Abstract
This study aimed to assess the effects of dietary Cyanus depressus (CD) extract on rainbow trout (Oncorhynchus mykiss) physiology and health. Fish with an average weight of 3.29 ± 0.34 g were allocated to four feeding groups, receiving diets containing CD extract at doses of 0 (Control), 0.5 (CD05), 1 (CD1), and 2 (CD2) g kg−1 for a period of 60 days. Results revealed significant improvements in final body weight, weight gain, and specific growth rate in groups supplemented with 1 and 2 g/kg of CD extract. Lipase activity increased notably in fish fed with CD0.5, CD1, and CD2 diets. The CD1 group exhibited the highest trypsin activity, while the groups receiving 1 g/kg and 2 g/kg of CD extract displayed the highest amylase activity. Analysis of gene expression levels for superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) showed marked increases across all groups compared to the control, with the most significant enhancement observed in fish fed with 2 g/kg of CD extract. Significant variations were observed in the expression of immunity-related genes among the treatments, with the highest expression levels of tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) found in groups fed a diet enriched with CD extract. The increased activity of immune-related genes was more prominent in fish fed 2 g/kg of CD extract than in the control group. Notably, in fish fed with CD extract, heat shock protein 70 (HSP70) gene expression increased in the CD2 group, decreased in the CD05 group, and remained statistically unchanged in the CD1 group compared to the control. Additionally, supplementation with CD extract altered the composition and abundance of the intestinal microbiota in rainbow trout. In conclusion, our study suggests that Cyanus depressus extract has significant potential for enhancing various physiological and health aspects of rainbow trout, particularly when supplemented at 2 g/kg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rainbow trout is esteemed as one of the foremost economically vital species in freshwater environments, renowned for its premium flesh quality and adaptability to diverse aquaculture systems (Hajirezaee et al. 2024). In recent years, the increasing demand for seafood products worldwide has led to the development of intensive aquaculture systems. However, under intensive production systems, fish farming compromises the immune systems of fish, resulting in the emergence of infectious diseases that decrease growth and survival rates, ultimately leading to significant economic losses and reduced fish production (Limbu et al. 2021; Tadese et al. 2022). In response to these challenges, antibiotics and synthetic growth promoters have become prevalent in aquaculture practices to stimulate growth, mitigate stress, and control bacterial infections (Ahmadifar et al. 2021a; Hajirezaee et al. 2024). These substances and antibiotics may increase fish growth and illness resistance, but fish and the ecosystem will inevitably suffer as a result (Rashidian et al. 2020a Therefore, the increasing concern about the widespread use of chemicals, especially antibiotics, in fish farming has led to regulatory interventions in many countries worldwide (Van Hai 2015; Santos and Ramos 2018). Although antibiotics generally prove successful in treating diseases, their excessive and improper use leads to the presence of antibiotic residues in aquatic products and the environment (Grenni et al. 2018; Elumalai et al. 2020; Limbu et al. 2021). Furthermore, inappropriate utilization of antibiotics for disease treatment or as growth promoters in aquaculture fosters the escalation of antimicrobial resistance (Awad and Awaad 2017; Tadese et al. 2022). On the other hand, the non-selective effect of antibiotics will adversely affect the gut microorganisms of aquatic animals and disrupt the balance of the microbial ecosystem (Limbu et al. 2021; Dien et al. 2023). Therefore, it is imperative to replace chemicals with alternative options that are more preferable in terms of public health and environmental considerations (Rahimi et al. 2022).
Feed additives are non-nutritive components that play a significant role in enhancing the quality of aquafeed (Mohan et al. 2022). Among the array of potential feed supplements, plant compounds emerge as promising alternatives due to their minimal adverse effects on organisms and the environment, absence of drug resistance, cost-effectiveness, and sustainability (Liu et al. 2021a, b). The utilization of plant components in aquatic species has gained substantial attention for their potential to bolster growth, improve digestibility and nutrient availability, modulate intestinal microbiota, and influence gene expression related to physiological processes (Rashidian et al. 2020a; Liu et al. 2021a, b; Mostafavi et al. 2022; Zhang et al. 2022).
The Asteraceae family genera are renowned for their diverse array of biologically active compounds, showcasing substantial potential for therapeutic applications (Ayad & Akkal 2019). Cyanus depressus (CD), also recognized as “gökbaş” in Türkiye, is a member of the Asteraceae family and is found in Western Asia, Iran, and the Caucasus region. This herbaceous annual plant features striking blue-purple flowers and can reach heights of up to 60 cm (Duman et al. 2022). Within Turkish traditional medicine, Cyanus species, including CD, are extensively utilized due to their recognized antidiarrheal, expectorant, antipyretic, and antidiabetic properties (Duman et al. 2022). Moreover, these species are employed in folk medicine for their purported hepatoprotective, wound healing, antidiabetic, antioxidant, anticarcinogenic, antiviral, anti-inflammatory, and antimicrobial activities, along with their traditional use in treating fever (Khammar & Djeddi 2012; Escher et al. 2018; Fattaheian-Dehkordi et al. 2021; Gawlik-Dziki et al. 2023). Despite this extensive therapeutic potential, as far as our current knowledge extends, no research has yet explored the effects of Cyanus depressus on aquatic species.
Given the recognized benefits of Cyanus depressus, we hypothesize that its incorporation into fish feed as an additive could offer advantages to the aquaculture industry. Such utilization can offer significant revelations regarding the advantageous impacts of Cyanus depressus on growth performance, intestinal microbiota, and gene expression associated with digestion, antioxidative processes, stress management, and immune reactions in rainbow trout.
Considering the economic significance of rainbow trout in the global aquaculture sector and the promising attributes of Cyanus depressus (CD) as a feed additive, this study was undertaken for the first time to evaluate the dietary impact of CD extract on rainbow trout’s growth performance, intestinal microflora composition, and gene expression profiles. The primary objective of this study is to assess the efficacy of Cyanus depressus extract as a dietary supplement in promoting the growth and health of rainbow trout, thereby contributing to the optimization of aquaculture practices. By elucidating the mechanisms underlying the observed effects, this research endeavors to provide valuable insights into the potential use of natural additives in aquafeed formulations, ultimately aiming to improve the sustainability and efficiency of rainbow trout production systems.
Materials and methods
Ethical approval
The Local Ethic Committee of Van Yüzüncü Yıl University approved (protocol no: 2023/06–11), and the experiment was carried out in accordance with standard ethics.
Preparation of CD extract
In May 2023, Cyanus depressus plants were collected from the campus of Van Yüzüncü Yıl University in Van, Turkey. Flower parts were utilized for the study. The lyophilized extract was prepared according to the procedure by Duman et al. (2022), with some modifications. The CD plants were first thoroughly cleaned with distilled water and then allowed to dry for ten days at room temperature in the shade. After that, a blender was used to grind the dried flower pieces into a powder. A total of 40 g of the flower powder sample was then mixed with 1 L of 80% ethanol in a shaker for 24 h. To separate the extract from solid particles, the resulting mixture was first passed through sterile cheesecloth and then subjected to centrifugation at 3500 rpm for 5 min. The filtered solution was further clarified by passing it through Whatman filter paper (Whatman Paper, No. 1). Following the mixture’s filtration, the extract was transferred to a rotary evaporator, where the alcohol was evaporated at 40 °C to obtain a concentrated extract. Finally, 20 ml of concentrated extract was obtained from 100 g of plant powder. After this, the concentrated extract underwent lyophilization in a freeze dryer at − 85 °C and 50 millitorr pressure until it was completely dry. The resulting lyophilized extracts were then stored at − 20 °C until they were utilized for the experimental application.
Phenolic composition of CD extract
At the Eastern Anatolia High Technology Application and Research Center (DAYTAM/Erzurum, Turkey), the concentration of phenolic compounds in the CD extract utilized in the study was measured. Chromatographic separation was carried out using a C18 column (Reversed Phase C18 Column) and an LC–MS/MS system (Agilent 6460 Triple Quad LC–MS/MS with 1290 Infinity UPLC system). The temperature in the column was maintained at 30 °C. Two different mobile phases were used: mobile phase A, which included ultra-pure water and formic acid, and mobile phase B, which included acetonitrile and formic acid. The injection volume was set at 5 μL, and the solvent flow rate was set at 0.4 mL/min.
Preparation of samples, formulation of diets, and rearing conditions
The research was conducted at Van Yüzüncü Yıl University’s Aquatic Creatures Trial Unit in Van, Türkiye. Rainbow trout (O. mykiss) were obtained from a local fish farm and kept for 2 weeks to acclimatize to the experimental conditions. After this period, 240 healthy fish weighing 3.29 ± 0.34 g were randomly distributed into 12 fiberglass tanks (volume of 300 L, 20 fish per tank, in triplicates). In the study, a commercial trout feed (Aquanorm, İzmir/Türkiye) was considered as a basal diet (Table 1). Fish in the control group were fed with the commercial trout feed, while the CD05, CD1, and CD2 groups received the commercial trout feed supplemented with 0.5, 1, and 2 g kg−1 of Cyanus depressus extract, respectively. By spraying, extracts were combined with fish feed (Bilen et al. 2021; Sönmez et al. 2022b). Fish were hand-fed three times at 08.00 am and 1.00 and 6.00 pm until satiation. Throughout the experiment, a central air pump was used to aerate the rearing tanks, and each morning, 20% of the tank’s water was replaced with new water. Regularly siphoned waste and other materials from the tank floor were also done. Every day, measurements were made of the water quality parameters, which included pH (8.13 ± 0.1), dissolved oxygen (8.48 ± 0.16 mg/L), and temperature (13.35 ± 0.25 °C). In addition, a photoperiod of 12 h of light and 12 h of darkness was performed for the study.
