Abstract
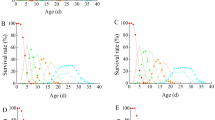
Breeding sachets and their advanced version (sheltered sachets) are commercially used for augmentative biological control of small pest arthropods in crop production. Sheltered sachets excel in not only protecting predatory mites from unsuitable environments (pesticides, drought, rain) but also in releasing more predators. Each sachet system can multiply predatory mites and gradually release them onto nearby plants over several weeks. However, the influence of ambient temperature on predator release by sachet systems remains poorly understood. In this study, we investigated the numbers of predatory mites (Neoseiulus californicus) released from breeding sachets and sheltered sachets at 12, 17, 22, 27, 32, and 37 ºC under laboratory conditions. Regardless of sachet type, significantly more predators were released at 17, 22, and 27 ºC than at 12, 32, and 37 ºC. More predators were dispersed from sheltered sachets than from breeding sachets at 12, 17, 22, and 27 ºC. These results indicate that both sachet systems, especially sheltered sachets, can perform well at around 17–27 ºC but may be handicapped by extreme temperature conditions. Next, we examined the short-term (one week) influence of the different temperatures on the densities of predators and prey mites (Lepidoglyphus destructor) inside breeding sachets and sheltered sachets. We found that numbers of either predators or prey, or both, began to decrease at 32 and 37 ºC. These results suggest that high temperatures may directly affect the breeding of predators that inhabit these sachet systems or indirectly affect them by reducing prey numbers.



Similar content being viewed by others
References
Addesso KM, Witcher AL, Fare DC (2018) Swirski mite controlled-release sachets as a pest management tool in container tree production. HortTechnology 28:391–398
Auger P, Tixier M, Kreiter S, Fauvel G (1999) Factors affecting ambulatory dispersal in the predaceous mite Neoseiulus californicus (Acari: Phytoseiidae). Exp Appl Acarol 23:235–250
Baxter I, Midthassel A, Stepman W, Fryer R, Garcia FP, Lewis J, Walker P, Hulshof J (2011) Field results of a sachet release system using the predator Amblyseius swirskii (Athias-Henriot) (Acari: Phytoseiidae) and the factitious prey, Suidasia medanensis Oudemans (Acari: Astigmata). IOBC/WPRS Bull 68:1–4
Buitenhuis R, Glemser E, Brommit A (2014) Practical placement improves the performance of slow release sachets of Neoseiulus cucumeris. Biocontrol Sci Technol 24:1153–1166
Buitenhuis R, Shipp L, Scott-Dupree C (2010) Dispersal of Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae) on potted greenhouse chrysanthemum. Biol Control 52:110–114
Calvo FJ, Knapp M, van Houten Y, Hoogerbrugge H, Belda J (2015) Amblyseius swirskii: What made this predatory mite such a successful biocontrol agent? Exp Appl Acarol 65:419–433
Canlas LJ, Amano H, Ochiai N, Takeda M (2006) Biology and predation of the Japanese strain of Neoseiulus californicus (McGregor) (Acari: Phytoseiidae). System Appl Acarol 11:141–157
Danielsen C, Stengård HL, Nachman G, Herling C (2004) The influence of temperature and relative humidity on the development of Lepidoglyphus destructor (Acari: Glycyphagidae) and its production of allergens: a laboratory experiment. Exp Appl Acarol 32:151–170
De Courcy Williams ME, Kravar-Garde L, Fenlon JS, Sunderland KD (2004) Phytoseiid mites in protected crops: the effect of humidity and food availability on egg hatch and adult life span of Iphiseius degenerans, Neoseiulus cucumeris, N. californicus and Phytoseiulus persimilis (Acari: Phytoseiidae). Exp Appl Acarol 32:1–13
El Taj HF, Jung C (2012) Effect of temperature on the life-history traits of Neoseiulus californicus (Acari: Phytoseiidae) fed on Panonychus ulmi. Exp Appl Acarol 56:247–260
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd edn. Sage, Thousand Oaks CA
Gallego JR, Solano-Rojas Y, Tiseyra B, Gamez M, Cabello T (2022) Population dynamics of mites in slow-release sachets used in biological control: a new study methodology. Exp Appl Acarol 87:325–335
Ghazy NA, Osakabe M, Negm MW, Schausberger P, Gotoh T, Amano H (2016) Phytoseiid mites under environmental stress. Biol Control 96:120–134
Gotoh T, Yamaguchi K, Mori K (2004) Effect of temperature on life history of the predatory mite Amblyseius (Neoseiulus) californicus (Acari: Phytoseiidae). Exp Appl Acarol 32:15–30
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50(3):346–363
Hubert J, Pekár S, Aulický R, Nesvorná M, Stejskal V (2013) The effect of stored barley cultivars, temperature and humidity on population increase of Acarus siro, Lepidoglyphus destructor and Tyrophagus putrescentiae. Exp Appl Acarol 60:241–252
Kim T, Ahn J, Lee JH (2009) Temperature-dependent developmental model of Neoseiulus californicus (McGregor) (Acari, Phytoseiidae). J Appl Entomol 133:284–291
Knapp M, van Houten Y, van Baal E, Groot T (2018) Use of predatory mites in commercial biocontrol: current status and future prospects. Acarologia 58:72–82
McMurtry JA, Croft BA (1997) Life-styles of phytoseiid mites and their roles in biological control. Ann Rev Entomol 42:291–321
Messelink G, van Steenpaal S, Ramakers P (2006) Evaluation of phytoseiid predators for control of western flower thrips on greenhouse cucumber. BioControl 51:753–768
Mikawa Y, Aizawa M, Uesugi R, Osakabe M, Mori K, Toyama M, Sonoda S (2020) Molecular monitoring of Neoseiulus californicus released from sheltered slow-release sachets for spider mite control in a Japanese pear greenhouse. Exp Appl Acarol 80:203–214
