Abstract
The use of intensive systems in aquaculture causes an increase in environmental stress agents, reducing water quality and facilitating the appearance of pathologies in individuals. In this context, the selection of ingredients is a key strategy in managing the quality of aquafeed and the cultivation of aquaculture species. Considering this, wine by-products can be a potential functional ingredient due to several particular characteristics, such as their low cost, large volumes produced, and for being a natural source of bioactive compounds with antioxidant activity. The present study developed different experiments focused on evaluating the benefits of feeding juvenile European sea bass during 5 weeks with feeds incorporating 0.4% red wine grape pomace (GP). At the end of the feeding period, potential modifications in metabolism, immunological and oxidative status, and functionality of intestinal microbiota were assessed as well as the potential protective effect against oxidation in fish fillets during 6 days of cold storage (4 °C). In addition, the preservative effect of GP on the feed when stored for a period of 12 weeks at two different temperatures (4 °C and 25 °C) was evaluated. The results demonstrated that the inclusion of GP in feeds for European sea bass prevents oxidation when stored at room temperature. In addition, a general improvement in the physiological and immunological status, as well as fillet quality, was evidenced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, a significant portion of aquaculture activities relies on intensive systems, which entail the presence of stressful situations for farmed animals together with a decrease in water quality that facilitates the development of pathologies. Moreover, temperature stress associated with global warming can have adverse effects on physiological status as well as growth performance of aquaculture species. Several economically significant species are impacted by these factors, as indicated by previous studies (Brodie et al. 2014; Gazeau et al. 2014; Paukert et al. 2016; Stévant et al. 2017; Stewart et al. 2019; Zhang et al. 2019). In addition, quality management, encompassing feed production and storage, as well as the maintenance of optimal nutritional properties in fish, plays a pivotal role in the production of high-quality aquaculture species. Unfortunately, several factors and processes can negatively affect aquafeed quality during storage at both ambient and low temperatures, with oxidation being a major concern (Hossen et al. 2013).
Taking this into account, the inclusion of antioxidant compounds in aquaculture diets to prevent lipid oxidation and extend their shelf-life without the need to be stored in refrigeration chambers is mandatory (Hamre et al. 2010; Hernández et al. 2014). Furthermore, antioxidants can mitigate the impact of reactive oxygen species (ROS) on fish metabolism, thereby safeguarding their health. Synthetic antioxidants such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) are routinely used in the feed industry but have limitations due to their toxicity and carcinogenic potential (Lourenço et al. 2019). Consequently, there is a growing interest in finding alternative antioxidants from natural sources that can be cost-effective (Li et al. 2009a; Filipe et al. 2023). In this regard, several agro-industrial by-products are emerging as valuable sources of such kind of bioactive compounds, particularly phenolics, with a wide range of potential applications in animal nutrition (Leyva-López et al. 2020; Dawood et al. 2022).
Within the wide array of agro-industrial by-products, it is worthwhile to highlight those stemming from the wine production industry that are widely present in regions such as the EU, USA, South America, China, and Australia. These by-products offer a cost-effective source of polyphenols, including anthocyanins, hydroxybenzoic acids, hydroxycinnamic acids, flavonols, and stilbenes (Bordiga et al. 2013). Among them, grape pomace (GP) stands out as the predominant by-product, constituting approximately 15–20% of the total grape weight, being formed by the solid residues remaining after pressing, including seeds, skins, and a minimal pulp content. Remarkably, it is estimated that nine million tons of GP waste are generated worldwide each year (Teixeira et al. 2014). Historically, GP has been employed as a source of fiber, carbohydrates, and minerals in the diet of terrestrial animals. Nevertheless, despite its widespread availability and profile of bioactive compounds, it is currently underutilized and frequently discarded. However, recent studies have unveiled the favorable impacts of GP phenolic compounds on the production of pigs, poultry, and ruminants (Chedea et al. 2018; Turcu et al. 2018; Costa et al. 2022). While research pertaining to aquaculture species is limited, preliminary findings suggest that grape polyphenols may also offer benefits, including prevention of liver diseases associated with oxidative stress (Souza et al. 2019), improvements in cellular energy homeostasis (Baldissera et al. 2019), enhanced growth and feed digestibility (Peña et al. 2020), or even to generate beneficial changes in the composition of intestinal microbiota (Pulgar et al. 2021).