Growth parameters
The fish were weighed before the feeding trial and at the conclusion of the 60-day period in order to measure growth parameters using the following standard formulae:
Weight gain (WG; g/fish) = final weight (g) − initial weight (g),
Body weight increase (BWI, %) = 100 × (final weight – initial weight)/initial weight,
Specific growth rate (SGR; %/day) = ((ln (final weight) – ln (initial weight))/days) × 100,
Daily weight gain (DWG; g/fish) = (final weight (g) − initial weight (g))/days,
Thermal growth coefficient (TGC) = (((final weight)1/3 – (initial weight)1/3)/temperature in °C × time in days) × 1000,
Feed conversion ratio (FCR) = total feed given (g)/weight gain (g),
Protein efficiency ratio (PER) = Weight gain (g)/protein intake (g),
Survival rate (SR; %) = (final number of fish/initial number of fish) × 100.
Gene expression analysis
In the study, the activities of digestion, antioxidant function, stress response, and immune response were evaluated at the gene level using molecular tests. Fish were anesthetized using clove powder at a concentration of 200 mg/L before sampling (Naderi et al. 2017). Three fish were then randomly selected from each tank (N = 9) following the feeding trial. The liver and intestinal tissues of the selected fish were subsequently stored in RNA later for further examination. Following the manufacturer’s instructions, the RNeasy Plus Mini Kit in a QIACUBE (Qiagen) device was used for total RNA isolation. The quality of the extracted RNAs was assessed at 260 and 280 nm using a thermonanospectrophotometer. Subsequently, isolated RNAs were diluted to a concentration of 1 ng/μl for each sample, and cDNAs were synthesized using the Qiagen RT2 First Strand Kit. Subsequently, qRT-PCR was analyzed using the RT2 SYBRGreen qPCR Master Mix (Qiagen) in the RotorGene Q 9000 (Qiagen) device. In the gene expression analysis, β-actin was chosen as the reference gene, while TRP, LPZ, AML, SOD, CAT, GPX, IL-1β, TNF-α, and HSP70 were designated as target genes. The PCR composition included 12 μL of SybrGreen qPCR Master Mix, 2.5 μL of forward and reverse assay primer, and 4 μL of H2O in a total volume of 21 μL, with 4 μL of cDNA added last. The PCR protocol involved 40 cycles, with initial incubation at 95 °C for 10 min, followed by annealing at 94 °C for 15 s and 60 °C for 30 s. Beta-actin was used as the reference gene to normalize the Ct values obtained from real-time PCR. Analysis of the real-time PCR data was conducted using the ΔΔCT method (Livak and Schmittgen 2001) (Table 2).
Intestinal microbiota analysis
At the end of the feeding trial, the intestines of 3 fish from each group were collected to obtain a diverse microbial flora (Liu et al. 2021a, b), and the total bacterial genomic DNA was extracted. The QIAamp DNA Extraction Mini Kit (Qiagen) was used for the extraction following the manufacturer’s protocol. The quality and concentration of DNA were assessed using a spectrophotometer (Qiaxpert; Qiagen). For bacterial diversity analysis, the V3–V4 regions of the 16S rRNA gene were amplified using the primer sequences F: 5'-CCTACGGGNGGCWGCAG-3' and R: 5'-GACTACHVGGGTATCTAATCC-3' with the SimpliAmp Thermal Cycler. The PCR conditions included pre-denaturation at 95 °C for 10 min; denaturation at 95 °C for 30 s, annealing at 53 °C for 30 s, extension at 72 °C for 90 s for 35 cycles, and final extension at 72 °C for 10 min. Prior to NGS analysis, purification was performed using the “Qiaseq beads Clean-Up Kit, Cat. No: 180795” from Qiagen. Library preparation for the 16S rRNA V3–V4 amplicons was carried out using Illumina’s “Nextera XT DNA Library Prep Kit, Cat. No.: FC-131–1096,” and indexing was done using the “TG Nextera XT Index Kit v2 Set A (96 Indices, 384 Samples), Cat. No: TG-131–2001.” The concentrations of the libraries were determined using the iQuant™ dsDNA HS Assay Kit (ABP Bioscience, USA). Sequencing was performed on the Illumina iSeq100 platform in paired-end (PE) 2 × 150 mode (Langmead and Salzberg 2012). Raw data reads were classified into OTUs using the Kraken Metagenomics system, which assigns taxonomic labels to short DNA sequences with high accuracy and speed (Wood and Salzberg 2014). The Kraken confidence filter was set at 0.05, minimum hit group at 2, and the RefSeq 2022.02 database was used, which includes archaea, bacteria, fungi, protozoa, and viruses. OTUs were clustered and defined at ≥ 97% identity and 97% similarity, and those representing at least 0.005% of the total reads were retained. Alpha diversity measurements were estimated using the Shannon and Simpson indices. Additionally, beta diversity was determined by principal coordinates analysis (PCoA). A Venn diagram was created using the web tool at https://bioinformatics.psb.ugent.be/webtools/Venn/ to identify and visualize shared and unique OTUs among groups (Rimoldi et al. 2019).
Statistical analysis
Statistical analyses were conducted using the SPSS 20 statistical package program. Before analysis, the data underwent checks for normality and homogeneity of variance. Differences among groups were assessed using one-way analysis of variance (ANOVA). Upon finding significant differences (p < 0.05), means were compared using the Duncan multiple-range test. Results were expressed as means ± standard error.
Results
Phenolic composition of CD extract
Table 3 displays the phenolic component concentration of the CD extract. Thirty-five distinct phenolic compounds were analyzed in the CD extract used in the study. After analyzing the data, it was found that the CD extract contained 15 of the 35 phenolic chemicals.
Growth performance
As delineated in Table 4, the CD1 and CD2 cohorts showcased the utmost concluding weight (FW) and the most minimal feed conversion ratio (FCR). Among the CD treatments, the CD2 group showed the most substantial increase in growth performance. Parameters of WG, BWI, SGR, DWG, TGC, and PER displayed no noteworthy variances between the control and CD05 groups (p > 0.05). Nonetheless, statistically significant differences (p < 0.05) were observed between these groups and the CD1 and CD2 groups. No mortality was recorded among the experimental groups throughout the 60-day feeding trial.
Gene expression related to digestion
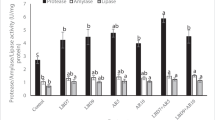
Below are the alterations in expression levels of genes that stimulate TRP-LPZ-AML enzymes linked to the digestive system (Fig. 1). According to these results, overexpression of TRP was observed in the CD1 group compared to the control group (p < 0.05). Significant downregulation was observed in the CD05 and CD2 groups compared to the control group (p < 0.05). Regarding LPZ expression results, upregulation was observed in all groups compared to the control group (p < 0.05). While the AML gene expression levels showed upregulation in the CD1 and CD2 groups compared to the control group, the CD05 group showed downregulation (p < 0.05).
Gene expression associated with antioxidants
The levels of gene expression related to antioxidants, namely SOD, CAT, and GPX, were assessed, and variations in their expression levels are depicted below (Fig. 2). In SOD gene expression levels, overexpression was observed in the CD2 group compared to the control group (p < 0.05). Upregulation was observed in the CD1 group compared to the control group (p < 0.05). In the CD05 group, insignificant downregulation was observed, similar to the control group. CAT results showed overexpression in the CD2 group compared to the control group, while upregulation was observed in the CD1 group (p < 0.05). In the CD05 group, upregulation similar to the control group was observed. In GPx expression levels, overexpression was observed in the CD2 group compared to the control group, and upregulation was observed in the CD1 and CD05 groups compared to the control group (p < 0.05).
Gene expression related to stress and immunity
Expression levels of IL-1β, TNF-α, and HSP70 genes used in the study related to immunity and stress are provided below (Fig. 3). According to these results, overexpression of the IL-1β gene was observed in the CD2 group compared to the control group, and significant upregulation was observed in the CD1 group samples (p < 0.05). The CD05 group was found to be downregulated compared to the control group (p < 0.05). In TNF gene expression results, overexpression was observed in all groups compared to the control group (p < 0.05). In HSP70 gene expression results, overexpression was observed in the CD2 group compared to the control group, downregulation was observed in the CD05 group, and significant upregulation similar to the control group was observed in the CD1 group (p < 0.05).
Effects of Cyanus depressus extract on intestinal microbiota of rainbow trout
16S-rRNA sequencing analysis was utilized to investigate the changes in the gut microbiota of rainbow trout. The average read numbers, observed OTU counts, and alpha diversity indices of the experimental groups are presented in Table 5. In the study, the number of observed OTUs in the rainbow trout gut flora ranged from 172 to 220, with the lowest OTU count observed in the CD2 group and the highest in the CD1 group. An increase in OTU counts was observed in the CD05 and CD1 groups compared to the control group. The read numbers varied between 14.595 and 34.877 among the groups. Additionally, alpha diversity indices based on read numbers were used to measure diversity, revealing a decrease in Shannon and Simpson indices in the other groups compared to the control group (Table 5).
