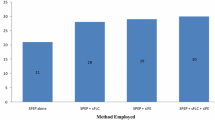
The diagnostic potentialities of complex immunochemical analysis of the serum and daily urine were evaluated in 118 patients with multiple myeloma. In 95 patients, we observed secretion of monoclonal intact immunoglobulins with heavy chains G (N=69), A (N=19), and M (N=4) and biclonal secretion of paraproteins G and A (N=3). Bence-Jones protein was detected in the sera and daily urine of 16 patients and Bence-Jones proteinuria alone was detected in 3 patients. The diagnostic sensitivity of serum immunoelectrophoresis in multiple myeloma is 94.1%. Analysis of paraproteinuria is particularly important in Bence-Jones myeloma, when paraprotein excretion may be not associated with paraproteinemia. Complex study by immunoelectrophoretic and immunoturbidimetric methods in multiple myeloma increases the diagnostic sensitivity to 99.2%.
Similar content being viewed by others
References
Lyubimova NV, Kadagidze ZG. Tumor markers. General characteristics, clinical significance, and recommendations for use. Biological Markers of Tumors: Fundamental and Clinical Studies. Kushlinskii NE, Krasil’nikov MA, eds. Moscow, 2017. P. 142-196. Russian.
Lubimova NV, Turko TA, Votyakova OM, Kushlinskii NE. Serum immunoglobulin free light chains in patients with monoclonal gammapathies. Bull. Exp. Biol. Med. 2012;153(2):249-254.
Bradwell AR. Serum free light chain measurements move to center stage. Clin. Chem. 2005;51(5):805-807.
Bradwell A.R, Carr-Smith HD, Mead GP, Harvey TC, Drayson MT. Serum test for assessment of patients with Bence Jones myeloma. Lancet. 2003;361:489-491.
Bradwell AR, Carr-Smith HD, Mead GP, Tang LX, Showell PJ, Drayson MT, Drew R. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin. Chem. 2001;47(4):673-680.
Katzmann JA, Clark RJ, Abraham RS, Bryant S, Lymp JF, Bradwell AR, Kyle RA. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin. Chem. 2002;48(9):1437-1444.
Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, Kröger N, Einsele H, Vesole DH, Dimopoulos M, San Miguel J, Avet-Loiseau H, Hajek R, Chen WM, Anderson KC, Ludwig H, Sonneveld P, Pavlovsky S, Palumbo A, Richardson PG, Barlogie B, Greipp P, Vescio R, Turesson I, Westin J, Boccadoro M; International Myeloma Working Group. Monoclonal gammopathy of undetermined signigicance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24(6):1121-1127.
Misra A, Mishra J, Chandramohan J, Sharma A, Raina V, Kumar R, Soni S, Chopra A. Old but still relevant: high resolution electrophoresis and Immunofixation in multiple myeloma. Indian J. Hematol. Blood Transfus. 2016;32(1):10-17.
Murray DL, Ryu E, Snyder MR, Katzmann JA. Quantitation of serum monoclonal proteins: relationship between agarose gel electrophoresis and immunonephelometry. Clin. Chem. 2009;55(8):1523-1529.
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, Weiss B, LeLeu X, Zweegman S, Lonial S, Rosinol L, Zamagni E, Jagannath S, Sezer O, Kristinsson SY, Caers J, Usmani SZ, Lahuerta JJ, Johnsen HE, Beksac M, Cavo M, Goldschmidt H, Terpos E, Kyle RA, Anderson KC, Durie BG, Miguel JF. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548.
Willrich MA, Katzmann JA. Laboratory testing requirements for diagnosis and follow-up of multiple myeloma and related plasma cell dyscrasias. Clin. Chem. Lab. Med. 2016;54(6):907-919.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 165, No. 1, pp. 99-103, January, 2018
Rights and permissions
About this article
Cite this article
Lyubimova, N.V., Timofeev, Y.S., Abaev, V.M. et al. Immunochemical Diagnosis of Multiple Myeloma. Bull Exp Biol Med 165, 84–87 (2018). https://doi.org/10.1007/s10517-018-4105-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-018-4105-y