Abstract
Over half a century has passed since Alexey Olovnikov's groundbreaking proposal of the end-replication problem in 1971, laying the foundation for our understanding of telomeres and their pivotal role in cellular senescence. This review paper delves into the intricate and multifaceted relationship between cellular senescence, the influence of telomeres in this process, and the far-reaching consequences of telomeres in the context of aging and age-related diseases. Additionally, the paper investigates the various factors that can influence telomere shortening beyond the confines of the end-replication problem and how telomeres can exert their impact on aging, even in the absence of significant shortening. Ultimately, this paper stands as a tribute to the pioneering work of Olovnikov, whose seminal contributions established the solid foundation upon which our ongoing explorations of telomeres and the aging process are based.

Similar content being viewed by others
References
Aguado J, Sola-Carvajal A, Cancila V, Revêchon G, Ong PF, Jones-Weinert CW, Wallén Arzt E, Lattanzi G, Dreesen O, Tripodo C et al (2019) Inhibition of DNA damage response at telomeres improves the detrimental phenotypes of Hutchinson-Gilford Progeria Syndrome. Nat Commun 10:4990
Anderson R, Lagnado A, Maggiorani D, Walaszczyk A, Dookun E, Chapman J, Birch J, Salmonowicz H, Ogrodnik M, Jurk D et al (2019) Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J 38:e100492
Bae NS, Baumann P (2007) A RAP1/TRF2 complex inhibits nonhomologous end-joining at human telomeric DNA ends. Mol Cell 26:323–334
Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness AR, Jeganathan KB, Verzosa GC, Pezeshki A et al (2016) Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 530:184–189
Barnes RP, de Rosa M, Thosar SA, Detwiler AC, Roginskaya V, Van Houten B, Bruchez MP, Stewart-Ornstein J, Opresko PL (2022) Telomeric 8-oxo-guanine drives rapid premature senescence in the absence of telomere shortening. Nat Struct Mol Biol 29:639–652
Birch J, Anderson RK, Correia-Melo C, Jurk D, Hewitt G, Marques FM, Green NJ, Moisey E, Birrell MA, Belvisi MG et al (2015) DNA damage response at telomeres contributes to lung aging and chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 309:L1124–L1137
Blasco MA, Lee H-W, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW (1997) Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91:25–34
Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE (1998) Extension of life-span by introduction of telomerase into normal human cells. Science 279:349–352
Cai Z, Yan LJ, Ratka A (2013) Telomere shortening and Alzheimer’s disease. Neuromolecular Med 15:25–48
Campisi J, d’Adda di Fagagna F (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8:729–740
Carrel A (1912) On the permanent life of tissues outside of the organism. J Exp Med 15:516–528
Cassidy LD, Young ARJ, Young CNJ, Soilleux EJ, Fielder E, Weigand BM, Lagnado A, Brais R, Ktistakis NT, Wiggins KA et al (2020) Temporal inhibition of autophagy reveals segmental reversal of ageing with increased cancer risk. Nat Commun 11:307–307
Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA (2003) Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361:393–395
Chandra A, Lagnado AB, Farr JN, Monroe DG, Park S, Hachfeld C, Tchkonia T, Kirkland JL, Khosla S, Passos JF et al (2020) Targeted reduction of senescent cell burden alleviates focal radiotherapy-related bone loss. J Bone Miner Res 35:1119–1131
Choudhury AR, Ju Z, Djojosubroto MW, Schienke A, Lechel A, Schaetzlein S, Jiang H, Stepczynska A, Wang C, Buer J et al (2007) Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat Genet 39:99–105
Coppé J-P, Desprez P-Y, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118
Correia-Melo C, Marques FD, Anderson R, Hewitt G, Hewitt R, Cole J, Carroll BM, Miwa S, Birch J, Merz A et al (2016) Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J 35:724–742
Correia-Melo C, Birch J, Fielder E, Rahmatika D, Taylor J, Chapman J, Lagnado A, Carroll BM, Miwa S, Richardson G et al (2019) Rapamycin improves healthspan but not inflammaging in nfκb1(-/-) mice. Aging Cell 18:e12882
Crabbe L, Verdun RE, Haggblom CI, Karlseder J (2004) Defective telomere lagging strand synthesis in cells lacking WRN helicase activity. Science 306:1951–1953
d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, von Zglinicki T, Saretzki G, Carter NP, Jackson SP (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426:194–198
de Lange T (2005) Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 19:2100–2110
Demanelis K, Jasmine F, Chen LS, Chernoff M, Tong L, Delgado D, Zhang C, Shinkle J, Sabarinathan M, Lin H et al (2020) Determinants of telomere length across human tissues. Science 369:eaaz6876
Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dollé ME et al (2014) An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31:722–733
Doksani Y, Wu JY, de Lange T, Zhuang X (2013) Super-resolution fluorescence imaging of telomeres reveals TRF2-dependent T-loop formation. Cell 155:345–356