The PCoA analysis revealed a differentiation in the structure of the intestinal microbiota following the application of CD extract. As shown in Fig. 4a, the first principal coordinate (PC1) and second principal coordinate (PC2) accounted for 35% and 32% of the microbial community structure variance, respectively, indicating differences among the groups. The results from the PCoA plot illustrate that the control group was distinct from the groups fed with CD extract, clustering separately. Furthermore, it was observed that the groups fed with CD extract also clustered differently among themselves. According to the Venn diagram (Fig. 4b) illustrating sample similarity and overlap, a total of 687 unique non-singleton OTUs were identified in the rainbow trout gut microbiota. The numbers of unique OTUs in control, CD05, CD1, and CD2 were found to be 133, 168, 178, and 126, respectively.
A general overview of the taxonomic profile is presented in Fig. 5. Overall, the OTUs were assigned to 23 phyla, 37 classes, 83 orders, and 169 families. The most prevalent microbiota phyla and families in the intestine were determined. Proteobacteria and Firmicutes were found to be the most prevalent phyla in the study, constituting between 91.9 and 98.3% of the total gut microbiota (Fig. 5a). The relative abundance of Proteobacteria increased in the CD05 and CD2 groups compared to the control group, while it decreased in the CD1 group. In the CD1 group, Firmicutes was the most abundant phylum. Additionally, the third most abundant phylum, with the highest bacterial density, was Bacteroidota in the control, CD05, and CD1 groups, whereas it was Actinobacteria in the CD2 group at the phylum level. At the family level, Enterobacteriaceae in the control and CD2 groups, Erwiniaceae in the CD05 group, and Streptococcaceae in the CD1 group were found to have the highest relative abundances (Fig. 5b). The abundance of Enterobacteriaceae was observed to increase in the CD2 group compared to the control group.
Discussion
Numerous medicinal plants and their active compounds have been utilized in aquaculture for many years due to their advantageous effects, including promoting growth, stimulating appetite, enhancing immunity, improving antioxidant status, increasing immunity against diseases, and reducing stress in aquatic species (Bilen et al. 2021; Liu et al. 2021a, b; Dadras et al. 2023). To our knowledge, there has been no prior investigation into the impacts of Cyanus depressus on fish. Therefore, this research was designed to assess the growth performance, intestinal microbiota, and gene activity associated with the process of digestion, antioxidants, stress, and immunity in rainbow trout subjected to diets supplemented with Cyanus depressus.
Flavonoids and phenolic acids are significant phytochemicals derived from various plants (Nwozo et al. 2023). In vitro and in vivo pharmacological investigations have demonstrated that these compounds serve as natural growth promoters (Awad and Awaad 2017; Ahmadifar et al. 2021b; Tadese et al. 2022), antioxidants exhibiting potent free radical scavenging properties (Kaurinovic and Vastag 2019), and possess immune-stimulating effects (Tungmunnithum et al. 2018; Elumalai et al. 2020). The LC–MS/MS analysis results of the CD extract (Table 3) indicate the presence of various flavonoids and phenolic acids such as quinic acid, chlorogenic acid, vanillic acid, luteolin, fumaric acid, gallic acid, 4-OH-benzoic acid, and apigenin. Similar flavonoid and phenolic compounds in the CD extract have been reported in previous studies (Mishio et al. 2015; Boğa et al. 2016; Duman et al. 2022). Also, various studies report the positive effects of these compounds on the health and performance of aquatic animals (das Neves et al. 2021; Ghafarifarsani et al. 2023; Jin et al. 2023; Zhang et al. 2023; Lin et al. 2024). These compounds found in the CD extract, as in many plants, have been reported to potentially reduce inflammatory propagation by inhibiting nuclear factor kappa B (NF-κB), Janus kinase (JAK), and mitogen-activated protein kinase (MAPK) pathways, thereby potentially preventing inflammation-associated tissue damage (Vendrame and Klimis-Zacas 2015; Yin et al. 2021; Alharbi et al. 2022; Hamsalakshmi et al. 2022). Additionally, it has been noted that these compounds may contribute to the clearance of excessive cellular free radicals by concurrently upregulating several important antioxidant factors, such as heme oxygenase-1 (HO-1) and NAD(P)H quinone oxidoreductase (NQO1), either dependent or independent of nuclear factor erythroid 2 (NF-E2)–related factor 2 (Nrf2) pathways (Bajpai et al. 2018; Iranshahy et al. 2018; Bhattacharjee and Dashwood 2020). On the other hand, these compounds have been reported to potentially regulate and support metabolic homeostasis of lipids and glucose by modulating glucose release and absorption, as well as lipid synthesis, through activation of the AMP-activated protein kinase (AMPK) pathway (Marín-Aguilar et al. 2017; Herath et al. 2018; Pietrzyk et al. 2021). On the other hand, the growth-promoting effects of flavonoids and phenolic acids observed in animals may be attributed to various mechanisms resulting from the diverse biological activities of phytochemicals (Valenzuela-Grijalva et al. 2017). For instance, it has been reported that various biological activities related to intestinal function, such as increased digestive secretions, nutrient absorption, and intestinal morphology improvement, could enhance the growth performance of animals (Mahfuz et al. 2021; Waqas et al. 2023).
The growth efficiency of fish stands as a critical determinant for successful production in aquaculture (Bilen et al. 2021). The findings from the present study indicate a beneficial impact on growth performance in fish following a 60-day feeding period with diets incorporating varying levels of CD extract. This could be attributed to the bioactive constituents (Table 3), which increased the attractiveness of the feed, leading to increased intake of the diets. Previous studies have reported that compounds such as chlorogenic acid, luteolin, gallic acid, and fumaric acid found in CD extract have positive effects on the growth performance of aquatic animals (das Neves et al. 2021; Ghafarifarsani et al. 2023; Jin et al. 2023; Zhang et al. 2023; Lin et al. 2024). Moreover, these bioactive compounds may enhance the production of digestive enzymes (Fig. 1), consequently improving feed digestibility and nutrient uptake. The enhancements observed in fish growth performance might be attributed to the activation of digestive enzymes and improved nutrient absorption (Jeney et al. 2015; Yousefi et al. 2021). Establishing the suitable dosage of medicinal herbs is a critical aspect of their administration. Typically, guidance on the optimal concentration of medicinal herbs is derived from in vivo experiments, often using a “trial and error” method (Adel et al. 2020). In our investigation, among the assessed concentrations, the CD 2% treatment displayed the most advantageous impact on growth rate, implying that a particular dosage level influences the optimal condition of growth performance. As of now, there is no available data regarding the function of CD as a growth enhancer in the rainbow trout aquaculture sector. Nonetheless, previous research has documented the enhancement of rainbow trout growth performance with the use of various herbal supplements such as Coriandrum sativum (Farsani et al. 2019), Malvae sylvestris (Rashidian et al. 2020b), Polygonum minus (Adel et al. 2020), Plantago lanceolata (Elbesthi et al. 2020), Ziziphora clinopodioides (Oroji et al. 2021), Viscum album (Yousefi et al. 2021), Allium hirtifolium (Ghafarifarsani et al. 2022), Astragalus caudiculosus (Sönmez et al. 2022b), and Taraxacum officinale (Mostafavi et al. 2022). These investigations reported comparable growth-promoting effects to our chosen herb in rainbow trout. Conversely, Sönmez et al. (2022a) noted no influence on the growth performance of rainbow trout when administered extracts from peel (Punica granatum) and Veratrum (Veratrum album). Likewise, Salem et al. (2021) documented no differences in growth performance among rainbow trout supplied with extracts from Taraxacum officinalis and Usnea barbata.
It is well recognized that medicinal plants can boost the function of digestive enzymes, thereby enhancing the digestibility and accessibility of nutrients, ultimately leading to improved food utilization, protein synthesis, and growth rates (Citarasu 2010; Awad et al. 2012; Beltrán et al. 2020). The heightened functionality of digestive enzymes contributes to achieving better fish growth (Bilen et al. 2021). The findings of our study demonstrated that CD extract effectively enhances the digestive capabilities of rainbow trout. In the present study, CD extract notably influenced the expression of genes responsible for promoting digestive enzyme activity. These observations suggest that the CD extract elicits beneficial effects on the appetite of rainbow trout, consequently augmenting digestive efficiency. This result might be explained by the fact that CD extract contains a variety of flavonoids and phenolic acids. Also, bioactive compounds in plants typically serve as tonics for the secretion of digestive enzymes and exert a direct effect on intestinal microflora (Vijayaram et al. 2022). Previous studies have reported that flavonoids and phenolic acids stimulate the activity of digestive enzymes (Ahmadifar et al. 2021a, b; Tadese et al. 2022). Our study aligns with previous research, which has consistently reported improvements in the digestive enzyme activity of rainbow trout through the use of various medicinal plants, including Quercus brantii (Rashidian et al. 2020a), Dioscorea oppositifolia (Wang et al. 2020), Malvae sylvestris (Rashidian et al. 2020b), Viscum album (Yousefi et al. 2021), Ziziphus jujuba (Liu et al. 2021a, b), and Taraxacum officinale (Mostafavi et al. 2022). These findings align closely with our research. The congruence in findings across different studies reinforces the notion that these medicinal plants possess a shared potential to positively impact the digestive capabilities of rainbow trout.
