on plants in weeks 3, 4, and 5 and Artemia cysts were provided every two weeks as supplementary food, starting in week 3. Scirtothrips inermis was released in weeks 5 and 6. The average number (± SE) of S. inermis is shown through time per sample (= one young, one old trifoliate and two flowers with petioles). Different letters denote significant differences in the population of S. inermis through time in the different treatments (Tukey’s HSD, P < 0.05)
Densities of the different predatory mite stages also showed similar patterns and were pooled together (data not shown). Predator species had a significant effect on predator population (χ2 = 87.48, df = 3, P < 0.001), with A. swirskii establishing in highest numbers, followed by A. limonicus, T. montdorensis and lastly N. cucumeris (Fig. 3).
Population dynamics of predatory mites (all stages). Predators were released on plants in weeks 3, 4, and 5 and Artemia cysts were provided every two weeks as supplementary food, starting in week 3. Scirtothrips inermis was released in weeks 5 and 6. The average number (± SE) of mites is shown through time per sample (= one young, one old trifoliate and two flowers with petioles). Different letters denote significant differences in the population of mites through time in the different treatments (Tukey’s HSD, P < 0.05)
A limited number of Orius mobile individuals were found in the samples collected from the cages, as these would move and hide when disturbed during sampling (data not shown). We thus present here only the number of unhatched eggs counted in the plant tissue samples through time. No significant differences were found between O. laevigatus and O. limbatus (χ2 = 0.196, df = 3, P = 0.658) (Supplementary Fig. S3). However, we did find significantly more mobile stages of O. limbatus (65.2 ± 13.5) compared to O. laevigatus (29.4 ± 6.1) during the destructive sampling at the end of the experiment (χ2 = 69.46, df = 1, P = 0.009).
Discussion
The results of this study show that all predatory mites species tested when released preventively have the potential to control Scirtothrips in strawberry, and the strength of their control efficacy directly matched their performance in the laboratory experiments. Predation rates recorded for the predatory mites did not differ between S. dorsalis and S. inermis, showing that both are equally susceptible to predation from phytoseiid mites. Unlike other thrips species such as F. occidentalis and Thrips tabaci Lindeman that exhibit strong defensive behavior including abdomen swinging and the production of rectal fluids (Bakker and Sabelis 1989; Teerling et al. 1993), no such behavior is known for Scirtothrips larvae, and we further did not observe strong defensive behaviors during the experiments. Possibly due to the small size of Scirtothrips larvae, these were easily subdued by all predators tested, and even adult stages of S. dorsalis can be subdued by predatory mites (Arthurs et al. 2009; Schoeller et al. 2020), and observations during our experiments suggest that this is also the case for S. inermis. The highest predation and oviposition rates were recorded for A. limonicus and A. swirskii, while the lowest performance was found for N. cucumeris. Schoeller et al. (2020) found similarly high predation rates for A. limonicus and A. swirskii on S. dorsalis larvae, but much lower oviposition rates than those we report in this study. This led Schoeller et al. (2020) to suggest that S. dorsalis may represent a poor nutritional food source for the predators. However, results from our study clearly show that larvae of both Scirtothrips led to high oviposition rates for both A. limonicus and A. swirskii, similar to those obtained on larvae of F. occidentalis (van Houten et al. 1995; Leman and Messelink 2015) and in agreement with other studies on S. dorsalis (Arthurs et al. 2009), suggesting that other factors, such as population genetics, may have led to the poor reproduction in the study of Schoeller et al. (2020). Neoseiulus cucumeris was the least voracious and laid the fewest number of eggs in our trial compared with the rest of the predators tested, in contrast with a previous study on S. dorsalis larvae on sweet pepper leaf discs (Arthurs et al. 2009). This difference may be due to different leaf architecture, as the high trichome density of the abaxial side of strawberry leaves may have hindered the foraging of the “sit-and-wait” predator N. cucumeris, compared to the more aggressive A. swirskii and A. limonicus (Messelink et al. 2006). Furthermore, here we report for the first time T. montdorensis successfully predates and reproduces on Scirtothrips larvae, albeit at a lower rate than A. swirskii and A. limonicus. No direct comparisons of its laboratory performance can be made, as this rather new predator has mostly been studied in field releases in literature (but see Steiner et al. 2003).