This study aims to assess the potential benefits of incorporating red GP as a functional ingredient in aquafeeds for European sea bass juveniles (Dicentrarchus labrax). The investigation encompasses three key aspects: (i) preservative effect, evaluating red GP potential in preventing oxidation of stored aquafeeds; (ii) the potential influence of red GP on basal metabolism, immune status, and cultivable gut microbiota of the fish; and (iii) fillet oxidation protection, where the possible protective effects of red GP on fish fillet oxidation during storage at low temperatures (4 °C) were explored. Altogether, these aspects will shed light on the multifaceted advantages that red GP could offer in enhancing the aquaculture industry, contributing to the circular blue economy and sustainability.
Material and methods
Processing and composition of grape pomace
The red wine GP used in the present study was obtained from an artisanal local winery (Fondón, Almería, Spain). It was dried, once collected, in an oven for 72 h at 60 °C and subsequently finely grounded until obtaining a fine powder that was used in the assays. A preliminary quantification of total phenolic compounds and antioxidant capacity was carried out in the by-product by means of specific methods (Brand-Williams et al. 1995; Graça et al. 2005). This information was used as the basis for determining suitable amounts of GP to be included in the experimental diets.
Experimental diets
Two experimental diets were formulated for the study: a control diet (C) and a supplemented diet that incorporated GP at a concentration of 0.4%. The selected dosage, although lower than that utilized in previous studies involving aquaculture species (Peña et al. 2020; Martínez-Antequera et al. 2023), was chosen to evaluate the additive’s impact at a more consistent level with those used for similar compounds in commercially produced aquafeeds. The diets were prepared at the Service of Experimental Diets, located at the University of Almeria, Spain (CEIMAR), using a laboratory-scale extrusion machine equipped with a 2.5 mm mesh. Following preparation, the diets were dried and stored at 4 °C until be used. The formulation and proximate composition of the diets are detailed in Table 1.
Shelf-life performance of aquafeeds
To monitor potential alterations in the antioxidant content of the aquafeeds during storage, samples of the experimental diets were kept in darkness under two distinct temperatures: room temperature (with an average of 25 ± 1 °C) and refrigerated storage at 4 °C. Replicate samples of each diet were collected on week 0 and at intervals of 1, 2, 4, 6, 9, 10, and 12 weeks. These samples were promptly preserved at − 80 °C for subsequent analysis.
Upon the conclusion of the sampling period, specific analyses were carried out to provide insights into the changes in antioxidant activity and lipid peroxidation in the stored aquafeeds over time. Total antioxidant capacity, expressed in terms of the molar Trolox equivalent antioxidant capacity (TEAC), was determined through the DPPH method as outlined by Brand-Williams et al. (1995) and expressed as µmol TEAC/g feed. The iron-induced thiobarbituric acid reactive substances (i-TBARS) index was calculated according to a modification of the method described by Carreras et al. (2004). Briefly, each sample was incubated in duplicate at 37 °C for 0 (TBARS) and 100 min (induced TBARS called as i-TBARS), after which time the TBARS were measured. The degree of oxidation of the experimental diets was expressed in µM of malondialdehyde (MDA)/g of diet at 0 and 100 min of incubation.
Biological assay
This short-term feeding trial aimed to assess potential variations in metabolism, immune status, and cultivable gut microbiota of juvenile European seabass (D. labrax) when fed on a diet including GP. In addition, although it was not the main objective of the assay due to the limited duration of the experimental period, some indicators of growth performance and feed utilization were also estimated.