Antioxidants have a crucial function in combating oxidative damage and are often influenced by the conditions of culture (Ashour et al. 2021). The principal antioxidants within the antioxidant defense system encompass SOD, GPx, and CAT. In fish, the activities of antioxidant enzymes are linked to nutritional aspects (Habte-Tsion et al. 2020). In this study, the gene expression profiles of SOD, CAT, and GPx exhibited a significant rise across all groups in comparison to the control group. It is widely acknowledged that the beneficial effects of plant extracts are related to their antioxidant properties (Ghafarifarsani et al. 2022). The noted elevation in SOD, CAT, and GPx levels indicates that CD extract contains bioactive compounds capable of modulating the immunity of rainbow trout. The phenolic compound concentration found in CD extract, as presented in Table 3, provides evidence to support this conclusion. These results indicate that CD extract may enhance the capacity of the antioxidant system and shield organisms from oxidative harm. Additionally, previous studies have reported that compounds such as chlorogenic acid, luteolin, and gallic acid found in CD extract have positive effects on the antioxidant capacity of aquatic animals (Ghafarifarsani et al. 2023; Jin et al. 2023; Zhang et al. 2023; Lin et al. 2024). While there is a lack of prior reports on the effects of CD on fish antioxidant parameters, the current findings align with studies conducted using other herbal substances. Consistent with our findings, Ghafarifarsani et al. (2022) documented a significant rise in SOD, CAT, and GPX activity in rainbow trout fed Allium hirtifolium compared to the control group. In a separate investigation, it was reported that the addition of Ziziphus jujuba extract to diets could increase the expressions of SOD and CAT in rainbow trout (Liu et al. 2021a, b). Similarly, in the study by Mostafavi et al. (2022), it was reported that the addition of Taraxacum officinale, flower extract to the diet of rainbow trout up to 4 g/kg improved the SOD, CAT, and GPx activities. These findings may be linked to the existence of bioactive compounds possessing antioxidant properties identified in medicinal plants (Citarasu 2010).
Pro-inflammatory cytokines such as TNF-α and IL-1β are vital for the modulation of the innate immune response, and thus, several studies proposed that studying their levels of expression could be used to predict changes in immune responses (Hoseinifar et al. 2020, 2022). In our investigation, notable variations were noted in the expression of genes associated with immunity among treatments, with the highest expression levels of TNF-α and IL-1β observed in rainbow trout receiving a diet supplemented with CD extract. Increasing in the TNF-α and IL-1β levels may be attributed to the activity of the compounds in CD extract like flavonoids and antioxidants. Indeed, previous studies have reported that some of the phenolic compounds listed in Table 3 strengthen immunity (Jin et al. 2023; Zhang et al. 2023; Lin et al. 2024). Various studies indicate that the expression of genes related to immunity in rainbow trout is influenced by medicinal plants. For example, Adel et al. (2020) found that incorporating Polygonum minus extract into the diet significantly boosted gene expressions related to immunity and disease resistance in rainbow trout. Similarly, Hoseinifar et al. (2020) documented that adding 2% Aloysia citrodora meal to the diet notably increased the transcriptional levels of IL-1β and TNF-α. Likewise, Shekarabi et al. (2021) noted substantial increases in TNFα and IL-1β cytokine gene expression levels in rainbow trout after a bacterial challenge when provided with a diet incorporating 3-g/kg Taraxacum officinale extract compared to other treatments and the control group. The incorporation of caper extract (Capparis spinosa) into the rainbow trout diet led to heightened transcription of cytokine genes (TNF and IL-1), bolstering the immune response, as detailed by Bilen et al. (2016). Yousefi et al. (2022) also noted the positive impact of dietary Hyssopus officinalis extract inclusion on the expression of IL-1β and TNF-α genes in rainbow trout. In our current study, the elevated levels of selected immune genes indicate the immunostimulatory effect of dietary CD at the molecular level in rainbow trout.
In general, HSP70 serves multiple roles that enhance the immune response and aid in safeguarding cytoplasmic components during diverse stressful situations (Basu et al. 2002). Numerous investigations have shown that medicinal plants upregulate the relative gene expression of HSP70 in species such as caspian roach (Paknejad et al. 2020), rainbow trout (Sheikhzadeh et al. 2019), red swamp crayfish (Liu et al. 2020), Pacific white shrimp (Lei and Zeng 2008; Anirudhan et al. 2021), hybrid grouper (Sun et al. 2018), and golden pompano (Zhou et al. 2015). Additionally, some studies have reported that medicinal plants downregulate (Tan et al. 2017; Rajabiesterabadi et al. 2020; Espinosa et al. 2020; Giri et al. 2020) or do not alter (Giri et al. 2019; Khieokhajonkhet et al. 2023) HSP70 gene expression. In our current study, the increased HSP70 expression in fish fed with 2 g/kg of CD extract may be considered a positive response that enhances the cells’ ability to cope with stress factors. Furthermore, Liu et al. (2021b) reported that upregulated HSP70 expression results in increased immunity. The increased expression of immune-related TNFα and IL-1β genes in our study supports this finding. It is possible that certain chemical compounds in the CD extract contributed to the increased HSP70 expression observed in the fish. Additionally, different application doses of the CD extract in the fish diet could have also played a role. However, additional research is warranted to validate these hypotheses, as there is a lack of information regarding the impacts of dietary plant components on stress and HSP70 pathways and functions in rainbow trout.
Biotic factors such as genotype, physiological status, pathobiology, and lifestyle, along with abiotic factors like environmental conditions, have the potential to impact the composition and diversity of the fish gut microbiota (Yukgehnaish et al. 2020). Several investigations have demonstrated notable impacts of medicinal plants and their extracts on the intestinal microbiota (Ye et al. 2019; Liu et al. 2021a; Zhang et al. 2022; Kamble et al. 2024). In this study, considering the positive results obtained from growth, digestion, antioxidant, and immune parameters, it is believed that the CD extract affects the fish intestinal microbiota. However, when the diversity of the intestinal microbiota obtained in the study was evaluated, it was unexpectedly observed that bacterial diversity narrowed but bacterial density increased an elevation in comparison to the control group. In our investigation, a different result was obtained from other studies reporting an increase in bacterial diversity of the intestinal microbiota with dietary plant supplements (Desai et al. 2012; Xu et al. 2022; Chen et al. 2024; Kamble et al. 2024). However, the lack of an effect of the CD extract used in our study on bacterial diversity could be viewed as a beneficial result, as decreased diversity might entail decreased competition for opportunistic or invasive pathogens. The studies by Apper et al. (2016) and Rimoldi et al. (2018) support our findings.
In this study, Proteobacteria and Firmicutes were found to be the two most common phyla in the intestines of rainbow trout. Despite the observed plasticity in bacterial diversity and composition, most studies resembling our findings have indicated that the microbiome is primarily influenced by these two phyla (Mougin et al. 2023; Singh et al. 2024). However, contrasting results from other investigations have shown these two phyla to be present in considerably lower quantities compared to more prevalent organisms such as Bacteroidota, Actinobacteria, Fusobacteria, and Tenericutes (Wang et al. 2020; Hines et al. 2021; Kamble et al. 2024). Moreover, the ratio of Firmicutes to Bacteroidota (F/B) in the intestinal microbiota is regarded as a key biomarker linked to weight regulation and the maintenance of intestinal balance in humans (Zhao et al. 2023). The changes observed in the abundance of Firmicutes/Bacteroidota in fish fed with CD extract in this study can indicate a potential association with weight gain.
At the family level, it was observed that the dominant families changed in the groups fed with CD extract (especially CD05 and CD1 groups), and their abundances increased compared to the control group. The dominance or increased abundance of different families in each group is thought to be due to the different doses of CD extract affecting the fish microbiota. Erwiniaceae, Enterococcaceae, and Streptococcaceae are bacterial families commonly found in both animal and fish intestinal floras. Similar to our study, various feeding trials in fish have reported that these bacterial families can become dominant (Hartviksen et al. 2014; Araújo et al. 2015; Hines et al. 2022). It is assumed that Erwiniaceae bacteria play a nutritious role by hydrolyzing plant biomass and fixing nitrogen. Additionally, Erwiniaceae bacteria are known to produce antibiotics (Cambronero-Heinrichs et al. 2023). Members of the Streptococcaceae and Enterobacteriaceae families include various bacterial species involved in the anaerobic breakdown of complex carbohydrates. This breakdown yields short-chain fatty acids (SCFAs) as end products, which are readily assimilated by the host and enhance food energy utilization efficiency (Rimoldi et al. 2018). The positive growth data obtained in the CD1 and CD2 groups support this. Furthermore, in all groups (especially CD1 and CD2 groups) compared to the control group, the abundance of the Sphingomonadaceae family increased. Some bacteria in the Sphingomonadaceae family are known to break down aromatic compounds that can have negative effects on fish (Asaf et al. 2020; Hao et al. 2020). Ma et al. (2022) reported that the Vicia faba extract increased the abundance of Sphingomonadaceae in the intestine, promoting the growth of grass carp. Similarly, it is estimated that in our study, the CD extract possibly promoted the growth of rainbow trout by increasing the abundance of Sphingomonadaceae in the intestine. Further studies are needed to evaluate the effects of CD extract on fish.