All predatory mites tested in the laboratory successfully laid eggs when offered hydrated decapsulated Artemia cysts as a food source, and the oviposition rate roughly equaled that when offered Scirtothrips larvae as prey. Artemia cysts are known to be an excellent food source for A. swirskii (Vangansbeke et al. 2016b) and A. limonicus (Vangansbeke et al. 2014). To the best of our knowledge, this is the first report of N. cucumeris and T. montdorensis successfully feeding and ovipositing on Artemia cysts. It is important to note here that the nutritional quality of Artemia cysts may differ substantially among different strains and origins (De Clercq et al. 2005; Vangansbeke et al. 2016a), and the high-quality product used in our study currently comes at an elevated cost comparable to that of E. kuehniella eggs (Amir Grosman, BioBee, personal communication). However, Artemia cysts maintain high nutritional value for a longer timespan compared to E. kuehniella eggs when applied in crops, which merits their inclusion in pest control strategies where food supplementation for generalist predators is applied (Pijnakker et al. 2020).
Preventive releases of all predatory mites on strawberry in the greenhouse experiment successfully suppressed the population and crop damage by S. inermis, but to a different extent and in a similar magnitude to the results of the laboratory experiments. Amblyseius swirskii and A. limonicus allowed for very little population development of S. inermis in the crop, and symptoms of thrips-feeding were hardly visible on the crop at the end of the trial. Furthermore, both predators built up high population on the crop under the warm studied conditions, and population spikes occurred the weeks after food supplementation, highlighting the benefits gained through feeding on Artemia cysts. Both mite species are known to be excellent thrips predators (Messelink et al. 2006) and can successfully control S. dorsalis in sweet pepper, roses, and strawberry in Florida USA (Arthurs et al. 2009; Doĝramaci et al. 2011; Lahiri and Yambisa 2021; Schoeller et al. 2022). The limited adoption of A. limonicus in biological control programs compared to A. swirskii is mostly due to the increased cost of the former due to difficulties in its mass rearing (Knapp et al. 2013). However, it has been suggested for use in lower temperature regimes in Northern Europe in the strawberry crop, where A. swirskii might have limited population growth (Hoogerbrugge et al. 2011; Vervoort et al. 2017). Furthermore, T. montdorensis showed significant control of S. inermis but limited population build-up in our study, perhaps due to this predator performing better in lower temperature regimes (Labbé et al. 2019; Téllez et al. 2020). Interestingly, recent studies have shown that both A. swirskii and A. limonicus may successfully predate on thrips eggs oviposited endophytically, but this was not the case for T. montdorensis (Vangansbeke et al. 2022), which may partly explain the reduced efficacy of the latter predator in this study as well. Finally, N. cucumeris had the weakest effect on thrips suppression and built up only a limited population, allowing for significant damage to be visible on the crop at the end of the experiment. The limited effect of N. cucumeris compared to other phytoseiid predators against thrips in greenhouse crops has been reported before for S. dorsalis (Arthurs et al. 2009) and other thrips pests (van Houten et al. 2005; Messelink et al. 2006). However, its strength lies in numbers as it is by far the cheapest predatory mite to mass produce tested in this trial, thus maintaining a relevant role in the control of thrips today.