Farr JN, Fraser DG, Wang H, Jaehn K, Ogrodnik MB, Weivoda MM, Drake MT, Tchkonia T, LeBrasseur NK, Kirkland JL et al (2016) Identification of senescent cells in the bone microenvironment. J Bone Miner Res: off J Am Soc Bone Miner Res 31:1920–1929
Farr JN, Saul D, Doolittle ML, Kaur J, Rowsey JL, Vos SJ, Froemming MN, Lagnado AB, Zhu Y, Weivoda M et al (2023) Local senolysis in aged mice only partially replicates the benefits of systemic senolysis. J Clin Investig 133:e162519
Fielder E, Tweedy C, Wilson C, Oakley F, LeBeau FEN, Passos JF, Mann DA, von Zglinicki T, Jurk D (2020) Anti-inflammatory treatment rescues memory deficits during aging in nfkb1(-/-) mice. Aging Cell 19:e13188
Fumagalli M, Rossiello F, Clerici M, Barozzi S, Cittaro D, Kaplunov JM, Bucci G, Dobreva M, Matti V, Beausejour CM et al (2012) Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol 14:355–365
Gilgun-Sherki Y, Melamed E, Offen D (2004) The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol 251:261–268
Gomes NM, Ryder OA, Houck ML, Charter SJ, Walker W, Forsyth NR, Austad SN, Venditti C, Pagel M, Shay JW et al (2011) Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell 10:761–768
Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G et al (2019) Cellular senescence: defining a path forward. Cell 179:813–827
Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T (1999) Mammalian telomeres end in a large duplex loop. Cell 97:503–514
Gurkar AU, Gerencser AA, Mora AL, Nelson AC, Zhang AR, Lagnado AB, Enninful A, Benz C, Furman D, Beaulieu D et al (2023) Spatial mapping of cellular senescence: emerging challenges and opportunities. Nat Aging 3:776–790
Harley CB, Futcher AB, Greider CW (1990) Telomeres shorten during ageing of human fibroblasts. Nature 345:458–460
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621
Herbig U, Jobling WA, Chen BPC, Chen DJ, Sedivy JM (2004) Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol Cell 14:501–513
Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM (2006) Cellular senescence in aging primates. Science 311:1257
Hewitt G, Jurk D, Marques FDM, Correia-Melo C, Hardy T, Gackowska A, Anderson R, Taschuk M, Mann J, Passos JF (2012) Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun 3:708
Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, Herrmann SM, Jensen MD, Jia Q, Jordan KL et al (2019) Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 47:446–456
Igarashi H, Takahashi T, Abe H, Nakano H, Nakajima O, Nagase S (2016) Poor embryo development in post-ovulatory in vivo-aged mouse oocytes is associated with mitochondrial dysfunction, but mitochondrial transfer from somatic cells is not sufficient for rejuvenation. Hum Reprod 31:2331–2338
Jacobs JJ, de Lange T (2004) Significant role for p16INK4a in p53-independent telomere-directed senescence. Curr Biol 14:2302–2308
Jun JI, Lau LF (2010) The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12:676–685
Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R et al (2014) Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun 2:4172
Kang T-W, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A et al (2011) Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479:547–551
Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW (2008) Senescence of activated stellate cells limits liver fibrosis. Cell 134:657–667
Lagnado A, Leslie J, Ruchaud-Sparagano MH, Victorelli S, Hirsova P, Ogrodnik M, Collins AL, Vizioli MG, Habiballa L, Saretzki G et al (2021) Neutrophils induce paracrine telomere dysfunction and senescence in ROS-dependent manner. Embo J 40:e106048
Lakin ND, Jackson SP (1999) Regulation of p53 in response to DNA damage. Oncogene 18:7644–7655
Lee PJ, Benz CC, Blood P, Börner K, Campisi J, Chen F, Daldrup-Link H, De Jager P, Ding L, Duncan FE et al (2022) NIH SenNet Consortium to map senescent cells throughout the human lifespan to understand physiological health. Nat Aging 2:1090–1100
Muñoz-Espín D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M et al (2013) Programmed cell senescence during mammalian embryonic development. Cell 155:1104–1118
Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, Day CP, Burt A, Palmer A, Anstee QM et al (2017) Cellular senescence drives age-dependent hepatic steatosis. Nat Commun 8:15691
Ogrodnik M, Zhu Y, Langhi LGP, Tchkonia T, Krüger P, Fielder E, Victorelli S, Ruswhandi RA, Giorgadze N, Pirtskhalava T et al (2019) Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab 29:1061–1077
Oikawa S, Tada-Oikawa S, Kawanishi S (2001) Site-specific DNA damage at the GGG sequence by UVA involves acceleration of telomere shortening. Biochemistry 40:4763–4768
Olovnikov AM (1971) Principle of marginotomy in template synthesis of polynucleotides. Dokl Akad Nauk SSSR 201:1496–1499
Olovnikov AM (1996) Telomeres, telomerase, and aging: origin of the theory. Exp Gerontol 31:443–448
Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, Yosef R, Sagiv A, Agrawal A, Shapira A et al (2018) Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun 9:5435–5435
Passos JF, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, Wappler I, Birkett M, Harold G, Schaeuble K et al (2007) Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol 5:e110
Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A et al (2010) Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol 6:347
Petersen S, Saretzki G, von Zglinicki T (1998) Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp Cell Res 239:152–160
Richter T, Zglinicki TV (2007) A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp Gerontol 42:1039–1042
Ritschka B, Storer M, Mas A, Heinzmann F, Ortells MC, Morton JP, Sansom OJ, Zender L, Keyes WM (2017) The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev 31:172–183
Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G et al (2016) Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15:973–977
Rossiello F, Aguado J, Sepe S, Iannelli F, Nguyen Q, Pitchiaya S, Carninci P, d’Adda di Fagagna F (2017) DNA damage response inhibition at dysfunctional telomeres by modulation of telomeric DNA damage response RNAs. Nat Commun 8:13980
Rossiello F, Jurk D, Passos JF, d’Adda di Fagagna F (2022) Telomere dysfunction in ageing and age-related diseases. Nat Cell Biol 24:135–147
Samper E, Flores JM, Blasco MA (2001) Restoration of telomerase activity rescues chromosomal instability and premature aging in Terc-/- mice with short telomeres. EMBO Rep 2:800–807
Saretzki G, Murphy MP, von Zglinicki T (2003) MitoQ counteracts telomere shortening and elongates lifespan of fibroblasts under mild oxidative stress. Aging Cell 2:141–143
Serra V, von Zglinicki T, Lorenz M, Saretzki G (2003) Extracellular superoxide dismutase is a major antioxidant in human fibroblasts and slows telomere shortening. J Biol Chem 278:6824–6830
Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593–602
Singh A, Kukreti R, Saso L, Kukreti S (2019) Oxidative stress: role and response of short guanine tracts at genomic locations. Int J Mol Sci 20:4258
Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J et al (2013) Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155:1119–1130
Stroik S, Hendrickson EA (2020) Telomere replication-when the going gets tough. DNA Repair 94:102875
Takai H, Smogorzewska A, de Lange T (2003) DNA damage foci at dysfunctional telomeres. Curr Biol 13:1549–1556
van der Reest J, Nardini Cecchino G, Haigis MC, Kordowitzki P (2021) Mitochondria: their relevance during oocyte ageing. Ageing Res Rev 70:101378
Victorelli S, Lagnado A, Halim J, Moore W, Talbot D, Barrett K, Chapman J, Birch J, Ogrodnik M, Meves A et al (2019) Senescent human melanocytes drive skin aging via paracrine telomere dysfunction. EMBO J 38:e101982
von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344
von Zglinicki T, Pilger R, Sitte N (2000) Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Rad Biol Med 28:64–74
Wang Y, Ferrucci L, Seidman MM, Liu Y (2022) An optimized proximity ligation assay to detect telomere dysfunction induced foci in human and mouse cells. STAR Protoc 3:101610
Watson JD (1972) Origin of concatemeric T7 DNA. Nat New Biol 239:197–201
Whittemore K, Vera E, Martínez-Nevado E, Sanpera C, Blasco MA (2019) Telomere shortening rate predicts species life span. Proc Natl Acad Sci USA 116:15122–15127
Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N et al (2015) Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife 4:e12997
Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG et al (2018) Senolytics improve physical function and increase lifespan in old age. Nat Med 24:1246–1256
Zhang X, Wu X, Tang W, Luo Y (2012) Loss of p16(Ink4a) function rescues cellular senescence induced by telomere dysfunction. Int J Mol Sci 13:5866–5877
Zhang L, Pitcher LE, Prahalad V, Niedernhofer LJ, Robbins PD (2021) Recent advances in the discovery of senolytics. Mech Ageing Dev 200:111587
Zhang X, Habiballa L, Aversa Z, Ng YE, Sakamoto AE, Englund DA, Pearsall VM, White TA, Robinson MM, Rivas DA et al (2022) Characterization of cellular senescence in aging skeletal muscle. Nat Aging 2:601–615
Zhu X, Han W, Xue W, Zou Y, Xie C, Du J, Jin G (2016) The association between telomere length and cancer risk in population studies. Sci Rep 6:22243
Acknowledgements
This work was funded by NIH grants R01AG068048 (JFP); UH3CA268103 (JFP) and R01AG082708 (JFP, SV) and The Glenn Foundation For Medical Research (JFP). SV would like to acknowledge funding from the Robert and Arlene Kogod Center for Aging Career Development Award.
Author information
Authors and Affiliations
Contributions
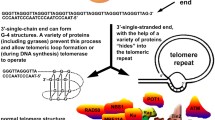
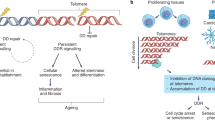
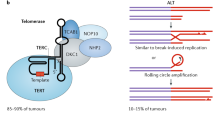
ME wrote the first draft of the manuscript, which was then edited and modified by JFP and SV. ME prepared Fig. 1. All authors had input into review or editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Competing interest
ME, JFP and SV declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eppard, M., Passos, J.F. & Victorelli, S. Telomeres, cellular senescence, and aging: past and future. Biogerontology 25, 329–339 (2024). https://doi.org/10.1007/s10522-023-10085-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-023-10085-4