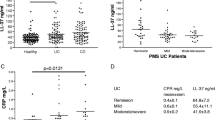
Zonulin content in blood serum of patients with colorectal cancer (CRC; n=152; 30-84 years) and patients with large bowel adenomas (n=32; 39-82 years) was measured by standardized kit IDK Zonulin ELISA (Immundiagnostik AG). The healthy control group (n=50) comprised volunteers (27 women, 23 men; 25-68 years); pathological control group (n=84) — patients (55 women, 29 men;18-84 years) with irritable bowel syndrome (n=29), Crohn’s disease (n=5), and ulcero-necrotic colitis (n=50). In comparison to healthy control group, the level of zonulin was significantly increased in CRC patients (p<0.0000001) and in patients with benign large bowel tumors (p<0.004), as well as in patients with inflammatory intestine diseases and with irritable bowel syndrome (p<0.0002). Zonulin level in blood serum of CRC patients was slightly, but significantly higher (p<0.05) than in the group of pathological control. ROC curve construction revealed that at optimal zonulin cut-off level (52.2 ng/ml), the diagnostic sensitivity of CRC detection was 66.7% and specificity relative to healthy control was 81.8%. The specificity relative to the combined control group (healthy control+non-tumor bowel diseases) was only 68.9%. Thus, no acceptable cut-off levels for differentiation between malignant and benign tumors, as well as between tumor and non-tumor large bowel pathologies were found. Analysis of the associations between serum zonulin level and the main clinical and pathological characteristics of CRC demonstrated that the level of this marker increased with disease progression (p<0.01; Kruskal—Wallis test), but was not associated with individual criteria of the TNM system, tumor localization, histological structure, and malignancy grade.
Similar content being viewed by others
References
Barbaro MR, Cremon C, Morselli-Labate AM, Di Sabatino A, Giuffrida P, Corazza GR, Di Stefano M, Caio G, Latella G, Ciacci C, Fuschi D, Mastroroberto M, Bellacosa L, Stanghellini V, Volta U, Barbara G. Serum zonulin and its diagnostic performance in non-coeliac gluten sensitivity. Gut. 2020;69(11):1966-1974. doi: https://doi.org/10.1136/gutjnl-2019-319281.
Di Pierro M, Lu R, Uzzau S, Wang W, Margaretten K, Pazzani C, Maimone F, Fasano A. Zonula occludens toxin structure-function analysis. Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J. Biol. Chem. 2001;276(22):19160-19165. doi: https://doi.org/10.1074/jbc.M009674200
Dowling P, Meleady P, Henry M, Clynes M. Recent advances in clinical proteomics using mass spectrometry. Bioanalysis. 2010;2(9):1609-1615. doi: https://doi.org/10.4155/bio.10.69
Esnafoglu E, Cırrık S, Ayyıldız SN, Erdil A, Ertürk EY, Daglı A, Noyan T. Increased Serum Zonulin Levels as an Intestinal Permeability Marker in Autistic Subjects. J. Pediatr. 2017;188:240-244. doi: https://doi.org/10.1016/j.jpeds.2017.04.004
Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. 2020;9:F1000 Faculty Rev-69. doi: https://doi.org/10.12688/f1000research.20510.1
Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011;91(1):151-175. doi: https://doi.org/10.1152/physrev.00003.2008
Hensley-McBain T, Manuzak JA. Zonulin as a biomarker and potential therapeutic target in multisys tem inflammatory syndrome in children. J. Clin. Invest. 2021;131(14):e151467. doi: https://doi.org/10.1172/JCI151467
Lai CH, Chang NW, Lin CF, Lin CD, Lin YJ, Wan L, Sheu JJ, Chen SY, Huang YP, Sing YT, Tao TW, Lai CK, Tsai MH, Chan HL, Jou YJ, Lin CW. Proteomics-based identification of haptoglobin as a novel plasma biomarker in oral squamous cell carcinoma. Clin. Chim. Acta. 2010;411(13-14):984-991. doi: https://doi.org/10.1016/j.cca.2010.03.028
Malíčková K, Francová I, Lukáš M, Kolář M, Králíková E, Bortlík M, Ďuricová D, Štěpánková L, Zvolská K, Pánková A, Zima T. Fecal zonulin is elevated in Crohn’s disease and in cigarette smokers. Pract. Lab. Med. 2017;9:39-44. doi: https://doi.org/10.1016/j.plabm.2017.09.001
Meira de-Faria F, Bednarska O, Ström M, Söderholm JD, Walter SA, Keita ÅV. Colonic paracellular permeability and circulating zonulin-related proteins. Scand. J. Gastroenterol. 2021;56(4):424-431. doi: https://doi.org/10.1080/00365521.2021.1879247
Skardelly M, Armbruster FP, Meixensberger J, Hilbig H. Expression of Zonulin, c-kit, and Glial Fibrillary Acidic Protein in Human Gliomas. Transl. Oncol. 2009;2(3):117-120. doi: https://doi.org/10.1593/tlo.09115
Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016;4(4):e1251384. doi: https://doi.org/10.1080/21688370.2016.1251384
Sun ZL, Zhu Y, Wang FQ, Chen R, Peng T, Fan ZN, Xu ZK, Miao Y. Serum proteomic-based analysis of pancreatic carcinoma for the identification of potential cancer biomarkers. Biochim. Biophys. Acta. 2007;1774(6):764-771. doi: https://doi.org/10.1016/j.bbapap.2007.04.001
Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, Antalis TM, Vogel SN, Zhao A, Yang S, Arrietta MC, Meddings JB, Fasano A. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc. Natl Acad. Sci. USA. 2009;106(39):16799-16804. doi: https://doi.org/10.1073/pnas.0906773106
Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J. Cell. Sci. 2000;113(Pt 24):4435-4440.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 173, No. 3, pp. 375-379, March, 2022
Rights and permissions
About this article
Cite this article
Kushlinskii, N.E., Gershtein, E.S., Zybina, N.N. et al. Blood Serum Zonulin in Colorectal Cancer, Autoimmune Bowel Diseases, and Irritable Bowel Syndrome. Bull Exp Biol Med 173, 376–379 (2022). https://doi.org/10.1007/s10517-022-05552-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-022-05552-w