Orius omnivorous predators represent a cornerstone in the biological control of flower thrips in vegetable crops, being widely employed in flowering crops that produce pollen, allowing their persistence when prey is scarce (van Lenteren 2012). They may also feed on foliar pests such as aphids (Messelink et al. 2011), whiteflies (Montserrat et al. 2004) and Echinothrips americanus Morgan (Mouratidis et al. 2022), and Orius insidiosus Say has been reported before to control S. dorsalis in sweet pepper (Doĝramaci et al. 2011). Here, we report that both O. laevigatus and O. limbatus are also effective predators of S. inermis and successfully established in the strawberry crop through supplementation with Artemia cysts. Orius predators are increasingly employed in several cropping systems including strawberry using banker plants or food supplementation (Zuma et al. 2023). However, it is important to note that most commercial anthocorid predators are highly anthophilous, and while very effective against flower thrips, they may abandon the plants looking for prey spatially overlapping with their preferred habitat, thus not completely controlling foliar Scirtothrips pests. While the oviposition rate of both Orius species showed similar patterns during the greenhouse experiment, considerably higher numbers of O. limbatus compared to O. laevigatus were found at the end of the study. Orius limbatus is endemic to the Canary Islands and very little is known about its life history (Carnero et al. 1993). We hypothesize that the endemic O. limbatus performed better under the high-temperature predators experienced during the study, leading to a higher population build-up. Based on the results of this study, further research on this understudied yet abundant in the Canary Islands predator is needed, especially in the context of conservation biological control to encourage the spontaneous occurrence of O. limbatus in crops.
Trophic interaction studies between Scirtothrips and co-occurring pests in crops are lacking and are needed to design effective biological control strategies. In strawberry, Scirtothrips may co-occur with spider mites (Acari: Tetranychidae), and it is not known how the two pests may interact. A report from S. citri in cotton found predation of this thrips species on spider mite eggs (Mollet and Sevacherian 1985), but this did not lead to enhanced performance of the thrips, unlike what other studies have shown for F. occidentalis (Agrawal et al. 1999). Thus, it appears that predation may be opportunistic in nature to remove a competitor rather than gain a direct nutritional benefit. The potential of S. dorsalis or other Scirtothrips species to engage in this behavior needs to be evaluated, especially against eggs of phytoseiid predators, as counter-attacking behavior may limit the effectiveness of biological control programs based on predatory mites (Janssen et al. 2003). Furthermore, biological control of Scirtothrips in flowering crops will need to be evaluated in the presence of the ubiquitous flower thrips F. occidentalis. While resource competition between the two thrips may be limited due to niche segregation on flowering crops, the two thrips may interact indirectly through their shared anthocorid or phytoseiid predators (Chailleux et al. 2014), potentially leading to one of the two thrips escaping predation (Reitz et al. 2006).
Summarizing, we conclude that generalist predatory mites have great potential in controlling S. dorsalis and S. inermis in strawberry crops, and that their performance in the laboratory may translate well in greenhouse conditions. The performance of predatory mites in strawberry, however, may be hindered on cultivars with high trichome densities (Fahim and El-Saiedy 2021), limiting their biological control potential as shown also in other crops (Tsuchida and Masui 2023). Thus, the application of predatory mites in strawberry for the control of thrips and other pests needs to be evaluated in conjunction with the varieties in question. Moreover, our study shows high-quality Artemia cysts to be an adequate food source for all predators tested, and their application in the greenhouse in combination with the pollen provided by strawberry plants maintained predator populations even in the absence of pests. In warm climates where Scirtothrips are typically most problematic, A. swirskii and A. limonicus may build higher populations and provide superior pest control to N. cucumeris and T. montdorensis when released in equal numbers. Orius predators were also found to effectively suppress S. inermis in the greenhouse. However, their effectiveness should be further evaluated under field conditions and in the presence of multiple pests, as these highly anthophilous predators may not extensively forage for Scirtothrips in the plant canopy in the presence of another food source (Desneux and O’Neil 2008). Pestiferous Scirtothrips species are expanding their world distribution due to global trade but also rising temperatures, and biological control practitioners need to be ready to tackle these emerging pests.