Nomikou M, Janssen A, Schraag R, Sabelis MW (2002) Phytoseiid predators suppress populations of Bemisia tabaci on cucumber plants with alternative food. Exp Appl Acarol 27:57–68
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/
Shimoda T, Hinomoto N (2018) A novel method for protecting slow-release sachets of predatory mites Neoseiulus californicus (McGregor) against environmental stresses and increasing release of predators in greenhouses integrated control of plant-feeding mites. IOBC/WPRS Bull 134:20–22
Shimoda T, Kagawa Y, Mori K, Hinomoto N, Hiraoka T, Nakajima T (2017) A novel method for protecting slow-release sachets of predatory mites against environmental stresses and increasing predator release to crops. BioControl 62:495–503
Shimoda T, Kagawa Y, Yoshizawa H, Nakano A, Matsuhira K, Yanagita H, Shimomoto M, Adachi-Hagimori T, Mori K, Hinomoto N, Hiraoka T, Nakajima T (2019) Moisturized sheltered sachets are potentially useful for the efficient release of selected predators in a wide range of humidity environments. BioControl 64:65–75
Solano-Rojas Y, Gallego JR, Gamez M, Lopez I, Castillo P, Cabello T (2022) Effect of relative humidity on the population dynamics of the predator Amblyseius swirskii and its prey Carpoglyphus lactis in the context of slow-release sachets for use in biological control in greenhouses. Plants 11:2493
Sugawara R, Ullah MS, Ho CC, Gotoh T (2018) Impact of temperature-mediated functional responses of Neoseiulus womersleyi and N. longispinosus (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae). Biol Control 126:26–35
van Lenteren JC (2012) The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. BioControl 57:1–20
van Lenteren JC, Hale A, Klapwijk J (2003) Guidelines for quality control of commercially produced natural enemies. In: van Lenteren JC (ed) Quality control and production of biological control agents Theory and testing procedures. CAB International, Wallingford, pp 265–303
Vangansbeke D, Audenaert J, Nguyen DT, Verhoeven R, Gobin B, Tirry L, De Clercq P (2015) Diurnal temperature variations affect development of a herbivorous arthropod pest and its predators. PLoS ONE 10(4):e0124898
Vangansbeke D, De Schrijver L, Spranghers T, Audenaert J, Verhoeven R, Nguyen DT, Gobin B, Tirry L, De Clercq P (2013) Alternating temperatures affect life table parameters of Phytoseiulus persimilis, Neoseiulus californicus (Acari: Phytoseiidae) and their prey Tetranychus urticae (Acari: Tetranychidae). Exp Appl Acarol 61:285–298
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Walzer A, Castagnoli M, Simoni S, Liguori M, Palevsky E, Schausberger P (2007) Intraspecific variation in humidity susceptibility of the predatory mite Neoseiulus californicus: Survival, development and reproduction. Biol Control 41:42–52
Acknowledgements
We thank Mrs. Yumi Kanai for helping with the experiments. This study was supported in part by the Science and Technology Research Promotion Program for the Agriculture, Forestry, Fisheries and Food Industry (Grant number: 26070C). We are grateful for the support of all the participants in the Japanese project, especially the members of the following organizations: NARO, Ishihara Sangyo Kaisha, Ltd. (Kusatsu, Shiga, Japan), ISK Biosciences K.K., Daikyo-Giken Kogyo Co., Ltd. (Sagamihara, Kanagawa, Japan), prefectural research institutes (Gunma, Tokushima, Kochi, Fukuoka, and Kagoshima prefectures), Japan Agricultural Development and Extension Association (Tokyo, Japan), and National Federation of Agricultural Cooperative Associations Zen-noh (Tokyo, Japan).
Funding
This study was supported in part by the Science and Technology Research Promotion Program for the Agriculture, Forestry, Fisheries and Food Industry (Grant number of the Japanese project: 26070C).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The corresponding author (Takeshi Shimoda) received research support (experimental materials) from Daikyo-Giken Kogyo Co., Ltd. An author (Yoshitake Kagawa: Ishihara Sangyo Kaisha, Ltd., now employed by ISK Biosciences K.K.) has received research support from the two companies. The two authors and the three companies joined the research project. All four authors have no relevant non-financial interests to disclose. Others: Yoshitake Kagawa holds patents “Natural enemy insect breeding device” (JP-Patent numbers: 5681334, 6700100, and 6901098) licensed to Ishihara Sangyo Kaisha, Ltd. and Daikyo-Giken Kogyo Co., Ltd.
Research involving human and animals rights
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Handling Editor: Marta Montserrat
Supplementary Information
Below is the link to the electronic supplementary material.
10526_2023_10223_MOESM1_ESM.pptx
Supplementary file1 (PPTX 1348 KB) (a) Two sachet systems (sheltered sachet and breeding sachet) used in this study. (b) A breeding sachet of Neoseiulus californicus, a black felt patch, and five water-absorbed polymers are encased in an outer water-resistant shelter to make a sheltered sachet. (c) A sealed transparent polyethylene bag, which contains a sheltered sachet and a moisture retention container, used in the predator release tests
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shimoda, T., Kagawa, Y., Yara, K. et al. Influence of temperature on the release of predatory mites from breeding and sheltered sachets. BioControl 68, 591–601 (2023). https://doi.org/10.1007/s10526-023-10223-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-023-10223-4