Conclusion
In conclusion, the findings of this study indicate that Cyanus depressus (CD) extract holds considerable potential for enhancing various aspects of rainbow trout physiology and health. Notably, the dietary supplementation of CD extract demonstrated promising outcomes in promoting growth, enhancing digestive enzyme activity, boosting antioxidant capacity, stimulating the immune response, and influencing the composition of the gut microbiota. Specifically, the supplementation with 2 g/kg of CD extract appeared to be a beneficial strategy for rainbow trout in this study. However, further investigations are warranted to delve deeper into the underlying mechanisms behind these effects and to optimize the utilization of CD extract in aquaculture practices. This study adds to the expanding literature on the utilization of medicinal plants in aquaculture, providing insights into potential strategies for improving fish health and productivity.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Adel M, Dawood MA, Shafiei S, Sakhaie F, Shekarabi SPH (2020) Dietary Polygonum minus extract ameliorated the growth performance, humoral immune parameters, immune-related gene expression and resistance against Yersinia ruckeri in rainbow trout (Oncorhynchus mykiss). Aquaculture 519:734738. https://doi.org/10.1016/j.aquaculture.2019.734738
Ahmadifar, E, Pourmohammadi Fallah, H, Yousefi, M, Dawood, MA, Hoseinifar, SH, Adineh, H, ... Doan, HV (2021a) The gene regulatory roles of herbal extracts on the growth, immune system, and reproduction of fish. Animals, 11(8), 2167. https://doi.org/10.3390/ani11082167
Ahmadifar, E, Yousefi, M, Karimi, M, Fadaei Raieni, R, Dadar, M, Yilmaz, S, ... Abdel-Latif, HM (2021b) Benefits of dietary polyphenols and polyphenol-rich additives to aquatic animal health: an overview. Reviews in Fisheries Science & Aquaculture, 29(4), 478–511. https://doi.org/10.1080/23308249.2020.1818689
Alharbi, KS, Afzal, O, Kazmi, I, Shaikh, MAJ, Thangavelu, L, Gulati, M, ... Gupta, G (2022) Nuclear factor-kappa B (NF-κB) inhibition as a therapeutic target for plant nutraceuticals in mitigating inflammatory lung diseases. Chemico-biological interactions, 354, 109842. https://doi.org/10.1016/j.cbi.2022.109842
Anirudhan, A, Okomoda, VT, Iryani, MTM, Andriani, Y, Abd Wahid, ME, Tan, MP, ... Sung, YY (2021) Pandanus tectorius fruit extract promotes Hsp70 accumulation, immune-related genes expression and Vibrio parahaemolyticus tolerance in the white-leg shrimp Penaeus vannamei. Fish & shellfish immunology, 109, 97–105. https://doi.org/10.1016/j.fsi.2020.12.011
Apper, E, Weissman, D, Respondek, F, Guyonvarch, A, Baron, F, Boisot, P, ... Merrifield, DL (2016) Hydrolysed wheat gluten as part of a diet based on animal and plant proteins supports good growth performance of Asian seabass (Lates calcarifer), without impairing intestinal morphology or microbiota. Aquaculture, 453, 40–48. https://doi.org/10.1016/j.aquaculture.2015.11.018
Araújo, C, Muñoz-Atienza, E, Nahuelquín, Y, Poeta, P, Igrejas, G, Hernández, PE, ... Cintas, LM (2015) Inhibition of fish pathogens by the microbiota from rainbow trout (Oncorhynchus mykiss, Walbaum) and rearing environment. Anaerobe, 32, 7–14. https://doi.org/10.1016/j.anaerobe.2014.11.001
Asaf S, Numan M, Khan AL, Al-Harrasi A (2020) Sphingomonas: from diversity and genomics to functional role in environmental remediation and plant growth. Crit Rev Biotechnol 40(2):138–152. https://doi.org/10.1080/07388551.2019.1709793
Ashour M, Mabrouk MM, Abo-Taleb HA, Sharawy ZZ, Ayoub HF, Van Doan H, ..., Goda AMA (2021) A liquid seaweed extract (TAM®) improves aqueous rearing environment, diversity of zooplankton community, whilst enhancing growth and immune response of Nile tilapia, Oreochromis niloticus, challenged by Aeromonas hydrophila. Aquaculture, 543, 736915. https://doi.org/10.1016/j.aquaculture.2021.736915
Awad E, Awaad A (2017) Role of medicinal plants on growth performance and immune status in fish. Fish Shellfish Immunol 67:40–54. https://doi.org/10.1016/j.fsi.2017.05.034
Awad E, Austin B, Lyndon A (2012) Effect of dietary supplements on digestive enzymes and growth performance of rainbow trout (Oncorhynchus mykiss, Walbaum). J Am Sci 8(12):858–864
Ayad R, Akkal S (2019) Phytochemistry and biological activities of Algerian Centaurea and related genera. Stud Nat Prod Chem 63:357–414. https://doi.org/10.1016/B978-0-12-817901-7.00012-5
Bajpai VK, Alam MB, Ju MK, Kwon KR, Huh YS, Han YK, Lee SH (2018) Antioxidant mechanism of polyphenol-rich Nymphaea nouchali leaf extract protecting DNA damage and attenuating oxidative stress-induced cell death via Nrf2-mediated heme-oxygenase-1 induction coupled with ERK/p38 signaling pathway. Biomed Pharmacother 103:1397–1407. https://doi.org/10.1016/j.biopha.2018.04.186
Basu N, Todgham AE, Ackerman PA, Bibeau MR, Nakano K, Schulte PM, Iwama GK (2002) Heat shock protein genes and their functional significance in fish. Gene 295(2):173–183. https://doi.org/10.1016/S0378-1119(02)00687-X
Beltrán JMG, Silvera DG, Ruiz CE, Campo V, Chupani L, Faggio C, Esteban MÁ (2020) Effects of dietary Origanum vulgare on gilthead seabream (Sparus aurata L.) immune and antioxidant status. Fish Shellfish Immunol 99:452–461. https://doi.org/10.1016/j.fsi.2020.02.040
Bhattacharjee S, Dashwood RH (2020) Epigenetic regulation of NRF2/KEAP1 by phytochemicals. Antioxidants 9(9):865. https://doi.org/10.3390/antiox9090865
Bilen S, Altunoglu YC, Ulu F, Biswas G (2016) Innate immune and growth promoting responses to caper (Capparis spinosa) extract in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 57:206–212. https://doi.org/10.1016/j.fsi.2016.08.040
Bilen S, Ali GAM, Amhamed ID, Almabrok AA (2021) Modulatory effects of laurel-leaf cistus (Cistus laurifolius) ethanolic extract on innate immune responses and disease resistance in common carp (Cyprinus carpio). Fish Shellfish Immunol 116:98–106. https://doi.org/10.1016/j.fsi.2021.07.001
Boğa, M., Alkan, H, Ertaş, A, Oral, EV, Yılmaz, MA, Yeşil, Y, ... Kolak, U (2016) Phytochemical profile and some biological activities of three Centaurea species from Turkey. Trop J Pharm Res, 15(9), 1865–1875. https://doi.org/10.4314/tjpr.v15i9.8
Cambronero-Heinrichs, JC, Battisti, A, Biedermann, PH, Cavaletto, G, Castro-Gutierrez, V, Favaro, L, ... Rassati, D (2023) Erwiniaceae bacteria play defensive and nutritional roles in two widespread ambrosia beetles. FEMS Microbiology Ecology, 99(12), fiad144. https://doi.org/10.1093/femsec/fiad144
Chen, Q, Wei, T, Li, M, Liu, S, Wu, J, Xu, G, ... Xie, S (2024) Effect of aqueous extract of Millettia speciosa Champ on intestinal health maintenance and immune enhancement of Cyprinus carpio. Fish & Shellfish Immunology, 144, 109227. https://doi.org/10.1016/j.fsi.2023.109227
Citarasu T (2010) Herbal biomedicines: a new opportunity for aquaculture industry. Aquacult Int 18(3):403–414. https://doi.org/10.1007/s10499-009-9253-7
Dadras F, Velisek J, Zuskova E (2023) An update about beneficial effects of medicinal plants in aquaculture: a review. Veterinární medicína 68(12):449. https://doi.org/10.17221/96/2023-VETMED
das Neves SC, da Silva SM, Costa GK, Correia ES, Santos AL, da Silva LC, Bicudo ÁJ (2021) Dietary supplementation with fumaric acid improves growth performance in Nile tilapia juveniles. Animals 12(1):8. https://doi.org/10.3390/ani12010008
Desai AR, Links MG, Collins SA, Mansfield GS, Drew MD, Van Kessel AG, Hill JE (2012) Effects of plant-based diets on the distal gut microbiome of rainbow trout (Oncorhynchus mykiss). Aquaculture 350:134–142. https://doi.org/10.1016/j.aquaculture.2012.04.005
Dien, LT, Ngo, TPH, Nguyen, TV, Kayansamruaj, P, Salin, KR, Mohan, CV, ... Dong, HT (2023) Non-antibiotic approaches to combat motile Aeromonas infections in aquaculture: Current state of knowledge and future perspectives. Reviews in Aquaculture, 15(1), 333–366. https://doi.org/10.1111/raq.12721
Duman, KE, Dogan, A, Kaptaner, B (2022) Ameliorative role of Cyanus depressus (M. Bieb.) Soják plant extract against diabetes-associated oxidative-stress-induced liver, kidney, and pancreas damage in rats. J Food Biochem, 46(10), e14314. https://doi.org/10.1111/jfbc.14314