References
Agrawal AA, Kobayashi C, Thaler JS (1999) Influence of prey availability and induced host-plant resistance on omnivory by western flower thrips. Ecology 80:518–523
Arthurs S, McKenzie CL, Chen J, Dogramaci M, Brennan M, Houben K, Osborne L (2009) Evaluation of Neoseiulus cucumeris and Amblyseius swirskii (Acari: Phytoseiidae) as biological control agents of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) on pepper. Biol Control 49:91–96
Bakker FM, Sabelis MW (1989) How larvae of Thrips tabaci reduce the attack success of phytoseiid predators. Entomol Exp Appl 50:47–51
Bara GT, Laing MD (2020) Attractiveness of different coloured sticky traps to the South African Citrus Thrips (Scirtothrips aurantii Faure) in Avocado, KwaZulu-Natal, South Africa. Entomol Soc S Afr 28:133–141
Carnero A, Peña M, Perez-Padrón F, Garrido C, Hernández-Garcia M (1993) Bionomics of Orius albidipennis and Orius limbatus. IOBC/WPRS Bull 16(2):27–30
Carrillo-Arámbula L, Infante F, Cavalleri A, Gómez J, Ortiz JA, Fanson BG, González FJ (2022) Colored sticky traps for monitoring phytophagous thrips (Thysanoptera) in mango agroecosystems, and their impact on beneficial insects. PLoS ONE 17(11):e0276865
Chailleux A, Mohl EK, Teixeira Alves M, Messelink GJ, Desneux N (2014) Natural enemy-mediated indirect interactions among prey species: potential for enhancing biocontrol services in agroecosystems. Pest Manag Sci 70:1769–1779
Coll M, Guershon M (2002) Omnivory in terrestrial arthropods: mixing plant and prey diets. Annu Rev Entomol 47:267–297
De Clercq P, Arijs Y, Van Meir T, Van Stappen G, Sorgeloos P, Dewettinck K, Rey M, Grenier S, Febvay G (2005) Nutritional value of brine shrimp cysts as a factitious food for Orius laevigatus (Heteroptera: Anthocoridae). Biocontrol Sci Technol 15:467–479
Desneux N, O’Neil RJ (2008) Potential of an alternative prey to disrupt predation of the generalist predator, Orius insidiosus, on the pest aphid, Aphis glycines, via short-term indirect interactions. Bull Entomol Res 98:631–639
Dickey AM, Kumar V, Hoddle MS, Funderburk JE, Morgan JK, Jara-Cavieres A, Shatters RG, Osborne LS, McKenzie CL (2015) The Scirtothrips dorsalis species complex: endemism and invasion in a global pest. PLoS ONE 10(4):e0123747
Doĝramaci M, Arthurs SP, Chen J, McKenzie C, Irrizary F, Osborne L (2011) Management of chilli thrips Scirtothrips dorsalis (Thysanoptera: Thripidae) on peppers by Amblyseius swirskii (Acari: Phytoseiidae) and Orius insidiosus (Hemiptera: Anthocoridae). Biol Control 59:340–347
Fahim SF, El-Saiedy ESM (2021) Life table parameters of Amblyseius swirskii and Neoseiulus californicus (Acari: Phytoseiidae) reared on two strawberry cultivars. Int J Acarol 47:568–574
Gilbert MJ, Samways MJ (2018) Mature larval dispersal and adult emergence of the economically significant pest, Scirtothrips aurantii Faure (Thysanoptera: Thripidae), in commercial citrus. J Insect Sci 18(2):32, 1–7
Grafton-Cardwell EE, Ouyang Y (1995) Augmentation of Euseius tularensis (Acari: Phytoseiidae) in citrus. Environ Entomol 24:738–747
Grafton-Cardwell EE, Ouyang Y, Striggow RA (1999) Predacious mites for control of citrus thrips, Scirtothrips citri (Thysanoptera: Thripidae) in nursery citrus. Biol Control 14:29–36
Grout TG, Richards GI (1992) Euseius addoensis addoensis, an effective predator of citrus thrips, Scirtothrips aurantii, in the Eastern Cape Province of South Africa. Exp Appl Acarol 15:1–13
Grout TG, Morse JG, Brawner OL (1986) Location of citrus thrips (Thysanoptera: Thripidae) pupation: tree or ground. J Econ Entomol 79:59–61
Hardin J, Hilbe J (2018) Generalized linear models and extensions. Stata Press, College Station
Hartig F (2022) Residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.4.5. http://florianhartig.github.io/DHARMa/
Hoddle M (2022) Scirtothrips dorsalis (chilli thrips). CABI Compend. https://doi.org/10.1079/cabicompendium.49065