Fish and feeding
A total of 120 juvenile seabass (D. labrax) with average body mass of 46.35 ± 0.12 g were provided by the Servicios Centrales de Investigación en Cultivos Marinos (SCI-CM, CASEM, University of Cádiz, Puerto Real, Cádiz, Spain; Facilities for Breeding, Supplying and Users of Experimental Animals; Spanish Operational Code REGA ES11028000312). Maintenance and handling of the fish followed the guidelines for experimental procedures in animal research from the Ethics and Animal Welfare Committee of the University of Cádiz, according to the Spanish (RD53/2013) and European Union (2010/63/UE) legislation. The Ethical Committee from the Autonomous Andalusian Government approved the experiments (Junta de Andalucía reference number 15/09/2021/132). Fish were distributed into six 400-L tanks (20 fish per tank) allocated in the SCI-CM experimental facilities under controlled environmental conditions of salinity (37 ‰), temperature (19 °C), and photoperiod (10L:14D). Each experimental diet was evaluated in triplicate. A daily ration was provided to visual satiation in 4 daily meals ensuring that the amount offered in each experimental unit was fully ingested. No mortality was recorded in any experimental group. The feeding trial lasted 5 weeks (February–March 2023). Water quality was continuously monitored; temperature, dissolved oxygen, and survival data were controlled daily, whereas ammonium, nitrite, and salinity levels were checked weekly.
Samplings
At the end of the short-feeding trial, overnight fasted fish (five fish per tank, fifteen per experimental condition) were randomly sampled and deeply anaesthetized with 2-phenoxyethanol in a lethal dose (1 mL/L sea water) prior to obtain samples of skin mucus, blood, and tissues. Mucus samples were taken by scraping on both dorsal-lateral sides of each fish. Blood was drawn from caudal vein with heparinized syringes and centrifuged at 3,000 g for 5 min at 4 °C to separate plasma, which was then snap-frozen in liquid nitrogen and stored at − 80 °C until used for further analysis. In addition, the hematocrit (Hc) was measured after centrifugation of blood in heparinized capillary tubes at 13,000 g for 5 min. Prior to blood centrifugation, an aliquot of 20 µL was introduced in an Eppendorf tube with 500 µL of Hb reactive 50 × and kept in dark and refrigerated to perform hemoglobin (Hb) measurements using a commercial kit adapted to 96-well microplates (Ref. 1,001,230; Spinreact, St. Esteve d’en Bas, Girona, Spain). After this, five fish were cervically sectioned to obtain biopsies of different tissues (muscle, liver, and mucus) which were snap-frozen in liquid nitrogen and stored at − 80 °C for subsequent biochemical analysis. The liver samples were previously divided into two aliquots for the analysis of metabolites and oxidative status. Samples from the distal intestine (the section between the distal end of the midgut and the anus) were taken from five individuals per tank under sterile conditions (15 fish per experimental diet). Intestinal samples were suspended and homogenized in sterile saline solution and diluted up to 10−3 maintaining sterile conditions (see more details below).
Growth performance and biometric parameters
The following growth parameters and organosomatic indices were evaluated and calculated as follows: (i) specific growth rate (SGR) = 100 × (ln final body weight − ln initial body weight)/days; (ii) weight gain (WG) = 100 × (body weight increase)/initial body weight; (iii) feed conversion ratio (FCR) = total feed intake/weight gain; (iv) condition factor (K) = (100 × body weight)/fork length3; (v) Hepatosomatic Index (HSI) = (100 × liver weight)/fish weight; and (vi) Intestine Length Index (ILI) = (100 × intestine length)/standard body length.
Functional diversity of cultivable gut microbiota
Functional biodiversity was determined by analyzing the physiological profile at the community level using Biolog EcoPlate™ microplates (Biolog, USA) according to Feigl et al. (2017) with some modifications according to the studied fish species. Dilutions of fresh samples of intestines described in “Samplings” were homogenized to obtain five pools (three individuals per pool; 15 individuals per diet), and then, 150 µL was pipetted into each well of the Biolog EcoPlate™ microplate and incubated at 30 °C for 120 h. After incubation, the optical density (OD) at 590 nm was determined using Gen5 software by Cytation (Biotek, USA). The ODi values and the number of substrates were used to calculate: (i) functional activity intensity as Average Well Color Development (AWCD = ΣODi/N);( ii) functional biodiversity as Shannon index (H′ = − Σpi ln(pi), where pi = ODi/ΣODi). The results were also expressed as Substrate Average Well Color Development (SAWCD) for each of substrate categories (Sala et al. 2010).
