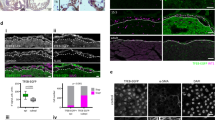
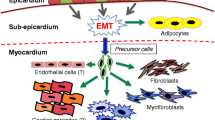
We evaluated the content of active form of TGF-β1 in the intact and post-infarction heart and the effect of this factor on the properties of epicardial cells. During the acute stage after myocardial infarction, the production of TGF-β1 in the heart increased, which closely correlated with activation of epicardial cells (appearance of a pool of Wt1+ epicardial cells entering the epithelial-mesenchymal transition). The role of TGF-β1 as the factor of epicardial activation was confirmed by the results of in vitro experiments: addition of recombinant TGF-β1 to cultured epicardial cells led to enhanced expression of genes of epithelial-mesenchymal transition and phenotypic transformation of these cells leading to the appearance of cells with markers of smooth muscle cells and fibroblasts. Our findings suggest that the regulatory axis “TGF-β1—epicardium cells” can be considered as an important link of the post-infarction reparative process and adaptive response during heart remodeling after myocardial infarction and as the target for therapeutic interventions.
Similar content being viewed by others
References
Dergilev KV, Komova AV, Tsokolaeva ZI, Beloglazova IB, Parfyonova YeV. Epicardium as a new target for regenerative technologies in cardiology. Geny Kletki. 2020;15(2):33-40. Russian.
Dergilev KV, Tsokolaeva ZI, Beloglazova IB, Ratner EI, Molokotina YuD, Parfenova EV. Angiogenic properties of myocardial c-kit+ cells. Geny Kletki. 2018;13(3):82-88. Russian.
Sergienko IV, Semenova AE, Masenko VP, Habibullina LI Gabrusenko SA, Kukharchuk VV, Belenkov YuN. Myocardial revascularization effects on vascular endothelial growth factor and transforming growth factor β-1 in coronary heart disease patients. Kardiovask. Ter. Prof. 2007;6(5):12-17. Russian.
Assoian RK, Fleurdelys BE, Stevenson HC, Miller PJ, Madtes DK, Raines EW, Ross R, Sporn MB. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc. Natl Acad. Sci. USA. 1987;84(17):6020-6024.
Burns WC, Thomas MC. The molecular mediators of type 2 epithelial to mesenchymal transition (EMT) and their role in renal pathophysiology. Expert Rev. Mol. Med. 2010;12:e17. doi: https://doi.org/10.1017/S1462399410001481
Cao J, Poss KD. The epicardium as a hub for heart regeneration. Nat. Rev. Cardiol. 2018;15(10):631-647.
Dettman RW, Denetclaw W Jr, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev. Biol. 1998;193(2):169-181.
Duim SN, Goumans MJ, Kruithof BPT. WT1 in cardiac development and disease. Wilms Tumor. van den Heuvel-Eibrink MM, ed. Brisbane, 2016. Chapter 13. P. 211-234.
Eghbali M. Cellular origin and distribution of transforming growth factor-beta in the normal rat myocardium. Cell Tissue Res. 1989;256(3):553-558.
El Kazzi M, Rayner BS, Chami B, Dennis JM, Thomas SR, Witting PK. Neutrophil-mediated cardiac damage after acute myocardial infarction: significance of defining a new target cell type for developing cardioprotective drugs. Antioxid. Redox Signal. 2020;33(10):689-712.
Gittenberger-de Groot AC, Vrancken Peeters MP, Bergwerff M, Mentink MM, Poelmann RE. Epicardial outgrowth inhibition leads to compensatory mesothelial outflow tract collar and abnormal cardiac septation and coronary formation. Circ. Res. 2000;87(11):969-971.
Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ. Res.1998;82(10):1043-1052.
Huynh ML, Fadok VA, Henson PM. Phosphatidylserinedependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J. Clin. Invest. 2002;109(1):41-50.
Ikeuchi M, Tsutsui H, Shiomi T, Matsusaka H, Matsushima S, Wen J, Kubota T, Takeshita A. Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc. Res. 2004;64(3):526-535.
Long CS. Autocrine and paracrine regulation of myocardial cell growth in vitro. The TGF-β1 paradigm. Trends Cardiovasc. Med. 1996;6(7):217-226.
Ma J, Sanchez-Duffhues G, Goumans MJ, Ten Dijke P. TGF-β-induced endothelial to mesenchymal transition in disease and tissue engineering. Front. Cell Dev. Biol. 2020;8:260. doi: https://doi.org/10.3389/fcell.2020.00260
Männer J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat. Rec. 1999;255(2):212-226.
Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev. Biol. 1996;174(2):221-232.
Olea-Flores M, Zuñiga-Eulogio MD, Mendoza-Catalán MA, Rodríguez-Ruiz HA, Castañeda-Saucedo E, Ortuño-Pineda C, Padilla-Benavides T, Navarro-Tito N. Extracellular-signal regulated kinase: a central molecule driving epithelial-mesen chymal transition in cancer. Int. J. Mol. Sci. 2019;20(12):2885. doi: https://doi.org/10.3390/ijms20122885
Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana J.L. Regulation of the polarity protein Par6 by TGF-β1 receptors controls epithelial cell plasticity. Science. 2005;307:1603-1609.
Parichatikanond W, Luangmonkong T, Mangmool S, Kurose H. Therapeutic Targets for the treatment of cardiac fibrosis and cancer: focusing on TGF-β1 signaling. Front Cardiovasc. Med. 2020;7:34. doi: https://doi.org/10.3389/fcvm.2020.00034
Pérez-Pomares JM, Carmona R, González-Iriarte M, Atencia G, Wessels A, Muñoz-Chápuli R. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int. J. Dev. Biol. 2002;46(8):1005-1013.
Poelmann RE, Lie-Venema H, Gittenberger-de Groot AC. The role of the epicardium and neural crest as extracardiac contributors to coronary vascular development. Tex. Heart Inst. J. 2002;29(4):255-261.
Rothenberg F, Hitomi M, Fisher SA, Watanabe M. Initiation of apoptosis in the developing avian outflow tract myocardium. Dev. Dyn. 2002;223(4):469-482.
Ruwhof C, van Wamel A. E, Egas JM, van der Laarse A. Cyclic stretch induces the release of growth promoting factors from cultured neonatal cardiomyocytes and cardiac fibroblasts. Mol. Cell. Biochem. 2000;208(1-2):89-98.
Schaefer KS, Doughman YQ, Fisher SA, Watanabe M. Dynamic patterns of apoptosis in the developing chicken heart. Dev. Dyn. 2004;229(3):489-499.
Suzuki HI. MicroRNA control of TGF-beta signaling. Int. J. Mol. Sci. 2018;19(7):1901. doi: https://doi.org/10.3390/ijms19071901
Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL. Activation of the Erk pathway is required for TGF-β1-induced EMT in vitro. Neoplasia. 2004;6(5):603-610.
Yu L, Hébert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002;21(14):3749-3759.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Kletochnye Tekhnologii v Biologii i Meditsine, No. 4, pp. 278-283, December, 2020
Rights and permissions
About this article
Cite this article
Dergilev, K.V., Tsokolaeva, Z.I., Beloglazova, I.B. et al. Transforming Growth Factor Beta (TGF-β1) Induces Pro-Reparative Phenotypic Changes in Epicardial Cells in Mice. Bull Exp Biol Med 170, 565–570 (2021). https://doi.org/10.1007/s10517-021-05107-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-021-05107-5