Hoddle MS, Mound LA (2003) The genus Scirtothrips in Australia (Insecta, Thysanoptera, Thripidae). Zootaxa 268:1–40
Hoddle MS, Robinson L (2004) Evaluation of factors influencing augmentative releases of Chrysoperla carnea for control of Scirtothrips perseae in California avocado orchards. Biol Control 31:268–275
Hoddle MS, Jetter KM, Morse JG (2003) The economic impact of Scirtothrips perseae Nakahara (Thysanoptera: Thripidae) on California avocado production. Crop Prot 22:485–493
Hoogerbrugge H, van Houten Y, Knapp M, Bolckmans K (2011) Biological control of thrips and whitefly on strawberries with Amblydromalus limonicus and Amblyseius swirskii. IOBC/WPRS Bull 68:65–69
Huang N, Enkegaard A, Osborne LS, Ramakers PMJ, Messelink GJ, Pijnakker J, Murphy G (2011) The banker plant method in biological control. Crit Rev Plant Sci 30:259–278
Janssen A, Willemse E, Van Der Hammen T (2003) Poor host plant quality causes omnivore to consume predator eggs. J Anim Ecol 72:478–483
Kaur G, Stelinski LL, Martini X, Boyd N, Lahiri S (2023) Reduced insecticide susceptibility among populations of Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) in strawberry production. J Appl Entomol 147:271–278
Knapp M, Van Houten Y, Hoogerbrugge H, Bolckmans K (2013) Amblydromalus limonicus (Acari: Phytoseiidae) as a biocontrol agent: literature review and new findings. Acarologia 53:191–202
Kumar V, Kakkar G, Seal DR, McKenzie CL, Osborne LS (2017) Evaluation of insecticides for curative, preventive, and rotational use on Scirtothrips dorsalis South Asia 1 (Thysanoptera: Thripidae). Fla Entomol 100:634–646
Labbé RM, Gagnier D, Shipp L (2019) Comparison of Transeius montdorensis (Acari: Phytoseiidae) to other phytoseiid mites for the short-season suppression of western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). Environ Entomol 48:335–342
Lacasa A, Llorens J, Sánchez JA (1996) Un Scirtothrips (Thysanoptera: Thripidae) causa daños en los cítricos en España. Bol San Veg Plagas 22:79–95
Lahiri S, Yambisa A (2021) Efficacy of a biopesticide and predatory mite to manage chilli thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) in strawberry. Fla Entomol 104:322–324
Leman A, Messelink GJ (2015) Supplemental food that supports both predator and pest: a risk for biological control? Exp Appl Acarol 65:511–524
Lenth RV (2021) emmeans: estimated marginal means, aka least-squares means. R package version 1.7.1-1. https://cran.r-project.org/package=emmeans
Marrero E, Mouratidis A, Cartaya N, Ramos A, Sánchez B, Hernández-Suárez EM (2023) Pasado, presente y futuro del cultivo de la fresa en Canarias. Avances en el manejo biológico de Scirtothrips inermis. Agropalca 61:25
McMurtry JA, Morse JG, Johnson HG (1992) Studies of the impact of Euseius species (Acari: Phytoseiidae) on citrus mites using predator exclusion and predator release experiments. Exp Appl Acarol 15:233–248
Messelink GJ, Van Steenpaal SEF, Ramakers PMJ (2006) Evaluation of phytoseiid predators for control of western flower thrips on greenhouse cucumber. BioControl 51:753–768
Messelink GJ, Bloemhard CM, Kok L, Janssen A (2011) Generalist predatory bugs control aphids in sweet pepper. IOBC/WPRS Bull 68:115–118
Messelink GJ, Bennison J, Alomar O, Ingegno BL, Tavella L, Shipp L, Palevsky E, Wäckers FL (2014) Approaches to conserving natural enemy populations in greenhouse crops: current methods and future prospects. BioControl 59:377–393
Modesto-Henández P, Fernández-Galván D, Carnero A (2010) Una plaga de thrips en mango. Granja Rev Agropecuaria 17:2–4
Mollet JA, Sevacherian V (1985) Scirtothrips citri (Moulton) (Thysanoptera: Thripidae) development on cotton leaves and/or eggs of Tetranychus cinnabarinus (Boisduval) (Acari: Tetranychidae). J Kansas Entomol Soc 58:532–533