Biochemical and immunological parameters
Plasmatic glucose, lactate, triglycerides, and total cholesterol were analyzed using commercial kits (Refs. 1,001,200, 1,001,330, 1,001,311, and 41,021, respectively; Spinreact, St. Esteve d’en Bas, Girona, Spain), adapted to 96-well microplates. Plasma total protein concentration was determined with a BCA Protein Assay Kit (Ref. 23,225; Thermo Fisher Scientific Pierce, Waltham, MA, USA) using Bovine Serum Albumin (BSA) protein as the standard. Plasma cortisol levels were measured with the commercial Cortisol Enzyme Immunoassay Kit (Ref. K003-H1W; Arbor Assays) according to manufacturer’s indications. Prior to analyze biochemical parameters in liver, samples of frozen tissue were homogenized by ultrasonic disruption in 7.5 volumes of ice-cold 0.6 N perchloric acid, neutralized using 1 M KCO3 (Laiz-Carrión et al. 2005), and centrifuged (30 min at 3220 g and 4 °C); the supernatants were then isolated to determine tissue metabolites. Prior to centrifugation, one aliquot was taken for triglyceride analyses. Hepatic glucose, lactate, triglycerides, and total cholesterol were analyzed using commercial kits previously described. Glycogen concentration was quantified in liver homogenates using the method described by Decker and Keppler (1974), where glucose obtained after glycogen breakdown with amyloglucosidase (Ref. A7420; Sigma-Aldrich, St. Louis, MO, USA) was determined using the commercial kit described above. All assays were performed using a PowerWave™ 340 microplate spectrophotometer (Bio-Tek Instruments, Winooski, VT, USA) and the Gen5 data analysis software (Bio-Tek Instruments, Winooski, VT, USA) for Microsoft®.
The potential variations in the immunological status were assessed by measuring lysozyme and alkaline phosphatase activities in skin mucus. Lysozyme was measured using a commercial kit (Ref. E22013; Thermo Fisher Scientific, Waltham, Massachusetts, USA), adapted to 96-well microplates. One unit of activity was defined as one Relative Fluorescence Units (RFU). Alkaline phosphatase activity was determined using 4-methylumbelliferyl phosphate disodium salt (Ref. M8168; Sigma-Aldrich, St. Louis, MO, USA) as substrate following the method described by Fernley et al. (1965). One unit of activity was defined as 103 RFU.
Oxidative status
Livers were homogenized (1:10, w/v) in 100 mM phosphate-buffered saline (pH 7.4) at 4 °C using a mini handheld homogenizer (Ref. MT-13 K; Hangzhou Miu Instruments Co., Ltd., Hangzhou, China) for 1 min. Homogenates were centrifuged (12,000 g for 15 min at 4 °C), and supernatants were used to determine different enzyme activities by using different commercial kits, such as superoxide dismutase (SOD; Ref. CS0009; Sigma-Aldrich, St. Louis, MO, USA), catalase (CAT; Ref. EIACATC; Thermo Fisher Scientific, Waltham, MA, USA), and glutathione peroxidase (GPx; Ref. 703,102; Cayman Chemical, Ann Arbor, MI, USA); lipid peroxidation (TBARS) was measured according to the method of Buege and Aust (1978).
Changes in fillet quality during storage
Five fish per tank (15 fish per experimental diet) were reserved for the collection of muscle samples. These were obtained by careful dissection of portions of 4 × 1.5 cm from the longissimus dorsi. Samples were stored at 4 °C and analyzed after 0, 2, 4, and 6 days. Lipid peroxidation was determined using the method in “Oxidative status.” Samples (1 g each) were homogenized in a ratio of 1:4 (v/v) with distilled water. The mixture was then centrifuged (4,000 g, 15 min, 4 °C), and supernatants were mixed with 2-thiobarbituric acid (TBA) reagent, trichloroacetic acid (TCA), and BHT. The mixture was heated for 20 min, then centrifuged (4,000 g, 15 min, 4 °C), and the absorbance of supernatants was measured at 532 nm. The amount of TBARS was expressed as pmol of malonyl dialdehyde (MDA) per mg protein of muscle after comparing with the MDA standard.