Elbesthi RTA, Özdemir KY, Taştan Y, Bilen S, Sönmez AY (2020) Effects of ribwort plantain (Plantago lanceolata) extract on blood parameters, immune response, antioxidant enzyme activities, and growth performance in rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 46:1295–1307. https://doi.org/10.1007/s10695-020-00790-z
Elumalai P, Kurian A, Lakshmi S, Faggio C, Esteban MA, Ringø E (2020) Herbal immunomodulators in aquaculture. Rev Fisheries Sci Aquaculture 29(1):33–57. https://doi.org/10.1080/23308249.2020.1779651
Escher, GB, Santos, JS, Rosso, ND, Marques, MB, Azevedo, L, do Carmo, MAV, ... Granato, D (2018) Chemical study, antioxidant, anti-hypertensive, and cytotoxic/cytoprotective activities of Centaurea cyanus L. petals aqueous extract. Food and Chem Toxicol, 118, 439-453https://doi.org/10.1016/j.fct.2018.05.046
Espinosa C, Beltrán JMG, Messina CM, Esteban MÁ (2020) Effect of Jasonia glutinosa on immune and oxidative status of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 100:58–69. https://doi.org/10.1016/j.fsi.2020.02.068
Farsani MN, Hoseinifar SH, Rashidian G, Farsani HG, Ashouri G, Van Doan H (2019) Dietary effects of Coriandrum sativum extract on growth performance, physiological and innate immune responses and resistance of rainbow trout (Oncorhynchus mykiss) against Yersinia ruckeri. Fish Shellfish Immunol 91:233–240. https://doi.org/10.1016/j.fsi.2019.05.031
Fattaheian-Dehkordi, S, Hojjatifard, R, Saeedi, M, Khanavi, M (2021) A review on antidiabetic activity of Centaurea spp.: a new approach for developing herbal remedies. Evidence-Based Complementary Alternative Med, https://doi.org/10.1155/2021/5587938
Gawlik-Dziki U, Wrzesińska-Krupa B, Nowak R, Pietrzak W, Zyprych-Walczak J, Obrępalska-Stęplowska A (2023) Herbicide resistance status impacts the profile of non-anthocyanin polyphenolics and some phytomedical properties of edible cornflower (Centaurea cyanus L) flowers. Scientific Reports 13(1):11538. https://doi.org/10.1038/s41598-023-38520-z
Ghafarifarsani H, Yousefi M, Hoseinifar SH, Paolucci M, Lumsangkul C, Jaturasitha S, Van Doan H (2022) Beneficial effects of Persian shallot (Allium hirtifolium) extract on growth performance, biochemical, immunological and antioxidant responses of rainbow trout Oncorhynchus mykiss fingerlings. Aquaculture 555:738162. https://doi.org/10.1016/j.aquaculture.2022.738162
Ghafarifarsani H, Hoseinifar SH, Adhami B, Rohani MF, Van Doan H (2023) Dietary gallic acid influences serum enzymatic parameters and immunological responses in Cyprinus carpio exposed to crowding stress. Aquaculture Reports 30:101630. https://doi.org/10.1016/j.aqrep.2023.101630
Giri SS, Sukumaran V, Park SC (2019) Effects of bioactive substance from turmeric on growth, skin mucosal immunity and antioxidant factors in common carp, Cyprinus carpio. Fish Shellfish Immunol 92:612–620. https://doi.org/10.1016/j.fsi.2019.06.053
Giri, SS, Kim, HJ, Kim, SG, Kim, SW, Kwon, J, Lee, SB, ... Park, SC (2020) Effectiveness of the guava leaf extracts against lipopolysaccharide-induced oxidative stress and immune responses in Cyprinus carpio. Fish & Shellfish Immunology, 105, 164–176. https://doi.org/10.1016/j.fsi.2020.06.004
Grenni P, Ancona V, Caracciolo AB (2018) Ecological effects of antibiotics on natural ecosystems: a review. Microchem J 136:25–39. https://doi.org/10.1016/j.microc.2017.02.006
Habte-Tsion HM, Kolimadu GD, Rossi W Jr, Filer K, Kumar V (2020) Effects of Schizochytrium and micro-minerals on immune, antioxidant, inflammatory and lipid-metabolism status of Micropterus salmoides fed high-and low-fishmeal diets. Sci Rep 10(1):7457. https://doi.org/10.1038/s41598-020-64286-9
Hajirezaee S, Rohanizadehghadikolaei F, Afzali-Kordmahalleh A, Khanjani MH (2024) Effects of dietary common juniper (Juniperus communis) essential oil on growth, immunity, antioxidant status, and disease resistance in rainbow trout. Oncorhynchus Mykiss Aquaculture Reports 34:101895. https://doi.org/10.1016/j.aqrep.2023.101895
Hamsalakshmi, Alex, AM, Arehally Marappa, M, Joghee, S, Chidambaram, SB (2022) Therapeutic benefits of flavonoids against neuroinflammation: a systematic review. Inflammopharmacology, 1–26. https://doi.org/10.1007/s10787-021-00895-8
Hao YT, Guo R, Jia GW, Zhang Y, Xia H, Li XH (2020) Effects of enzymatic hydrolysates from poultry by-products (EHPB) as an alternative source of fish meal on growth performance, hepatic proteome and gut microbiota of turbot (Scophthalmus maximus). Aquac Nutr 26(6):1994–2006. https://doi.org/10.1111/anu.13141
Hartviksen, M, Vecino, JG, Ringø, E, Bakke, AM, Wadsworth, S, Krogdahl, Å, ... Kettunen, A (2014) Alternative dietary protein sources for Atlantic salmon (Salmo salar L.) effect on intestinal microbiota, intestinal and liver histology and growth. Aquaculture Nutrition, 20(4), 381–398. https://doi.org/10.1111/anu.12087
Herath, KHINM, Cho, J, Kim, A, Eom, TK, Kim, JS, Kim, JB, ... Jee, Y (2018) Phenolic acid and flavonoid-rich fraction of Sasa quelpaertensis Nakai leaves prevent alcohol induced fatty liver through AMPK activation. J Ethnopharmacol, 224, 335–348. https://doi.org/10.1016/j.jep.2018.06.008
Hines, IS, Ferguson, CS, Bushman, TJ, Gatlin III, DM, Jensen, RV, Smith, SA, ... Stevens, AM (2021) Impact of a yeast-based dietary supplement on the intestinal microbiome of rainbow trout, Oncorhynchus mykiss. Aquaculture Research, 52(4), 1594–1604. https://doi.org/10.1111/are.15011
Hines, IS, Santiago-Morales, KD, Ferguson, CS, Clarington, J, Thompson, M, Rauschenbach, M, ... Stevens, AM (2022) Steelhead trout (Oncorhynchus mykiss) fed probiotic during the earliest developmental stages have enhanced growth rates and intestinal microbiome bacterial diversity. Frontiers in Marine Science, 9, 1021647. https://doi.org/10.3389/fmars.2022.1021647
Hoseinifar SH, Fazelan Z, Bayani M, Yousefi M, Van Doan H, Yazici M (2022) Dietary red macroalgae (Halopithys incurva) improved systemic an mucosal immune and antioxidant parameters and modulated related gene expression in zebrafish (Danio rerio). Fish Shellfish Immunol 123:164–171. https://doi.org/10.1016/j.fsi.2022.02.047
Hoseinifar SH, Shakouri M, Van Doan H, Shafiei S, Yousefi M, Raeisi M, ..., Reverter M (2020) Dietary supplementation of lemon verbena (Aloysia citrodora) improved immunity, immune-related genes expression and antioxidant enzymes in rainbow trout (Oncorrhyncus mykiss). Fish & shellfish immunology, 99, 379–385. https://doi.org/10.1016/j.fsi.2020.02.006
Iranshahy M, Iranshahi M, Abtahi SR, Karimi G (2018) The role of nuclear factor erythroid 2-related factor 2 in hepatoprotective activity of natural products: a review. Food Chem Toxicol 120:261–276. https://doi.org/10.1016/j.fct.2018.07.024
Jeney, G, De Wet, L, Jeney, Z, Yin, G (2015) Plant extracts. In: Lee, C.-S., Lim, C., Webster, C.D. (Eds.), Dietary nutrients, additives, and fish health. Wiley-Blackwell, NJ, USA, pp. 321–333. https://doi.org/10.1002/9781119005568.ch16
Jin X, Su M, Liang Y, Li Y (2023) Effects of chlorogenic acid on growth, metabolism, antioxidation, immunity, and intestinal flora of crucian carp (Carassius auratus). Front Microbiol 13:1084500. https://doi.org/10.3389/fmicb.2022.1084500
Kamble, MT, Chaiyapechara, S, Salin, KR, Bunphimpapha, P, Chavan, BR, Bhujel, RC, ... Pirarat, N (2024) Guava and star gooseberry leaf extracts improve growth performance, innate immunity, intestinal microbial community, and disease resistance in Nile tilapia (Oreochromis niloticus) against Aeromonas hydrophila. Aquaculture Reports, 35, 101947. https://doi.org/10.1016/j.aqrep.2024.101947
Kaurinovic B, Vastag D (2019) Flavonoids and phenolic acids as potential natural antioxidants. IntechOpen, London, UK, pp 1–20
Khammar A, Djeddi S (2012) Pharmacological and biological properties of some Centaurea species. Eur J Sci Res 84(3):398–416
Khieokhajonkhet, A, Roatboonsongsri, T, Suwannalers, P, Aeksiri, N, Kaneko, G, Ratanasut, K, ... Phromkunthong, W (2023) Effects of dietary supplementation of turmeric (Curcuma longa) extract on growth, feed and nutrient utilization, coloration, hematology, and expression of genes related immune response in goldfish (Carassius auratus). Aquaculture Reports, 32, 101705. https://doi.org/10.1016/j.aqrep.2023.101705
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nature Methods 9(4):357–9. https://doi.org/10.1038/nmeth.1923