Montserrat M, Albajes R, Castañé C (2004) Behavioral responses of three plant-inhabiting predators to different prey densities. Biol Control 30:256–264
Morse JG, Hoddle MS (2005) Invasion biology of thrips. Annu Rev Entomol 51:67–89
Morse JG, Zareh N (1991) Pesticide-induced hormoligosis of citrus thrips (Thysanoptera: Thripidae) fecundity. J Econ Entomol 84:1169–1174
Mound LA (2005) Thysanoptera: diversity and interactions. Annu Rev Entomol 50:247–269
Mound LA, Palmer JM (1981) Identification, distribution and host-plants of the pest species of Scirtothrips (Thysanoptera: Thripidae). Bull Entomol Res 71:467–479
Mound LA, Stiller M (2011) Species of the genus Scirtothrips from Africa (Thysanoptera, Thripidae). Zootaxa 61:51–61
Mouratidis A, de Lima AP, Dicke M, Messelink GJ (2022) Predator–prey interactions and life history of Orius laevigatus and O. majusculus feeding on flower and leaf-inhabiting thrips. Biol Control 172:104954
Mouratidis A, Bastin S, Pomposo M, Marrero E, Goldarazena A, Hernández-Suárez EM (2023) First report of Scirtothrips dorsalis Hood in the Canary Islands. EPPO Bull. https://doi.org/10.1111/epp.12968
Panthi BR, Renkema JM, Lahiri S, Liburd OE (2021) The short-range movement of Scirtothrips dorsalis (Thysanoptera: Thripidae) and rate of spread of feeding injury among strawberry plants. Environ Entomol 50:12–18
Péricart J (1972) Hémiptères : Anthocoridae, Cimicidae et Microphysidae : de l’ouest-paléarctique. Masson et Cié, París
Pijnakker J, Vangansbeke D, Duarte M, Moerkens R, Wäckers FL (2020) Predators and parasitoids-in-first: from inundative releases to preventative biological control in greenhouse crops. Front Sustain Food Syst 4:595630
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.r-project.org/
Reitz SR, Funderburk JE, Waring SM (2006) Differential predation by the generalist predator Orius insidiosus on congeneric species of thrips that vary in size and behavior. Entomol Exp Appl 119:179–188
Sabelis MW (1990) How to analyse prey preference when prey density varies? A new method to discriminate between effects of gut fullness and prey type composition. Oecologia 82:289–298
Schoeller EN, McKenzie CL, Osborne LS (2020) Comparison of the phytoseiid mites Amblyseius swirskii and Amblydromalus limonicus for biological control of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae). Exp Appl Acarol 82:309–318
Schoeller EN, McKenzie CL, Osborne LS (2022) Chilli thrips rose management using an Amblyseius swirskii or Amblydromalus limonicus (Acari: Phytoseiidae) pepper banker plant. J Appl Entomol 146:1281–1292
Seal DR, Kumar V (2010) Biological response of chilli thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae), to various regimes of chemical and biorational insecticides. Crop Prot 29:1241–1247
Shibao M, Tanaka F, Tsukuda R, Fujisaki K (1991) Overwintering sites and stages of the chillie thrips, Scirtothrips dorsalis Hood (Thysanoptera, Thripidae) in grape fields. Jpn J Appl Entomol Zool 35:161–163
Steiner MY, Goodwin S, Wellham TM, Barchia IM, Spohr LJ (2003) Biological studies of the Australian predatory mite Typhlodromips montdorensis (Schicha) (Acari: Phytoseiidae), a potential biocontrol agent for western flower thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Aust J Entomol 42:124–130
Symondson WOC, Sunderland KD, Greenstone MH (2002) Can generalist predators be effective biocontrol agents? Annu Rev Entomol 47:561–594
Teerling CR, Pierce HD, Borden JH, Gillespie DR (1993) Identification and bioactivity of alarm pheromone in the western flower thrips, Frankliniella occidentalis. J Chem Ecol 19:681–697
Téllez MM, Cabello T, Gámez M, Burguillo FJ, Rodríguez E (2020) Comparative study of two predatory mites Amblyseius swirskii Athias-Henriot and Transeius montdorensis (Schicha) by predator–prey models for improving biological control of greenhouse cucumber. Ecol Model 431:109197