Statistical analysis
The normality of the data was evaluated using the Shapiro–Wilk test, and homoscedasticity analysis was conducted using the Brown–Forsythe test. Statistical analysis of the data obtained for experiments on preservative effect of feed and fillet quality was carried out using a RM ANOVA. The rest of data were evaluated using Fisher’s LSD test at p < 0.05. When required, data expressed in percentage were previously arcsin transformed. All the analyses were performed using the software Statgraphics Centurion (Statgraphics Corp. CA. EE.UU.) for Windows.
Results
Variations during storage
Time variations in the total antioxidant activity and oxidation measured in the two diets during storage at either 4 °C and 25 °C are resumed in Table 2. Total antioxidant capacity (TEAC) did not show any significant effect of time or the presence of GP, and values measured at the two storing temperatures were equivalent. On the other hand, no significant effect of GP dietary inclusion on the oxidation of diets measured by TBARS was evidenced, although significant variations were measured at different moments at both temperatures. In contrast, values of i-TBARS measured in samples stored at room temperature evidenced a significant effect of the inclusion of GP.
Growth and biometric parameters
Results of growth performance and biometric indices evaluated in the fish after 5 weeks of receiving the diets are presented in Table 3. No significant differences were observed in zootechnic nor somatic parameters measured, with the exception of the significant intestine length index (ILI) enhancement observed in GP group.
Functional diversity of cultivable gut microbiota
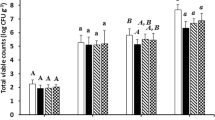
The impact of diets on the functional profiles of the cultivable aerobic gut microbiota is summarized in Figs. 1 and 2. A notable influence of GP on the microbial profile was observed, resulting in reduced biodiversity, which was characterized by a lower number of functional groups when compared to fish fed control diet (Fig. 1). Although no significant differences were observed among diets in terms of the preferred substrates used by microbial populations, the profile observed in fish fed GP exhibited some distinct characteristics compared to control diet (Fig. 2).
Variations in functional biodiversity of cultivable gut microbiota in fish fed on the different experimental diets (C: control; GP: 0.4% grape pomace). Values are presented as mean ± SD. Values not sharing a common letter differ significantly with p < 0.05. AWCD: average color development is an index of the total bioactivity; H′: Shannon index (H) is the functional biodiversity index
Preferred use of the diverse types of metabolic substrates by the aerobic cultivable intestinal microbiota of fish fed on the experimental diets (C: control; GP: 0.4% grape pomace). Expressed as relative abundance (SAWCD). Data are the mean, and values not sharing a common letter are significantly different with p < 0.05
Biochemical and immunological parameters
Biochemical and immunological parameters assessed in plasma, muscle, liver, and skin mucus of fish fed on the different experimental diets are detailed in Table 4. Significantly lower values of hematocrit were measured in fish fed on the GP diet with respect to the control. Also, significantly lower values of plasma cortisol levels were detected in the same group. In addition, fish fed on GP diet significantly increased hepatic glucose values concomitantly with diminished glycogen levels.
Oxidative status
The values for various parameters used to assess the oxidative status in fish are presented in Table 5. Fish receiving GP diet increased significantly SOD levels while decreasing GPx values.
Changes in fillet quality during storage
The progression of oxidation in stored fish fillets is illustrated in Fig. 3. At the initial time point (0 days), there were no significant differences in lipid oxidation of fillet between the fish fed both diets (C and GP). However, after 2 days of storage, fillet samples from fish fed GP diet exhibited significantly lower levels of measured reactive oxygen species (ROS) with respect to fish fed C diet. Fish fed with diet C maintained constant levels of fillet lipid oxidation during the 6 days, unlike fish fed with diet GP, where a significant decrease was observed from the second day compared to the initial time (0 days).