Lei AY, Zeng DG (2008) Effects of compound Chinese herbal on the expression of heat stress protein 70 gene in white shrimp (Litopenaeus vannamei). Guangxi Agricultural Sciences 6:830–833
Limbu SM, Chen LQ, Zhang ML, Du ZY (2021) A global analysis on the systemic effects of antibiotics in cultured fish and their potential human health risk: a review. Rev Aquac 13(2):1015–1059. https://doi.org/10.1111/raq.12511
Lin, Y, Li, S, Li, Y, Fang, L, Zhang, H, Wang, Q, Ruan, G (2024) Effects of luteolin supplementation on growth, histology, antioxidant capacity, non− specific immunity and intestinal microbiota of the red swamp crayfish (Procambarus clarkii). Animal Feed Sci Technol, 115986. https://doi.org/10.1016/j.anifeedsci.2024.115986
Liu F, Zhang Y, Wang F (2021) Effect of jujube (Ziziphus jujuba) extract on intestinal flora, digestion, and immunity of rainbow trout (Oncorhynchus mykiss). Aquaculture Reports 21:100890. https://doi.org/10.1016/j.aqrep.2021.100890
Liu, F, Geng, C, Qu, YK, Cheng, BX, Zhang, Y, Wang, AM, ... Chen, ZB (2020) The feeding of dietary Codonopsis pilosula polysaccharide enhances the immune responses, the expression of immune-related genes and the growth performance of red swamp crayfish (Procambarus clarkii). Fish & shellfish immunology, 103, 321–331. https://doi.org/10.1016/j.fsi.2020.05.034
Liu, F, Shao, GY, Tian, QQ, Cheng, BX, Shen, C, Wang, AM, ... Yu, YB (2021b) Enhanced growth performance, immune responses, immune-related gene expression and disease resistance of red swamp crayfish (Procambarus clarkii) fed dietary glycyrrhizic acid. Aquaculture, 533, 736202. https://doi.org/10.1016/j.aquaculture.2020.736202
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. methods, 25(4), 402–408. https://doi.org/10.1006/meth.2001.1262
Ma, LL, Zhang, JM, Kaneko, G, Xie, J, Sun, JH, Wang, GJ, ... Yu, EM (2022) Growth performance, intestinal microbiota and immune response of grass carp fed isonitrogenous and isoenergetic diets containing faba bean extracts. Aquaculture Reports, 22, 100924. https://doi.org/10.1016/j.aqrep.2021.100924
Mahfuz S, Shang Q, Piao X (2021) Phenolic compounds as natural feed additives in poultry and swine diets: a review. J Animal Sci Biotechnol 12:1–18. https://doi.org/10.1186/s40104-021-00565-3
Marín-Aguilar F, Pavillard LE, Giampieri F, Bullón P, Cordero MD (2017) Adenosine monophosphate (AMP)-activated protein kinase: a new target for nutraceutical compounds. Int J Mol Sci 18(2):288. https://doi.org/10.3390/ijms18020288
Mishio T, Takeda K, Iwashina T (2015) Anthocyanins and other flavonoids as flower pigments from eleven Centaurea species. Natural Product Communications 10(3):1934578X1501000318. https://doi.org/10.1177/1934578X15010003
Mohan K, Karthick Rajan D, Muralisankar T, Ramu Ganesan A, Marimuthu K, Sathishkumar P (2022) The potential role of medicinal mushrooms as prebiotics in aquaculture: a review. Rev Aquac 14(3):1300–1332. https://doi.org/10.1111/raq.12651
Mostafavi ZS, Shekarabi SPH, Mehrgan MS, Islami HR (2022) Amelioration of growth performance, physio-metabolic responses, and antioxidant defense system in rainbow trout, Oncorhynchus mykiss, using dietary dandelion, Taraxacum officinale, flower extract. Aquaculture 546:737296. https://doi.org/10.1016/j.aquaculture.2021.737296
Mougin, J, Lobanov, V, Danion, M, Roquigny, R, Goardon, L, Grard, T, ... Joyce, A (2023) Effects of dietary co-exposure to fungal and herbal functional feed additives on immune parameters and microbial intestinal diversity in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol, 137, 108773. https://doi.org/10.1016/j.fsi.2023.108773
Mousavi S, Sheikhzadeh N, Tayefi-Nasrabadi H, Alizadeh-Salteh S, Khani Oushani A, Firouzamandi M, Mardani K (2020) Administration of grape (Vitis vinifera) seed extract to rainbow trout (Oncorhynchus mykiss) modulates growth performance, some biochemical parameters, and antioxidant-relevant gene expression. Fish Physiol Biochem 46(3):777–786. https://doi.org/10.1007/s10695-019-00716-4
Naderi M, Keyvanshokooh S, Salati AP, Ghaedi A (2017) Effects of chronic high stocking density on liver proteome of rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 43(5):1373–1385. https://doi.org/10.1007/s10695-017-0378-8
Nwozo OS, Effiong EM, Aja PM, Awuchi CG (2023) Antioxidant, phytochemical, and therapeutic properties of medicinal plants: a review. Int J Food Prop 26(1):359–388. https://doi.org/10.1080/10942912.2022.2157425
Oroji E, Mehrgan MS, Islami HR, Sharifpour I (2021) Dietary effect of Ziziphora clinopodioides extract on zootechnical performance, immune response, and disease resistance against Yersinia ruckeri in Oncorhynchus mykiss. Aquaculture Reports 21:100827. https://doi.org/10.1016/j.aqrep.2021.100827
Paknejad H, Hosseini Shekarabi SP, Shamsaie Mehrgan M, Hajimoradloo A, Khorshidi Z, Rastegari S (2020) Dietary peppermint (Mentha piperita) powder affects growth performance, hematological indices, skin mucosal immune parameters, and expression of growth and stress-related genes in Caspian roach (Rutilus caspicus). Fish Physiol Biochem 46:1883–1895. https://doi.org/10.1007/s10695-020-00839-z
Pietrzyk N, Zakłos-Szyda M, Koziołkiewicz M, Podsędek A (2021) Viburnum opulus L fruit phenolic compounds protect against FFA-induced steatosis of HepG2 cells via AMPK pathway. J Functional Foods 80:104437. https://doi.org/10.1016/j.jff.2021.104437
Rahimi R, Mirahmadi SA, Hajirezaee S, Fallah AA (2022) How probiotics impact on immunological parameters in rainbow trout (Oncorhynchus mykiss)? A systematic review and meta-analysis. Rev Aquac 14(1):27–53. https://doi.org/10.1111/raq.12582
Rajabiesterabadi H, Ghelichi A, Jorjani S, Hoseini SM, Akrami R (2020) Dietary olive (Olea europaea) leaf extract suppresses oxidative stress and modulates intestinal expression of antioxidant-and tight junction-related genes in common carp (Cyprinus carpio). Aquaculture 520:734676. https://doi.org/10.1016/j.aquaculture.2019.734676
Rashidian G, Bahrami Gorji S, Farsani MN, Prokić MD, Faggio C (2020) The oak (Quercus brantii) acorn as a growth promotor for rainbow trout (Oncorhynchus mykiss): growth performance, body composition, liver enzymes activity and blood biochemical parameters. Nat Prod Res 34(17):2413–2423. https://doi.org/10.1080/14786419.2018.1538994
Rashidian G, Kajbaf K, Prokić MD, Faggio C (2020b) Extract of common mallow (Malvae sylvestris) enhances growth, immunity, and resistance of rainbow trout (Oncorhynchus mykiss) fingerlings against Yersinia ruckeri infection. Fish Shellfish Immunol 96:254–261. https://doi.org/10.1016/j.fsi.2019.12.018
Rimoldi S, Terova G, Ascione C, Giannico R, Brambilla F (2018) Next generation sequencing for gut microbiome characterization in rainbow trout (Oncorhynchus mykiss) fed animal by-product meals as an alternative to fishmeal protein sources. PLoS ONE 13(3):e0193652. https://doi.org/10.1371/journal.pone.0193652
Rimoldi S, Gini E, Iannini F, Gasco L, Terova G (2019) The effects of dietary insect meal from Hermetia illucens prepupae on autochthonous gut microbiota of rainbow trout (Oncorhynchus mykiss). Animals 9(4):143. https://doi.org/10.3390/ani9040143
Salem MOA, Salem TA, Yürüten Özdemir K, Sönmez AY, Bilen S, Güney K (2021) Antioxidant enzyme activities and immune responses in rainbow trout (Onchorhynchus mykiss) juveniles fed diets supplemented with dandelion (Taraxacum officinalis) and lichen (Usnea barbata) extracts. Fish Physiol Biochem 47(4):1053–1062. https://doi.org/10.1007/s10695-021-00962-5
Santos L, Ramos F (2018) Antimicrobial resistance in aquaculture: current knowledge and alternatives to tackle the problem. Int J Antimicrob Agents 52(2):135–143. https://doi.org/10.1016/j.ijantimicag.2018.03.010
Sheikhzadeh N, Nootash S, Khani Oushani A, Mousavi S, Tahapour K (2019) Expression analysis of IFN-γ, MX1, MX2, MX3 and HSP70 genes in rainbow trout (Oncorhynchus mykiss) administrated with green tea (Camellia sinensis). Iranian Veterinary J 15(3):32–40. https://doi.org/10.22055/IVJ.2018.105608.1983
Shekarabi SPH, Mostafavi ZS, Mehrgan MS, Islami HR (2021) Dietary supplementation with dandelion (Taraxacum officinale) flower extract provides immunostimulation and resistance against Streptococcus iniae infection in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 118:180–187. https://doi.org/10.1016/j.fsi.2021.09.004