Tsuchida Y, Masui S (2023) Efficacy of biocontrol of the yellow tea thrips and the Kanzawa spider mite with the generalist phytoseiid mite Euseius sojaensis differs between grape cultivars with different leaf morphological traits. BioControl 68:425–434
van Houten YM, Rijn PCJ, Tanigoshi LK, Stratum P, Bruin J (1995) Preselection of predatory mites to improve year-round biological control of western flower thrips in greenhouse crops. Entomol Exp Appl 74:225–234
van Houten YM, Østliem ML, Hoogerbrugge H, Bolckmans K (2005) Biological control of western flower thrips on sweet pepper using the predatory mites Amblyseius cucumeris, Iphiseius degenerans, A. andersoni and A. swirskii. IOBC/WPRS Bull 28:283–286
van Lenteren JC (2012) The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. BioControl 57:1–20
Vangansbeke D, Nguyen DT, Audenaert J, Verhoeven R, Gobin B, Tirry L, De Clercq P (2014) Performance of the predatory mite Amblydromalus limonicus on factitious foods. BioControl 59:67–77
Vangansbeke D, Nguyen DT, Audenaert J, Gobin B, Tirry L, De CP (2016a) Establishment of Amblyseius swirskii in greenhouse crops using food supplements. Syst Appl Acarol 21:1174–1184
Vangansbeke D, Nguyen DT, Audenaert J, Verhoeven R, Gobin B, Tirry L, De Clercq P (2016b) Supplemental food for Amblyseius swirskii in the control of thrips: feeding friend or foe? Pest Manag Sci 72:466–473
Vangansbeke D, Duarte MVA, Pijnakker J, Pekas A, Wäckers F (2022) Egg predation by phytoseiid predatory mites: is there intraguild predation towards predatory bug eggs? J Econ Entomol 115:1087–1094
Vervoort M, Melis P, Hanssens J, Craeye S, Pisman M, Smagghe G, Clymans R, Beliën T (2017) Thrips control with predatory mites A. limonicus and A. swirskii in different strawberry cultivation systems. Acta Hortic 1156:833–842
Wang Z, Mound LA, Hussain M, Arthurs SP, Mao R (2022) Thysanoptera as predators: their diversity and significance as biological control agents. Pest Manag Sci 78:5057–5070
Yee WL, Phillips PA, Rodgers JL, Faber BA (2001) Phenology of arthropod pests and associated natural predators on avocado leaves, fruit, and in leaf litter in southern California. Environ Entomol 30:892–898
Zuma M, Njekete C, Konan KAJ, Bearez P, Amiens-Desneux E, Desneux N, Lavoir AV (2023) Companion plants and alternative prey improve biological control by Orius laevigatus on strawberry. J Pest Sci 96:711–721
Acknowledgements
This research was partially funded by the Agricultural Research Advisory Council (CAIA) Project 2022-0003-07 “Development of integrated pest management strategies of pests of special interest for the Canary Islands”. Angelos Mouratidis was financially supported by a PhD grant from Stimuflori (Amsterdam, The Netherlands, Project Number 18.100), and an Erasmus mobility grant.
Author information
Authors and Affiliations
Contributions
AM: Conceptualization, methodology, investigation, data curation, formal analysis, writing—original draft. EM-D: Resources, investigation, writing—review and editing. BS-Á: Investigation, writing—review and editing. EH-S: Resources, funding acquisition, supervision, writing—review and editing. GJM: Conceptualization, methodology, funding acquisition, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
This research did not involve any studies with human participants or animals (vertebrates).
Consent for publication
All authors consent to publication.
Additional information
Handling Editor: Marta Montserrat.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mouratidis, A., Marrero-Díaz, E., Sánchez-Álvarez, B. et al. Preventive releases of phytoseiid and anthocorid predators provided with supplemental food successfully control Scirtothrips in strawberry. BioControl 68, 603–615 (2023). https://doi.org/10.1007/s10526-023-10232-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-023-10232-3