Muscle thiobarbituric acid reactive substance (TBARS) content in fillets of D. labrax fed on experimental diets (C: control; GP: 0.4% grape pomace) over cold storage (4 °C) at times: 0, 2, 4, and 6 days. Values are presented as mean ± SD. Comparisons of values between time within each diet are noted with small letter; comparisons of the same sampling points among different diets are noted with capital letter. Values not sharing a common letter differ significantly with p < 0.05
Discussion
The composition of aquafeeds affects their susceptibility to oxidation, being this mainly conditioned by their content of highly unsaturated lipids (Pezzuto and Park 2002; Siddhuraju and Becker 2003). The amount of peroxides increases with storage time and temperature depending on the type of raw material and oil content (Kop et al. 2019); and such effect can be prevented with the addition of natural antioxidants as previously described (see the “Introduction” section for further information). In the present study, surprisingly, the inclusion of grape pomace (GP) did not have a significant effect on total antioxidant capacity expressed as TEAC. This could be explained considering that the extra supply of antioxidant compounds provided by the addition of only 4 g GP per kg of diet could be masked by the basal antioxidant capacity due to other compounds already present in the diet (i.e., free amino acids), as previously suggested by Li et al. (2009b). According to our results, it would be interesting to determinate the optimal % of GP inclusion in aquafeed to have a positive effect on TEAC.
Regarding the course of the oxidation, while TBARS indicate the amount of lipid oxidation that has occurred based on MDA equivalents in the sample, i-TBARS provides information about their sensitivity to oxidation. Results of TBARS showed that aquafeeds maintained their oxidative status during at least 10 weeks, irrespective of storage temperature. In contrast, results of i-TBARS indicated that while preservation at low temperature did not induce significant differences in the appearance of oxidative products in both aquafeeds, this activity increased when stored at room temperature. In this case, the significantly lower values measured in GP feed strongly suggest the presence of compounds that are able to protect lipids to a greater extent than in C feed, mainly when aquafeeds are stored under running conditions. This agrees with results obtained by other authors who tested the potential preservative effect of extracts obtained from aromatic plants (Hernández et al. 2014) or from fermented mixtures with grape bagasse by fungi (Filipe et al. 2023).
Attending to the impact of GP on zootechnical parameters, our results showed no adverse effects on growth or feed conversion ratios, at least during the short-term period of the current 5-week feeding trial. This suggests that the inclusion of GP in diet was well-accepted by fish and was nutritionally equivalent to the control diet. However, a notable and surprising increase of almost 18% in intestine length index (ILI) among fish fed the GP diet when compared to those in control group (C) was observed. On the other hand, results obtained in the present study evidenced a significant reduction in the functional biodiversity of cultivable gut microbiota in fish receiving GP (Figs. 1 and 2). Although variations in the composition of microbial populations in fish fed GP can be only presumed, since they were not directly assessed using a genomic approach, it seems that they could be aligned to modifications in the intestinal absorptive capacity.
Dietary polyphenols can strongly influence diversity and functionality of gut microbiota (Catalkaya et al. 2020). Gastrointestinal tract microbiota can metabolize polyphenols, and conversely, polyphenols can modify composition, diversity, and activity of intestinal bacterial communities (Bustos et al. 2012). This intricate relationship between polyphenols and gut microbiota is influenced by several factors, including (i) concentration and structure of phenolic compounds, (ii) characteristics of bacterial strains, (iii) microbial environment, and/or (iv) composition of food matrix (Requena et al. 2010). On the other hand, the impact of polyphenols on the intestinal microbiota influences the growth and metabolism of bacteria, interfering with bacterial quorum sensing, affecting membrane permeability, and rendering bacteria more susceptible to xenobiotics (Plamada and Vodnar 2021). In this sense, Viveros et al. (2011) concluded that polyphenol-rich grape products can alter gut morphology and intestinal microbiota, leading to an increased degree of biodiversity in the intestinal bacteria of broiler chicks. In contrast, studies conducted in pigs (Choy et al. 2014; Fiesel et al. 2014) demonstrated that the administration of grape seed extract induced an ecological shift in the microbiome, reducing the amount of certain bacterial groups (such as Streptococcus and Clostridium) while increasing others (such as Lachnospiraceae, Lactobacillus, and Ruminococcus). In the case of fish, a study by Zhang et al. (2022) revealed that supplementing the diet of koi carp (Cyprinus carpio) with resveratrol increased gut bacterial richness, whereas no significant effect was observed with other polyphenols like curcumin or chlorogenic acid.