Singh, A, Vidakovic, A, Hjertner, B, Krikigianni, E, Karnaouri, A, Christakopoulos, P, ... Lundh, T (2024) Effects of dietary supplementation of lignocellulose-derived cello-oligosaccharides on growth performance, antioxidant capacity, immune response, and intestinal microbiota in rainbow trout (Oncorhynchus mykiss). Aquaculture, 578, 740002. https://doi.org/10.1016/j.aquaculture.2023.740002
Sönmez AY, Bïlen S, Taştan Y, Kenanoğlu ON, Terzi E (2022) Effects of dietary Astragalus caudiculosus (Boiss & Huet, 1856) supplementation on growth, hematology, antioxidant enzyme activities, and immune responses in rainbow trout (Oncorhynchus mykiss Walbaum, 1792). Fish Shellfish Immunol 122:366–375. https://doi.org/10.1016/j.fsi.2022.02.031
Sönmez, AY, Bilen, S, Özdemir, KY, Alagöz, K, Özçelik, H (2022a) Effect of aqueous methanolic extract of pomegranate peel (Punica granatum) and veratrum (Veratrum album) on oxidative status, immunity and digestive enzyme activity in rainbow trout (Oncorhynchus mykiss). J Agricultural Sci, 1–1. https://doi.org/10.15832/ankutbd.870923
Sun Z, Tan X, Ye H, Zou C, Ye C, Wang A (2018) Effects of dietary Panax notoginseng extract on growth performance, fish composition, immune responses, intestinal histology and immune related genes expression of hybrid grouper (Epinephelus lanceolatus♂× Epinephelus fuscoguttatus♀) fed high lipid diets. Fish Shellfish Immunol 73:234–244. https://doi.org/10.1016/j.fsi.2017.11.007
Tadese, DA, Song, C, Sun, C, Liu, B, Liu, B, Zhou, Q, ... Kevin, NT (2022) The role of currently used medicinal plants in aquaculture and their action mechanisms: a review. Reviews in Aquaculture, 14(2), 816–847. https://doi.org/10.1111/raq.12626
Tan, X, Sun, Z, Huang, Z, Zhou, C, Lin, H, Tan, L, ... Huang, Q (2017) Effects of dietary hawthorn extract on growth performance, immune responses, growth-and immune-related genes expression of juvenile golden pompano (Trachinotus ovatus) and its susceptibility to Vibrio harveyi infection. Fish & shellfish immunology, 70, 656–664. https://doi.org/10.1016/j.fsi.2017.09.041
Teimouri M, Yeganeh S, Mianji GR, Najafi M, Mahjoub S (2019) The effect of Spirulina platensis meal on antioxidant gene expression, total antioxidant capacity, and lipid peroxidation of rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 45(3):977–986. https://doi.org/10.1007/s10695-019-0608-3
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A (2018) Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines 5(3):93. https://doi.org/10.3390/medicines5030093
Valenzuela-Grijalva NV, Pinelli-Saavedra A, Muhlia-Almazan A, Domínguez-Díaz D, González-Ríos H (2017) Dietary inclusion effects of phytochemicals as growth promoters in animal production. J Animal Sci Technol 59:1–17. https://doi.org/10.1186/s40781-017-0133-9
Van Hai N (2015) The use of medicinal plants as immunostimulants in aquaculture: a review. Aquaculture 446:88–96. https://doi.org/10.1016/j.aquaculture.2015.03.014
Vazirzadeh A, Marhamati A, Rabiee R, Faggio C (2020) Immunomodulation, antioxidant enhancement and immune genes up-regulation in rainbow trout (Oncorhynchus mykiss) fed on seaweeds included diets. Fish Shellfish Immunol 106:852–858. https://doi.org/10.1016/j.fsi.2020.08.048
Vendrame S, Klimis-Zacas D (2015) Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-κB and mitogen-activated protein kinase signaling cascades. Nutr Rev 73(6):348–358. https://doi.org/10.1093/nutrit/nuu066
Vijayaram S, Sun YZ, Zuorro A, Ghafarifarsani H, Van Doan H, Hoseinifar SH (2022) Bioactive immunostimulants as health-promoting feed additives in aquaculture: a review. Fish Shellfish Immunol 130:294–308. https://doi.org/10.1016/j.fsi.2022.09.011
Wang F, Liu H, Liu F, Chen W (2020) Effects of Chinese yam (Dioscorea oppositifolia L.) dietary supplementation on intestinal microflora, digestive enzyme activity and immunity in rainbow trout (Oncorhynchus mykiss). Aquaculture research 51(11):4698–4712. https://doi.org/10.1111/are.14815
Waqas M, Salman M, Sharif MS (2023) Application of polyphenolic compounds in animal nutrition and their promising effects. J Animal and Feed Sci 32(3):233–256. https://doi.org/10.22358/jafs/159718/2023
Wood DE, Salzberg SL (2014) Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 15(3):1–12. https://doi.org/10.1186/gb-2014-15-3-r46
Xu, JR, Zheng, PH, Zhang, XX, Li, JT, Chen, HQ, Zhang, ZL, ... Dai, HF (2022) Effects of Elephantopus scaber extract on growth, proximate composition, immunity, intestinal microbiota and resistance of the GIFT strain of Nile tilapia Oreochromis niloticus to Streptococcus agalactiae. Fish & Shellfish Immunology, 127, 280–294. https://doi.org/10.1016/j.fsi.2022.06.032
Ye, Q, Feng, Y, Wang, Z, Zhou, A, Xie, S, Fan, L., ... Zou, J (2019) Effects of dietary Gelsemium elegans alkaloids on intestinal morphology, antioxidant status, immune responses and microbiota of Megalobrama amblycephala. Fish & Shellfish Immunology, 94, 464–478. https://doi.org/10.1016/j.fsi.2019.09.048
Yin Q, Wang L, Yu H, Chen D, Zhu W, Sun C (2021) Pharmacological effects of polyphenol phytochemicals on the JAK-STAT signaling pathway. Front Pharmacol 12:716672. https://doi.org/10.3389/fphar.2021.716672
Yousefi M, Farsani MN, Ghafarifarsani H, Hoseinifar SH, Van Doan H (2021) The effects of dietary supplementation of mistletoe (Viscum album) extract on the growth performance, antioxidant, and innate, immune responses of rainbow trout (Oncorhynchus mykiss). Aquaculture 536:736385. https://doi.org/10.1016/j.aquaculture.2021.736385
Yousefi M, Hoseini SM, Abtahi B, Vatnikov YA, Kulikov EV, Rodionova NY (2022) Effects of dietary methanolic extract of hyssop, Hyssopus officinalis, on growth performance, hepatic antioxidant, humoral and intestinal immunity, and intestinal bacteria of rainbow trout. Oncorhynchus Mykiss Frontiers in Marine Science 9:1026651. https://doi.org/10.3389/fmars.2022.1026651
Yukgehnaish K, Kumar P, Sivachandran P, Marimuthu K, Arshad A, Paray BA, Arockiaraj J (2020) Gut microbiota metagenomics in aquaculture: factors influencing gut microbiome and its physiological role in fish. Rev Aquac 12(3):1903–1927. https://doi.org/10.1111/raq.12416
Zhang Y, Liu F, Wang F (2022) Combined effects of jujube, Chinese yam and astragalus on digestion, immunity and intestinal microflora of rainbow trout. Aquac Res 53(13):4663–4675. https://doi.org/10.1111/are.15959
Zhang J, Wang Z, Shi Y, Xia L, Hu Y, Zhong L (2023) Protective effects of chlorogenic acid on growth, intestinal inflammation, hepatic antioxidant capacity, muscle development and skin color in channel catfish Ictalurus punctatus fed an oxidized fish oil diet. Fish Shellfish Immunol 134:108511. https://doi.org/10.1016/j.fsi.2022.108511
Zhao C, Men X, Dang Y, Zhou Y, Ren Y (2023) Probiotics mediate intestinal microbiome and microbiota-derived metabolites regulating the growth and immunity of rainbow trout (Oncorhynchus mykiss). Microbiol Spectr 11(2):e0398022. https://doi.org/10.1128/spectrum.03980-22
Zhou, C, Lin, H, Ge, X, Niu, J, Wang, J, Wang, Y, ... Tan, X (2015) The effects of dietary soybean isoflavones on growth, innate immune responses, hepatic antioxidant abilities and disease resistance of juvenile golden pompano Trachinotus ovatus. Fish & Shellfish Immunology, 43(1), 158–166. https://doi.org/10.1016/j.fsi.2014.12.014
Acknowledgements
The author would like to thank the SolverArge for generously providing access to their laboratory facilities, facilitating the experimental work essential to this research.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Boran KARATAŞ; Conceptualization; Data curation; Formal analysis; Methodology; Validation; Supervision; Writing – original draft; Investigation; Validation;
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karataş, B. Dietary Cyanus depressus (M. Bieb.) Soják plant extract enhances growth performance, modulates intestinal microbiota, and alters gene expression associated with digestion, antioxidant, stress, and immune responses in rainbow trout (Oncorhynchus mykiss). Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01548-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01548-7