This suggests that polyphenols can modify intestinal microbiota through direct and indirect interactions, either stimulating or inhibiting bacterial growth. In this sense, it is important to consider that certain bacteria may be inhibited by polyphenols present in GP, such as catechin, epicatechin, and quercetin (Catalkaya et al. 2020), and that a reduction in microbial diversity can be associated with a decrease in the intestinal capacity for absorption. Within this context, the observed increase in intestinal length in GP-fed fish could be interpreted as a compensatory response similar to that described when the dietary amount of vegetable ingredients is increased in aquafeeds for carnivorous species (Perera et al. 2020).
Regarding the biochemical parameters, the reduction on hematocrit values in GP group could reflect a protective role of antioxidant compounds, suggesting an increase in blood O2-carrying capacity associated with a slight reduction in hemoglobin. Moreover, the decrease in hepatic glycogen content can be associated with the general antioxidant state observed in specimens fed GP diet, since a link between antioxidant capacity and hepatic glycogen levels has been reported in fish (Lygren and Hemre 2001; Li et al. 2022). In addition, the free glucose enhancement observed in the liver of GP group could be related with its self-function as a scavenger of OH-radicals (Sagone et al. 1983), contributing to the improvement of antioxidant capacity.
Furthermore, data obtained on circulating cortisol levels revealed that either of the two experimental groups was not in a stressful situation judging by their low concentrations compared to several experimental models focused on the substitution or supplementation of different ingredients in aquaculture feeds, or even related to the basal levels considered normal recorded in other species including European sea bass (Montero et al. 2015; Gorissen and Flik 2016; Schreck and Tort 2016; Samaras et al. 2018; López-Patiño et al. 2021). Although cortisol levels were low in both treatments, the slightly significant increase observed in control fish could be related to lower antioxidant capacity compared to fish fed the GP diet. This suggests a protective role of cortisol against oxidative stress, rather than generate it. In fact, this hormone could increase antioxidant defenses through genomic pathways, in addition to affecting other mechanisms that limit the production of prooxidants such as ROS (Costantini et al. 2011). The fact about the low cortisol levels detected could be also important to be considered, as cause or consequence, on the homeostatic load detected in plasma metabolites of both experimental groups.
In relation to the oxidative status, it must be considered that the control of ROS levels is provided not only through its production, but also through elimination. Endogenous antioxidant systems are known to play a key role in cellular defense against oxidative damage (Schieber and Chandel 2014), being CAT, SOD, and GPx activities the first line of defense against ROS. The activation of these enzymes occurs to detoxify and counteract the harmful effects of ROS (Halliwell 2012). Numerous studies, as this described herein, have evaluated ROS system activation in the face of challenges present in routine aquaculture practices. Souza et al. (2019) assessed the impact of including GP (at levels of 0.03%) in diets for grass carp (Ctenopharyngodon idella) challenged with the pathogen Pseudomonas aeruginosa. Results indicated that dietary supplementation prevented P. aeruginosa-induced liver damage, and this protective effect occurred through prevention on excessive ROS and NOx production, as well as via prevention of lipid damage. A similar study by Harikrishnan et al. (2021) evaluated the impact of including GP in diets (at levels of 0.02 and 0.03%) for Labeo rohita that were challenged with another potential pathogen (Flavobacterium columnaris). In this case, results indicated that both control and challenged fish treated with a 0.02% inclusion improved significantly antioxidant status (with SOD and GPx activity enhancement in fish receiving GP diet) and immune defense mechanisms. Conversely, other studies (Arslan et al. 2018; Mousavi et al. 2020) investigated the effects of grape seed ethanolic extract on intestinal antioxidant gene expression in rainbow trout (Oncorhynchus mykiss). Despite not stimulating ROS system through any challenge, their findings indicated that fish receiving the lower dose of the extract enhanced gene expression of several intestinal antioxidant genes (CAT, GPx1, and GST). Interestingly, in our study, lower levels of GPx activity were observed in fish fed diets including GP. In line with this, Zhong et al. (2020) found that in black carp (Mylopharyngodon piceus), serum SOD content significantly increased in dietary vegetable polyphenol supplement groups, while contents of GSH, GPx, and MDA significantly decreased, which can improve the antioxidative capacity of specimens. This suggests that the low GPx activity observed in fish receiving aquafeed including GP could be due to a direct phenol inhibition of enzyme synthesis or increased hydroperoxide generation that may have inhibited enzyme activity (Gaur and Mathur 2017). These studies collectively highlight the potential of grape-derived compounds, such as those from grape pomace and grape seeds, to enhance antioxidant defenses and gene expression related to oxidative stress in various aquatic species. Such findings are valuable in understanding the potential benefits of incorporating ingredients, or by-products, rich in polyphenols into aquafeeds to boost the health and resilience of these species.
In relation to oxidation of cold-preserved muscle fillet, a protective effect against oxidation was clear in samples from fish receiving the diet that included GP compared to those fed the control diet. It seems clear that some compounds present in GP were absorbed and incorporated into the muscle of fish receiving aquafeed containing GP and exerted a protective effect on tissue oxidation, thus improving the preservation of muscle samples. In a similar study, Gai et al. (2015) evaluated the effect of incorporating red grape bagasse extracts on the lifespan of minced muscles of rainbow trout (Oncorhynchus mykiss). The results confirmed that grape bagasse extracts delayed lipid oxidation and cadaverine formation in the minced muscle of trout after 6 days of refrigerated storage. In line with this, Sánchez-Alonso et al. (2007) carried out a test in which they evaluated the stability of lipids by mixing grape bagasse with minced fish muscle (Trachurus trachurus) during frozen storage for a period of 6 months. This addition to the fillet significantly delayed lipid oxidation in minced mackerel muscle during the first 3 months of storage. In this sense, both the inclusion of a natural antioxidant such as grape bagasse in experimental diets and directly in fish muscle can improve the quality and useful life of various aquaculture species.
From all the previously described experiments, it can be concluded that the inclusion of 0.4% of GP in feeds for D. labrax has the potential to prevent their oxidation in the absence of other preservatives. Also, it generates different positive effects on the oxidative and immune status of the fish, as well as on the quality of fillets during storage. Regarding the observed changes in the antioxidant enzymes, future studies should go in depth in this aspect through the analysis of their gene expression as well as of protein levels of the key transcriptional factor Nrf2.
Data availability
All other data generated during the current study are available from the author on request.
References
Arslan G, Sönmez AY, Yan T (2018) Effects of grape Vitis vinifera seed oil supplementation on growth, survival, fatty acid profiles, antioxidant contents and blood parameters in rainbow trout Oncorhynchus mykiss. Aquac Res 49(6):2256–2266
Baldissera MD, Souza CF, Descovi SN, Verdi CM, Zeppenfeld CC, da Silva AS, Santos RCV, Baldisserotto B (2019) Grape pomace flour ameliorates Pseudomonas aeruginosa-induced bioenergetic dysfunction in gills of grass carp. Aquaculture 506:359–366
Bordiga M, Coïsson JD, Locatelli M, Arlorio M, Travaglia F (2013) Pyrogallol: an alternative trapping agent in proanthocyanidins analysis. Food Anal Methods 6(1):148–156
Brand-Williams W, Cuvelier ME, Berset CLWT (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28(1):25–30
Brodie J, Williamson CJ, Smale DA, Kamenos NA, Mieszkowska N, Santos R, Hall-Spencer JM (2014) The future of the northeast Atlantic benthic flora in a high CO2 world. Ecol Evol 4(13):2787–2798
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. In Methods in Enzymology; Academic Press: Cambridge. MA, USA 52:302–310
Bustos I, Garcia-Cayuela T, Hernandez-Ledesma B, Pelaez C, Requena T, Martínez-Cuesta MC (2012) Effect of flavan-3-ols on the adhesion of potential probiotic lactobacilli to intestinal cells. J Agric Food Chem 60(36):9082–9088
Carreras I, Castellari M, Regueiro JG, Guerrero L, Esteve-Garcia E, Sarraga C (2004) Influence of enrofloxacin administration and α-tocopheryl acetate supplemented diets on oxidative stability of broiler tissues. Poult Sci 83(5):796–802
Catalkaya G, Venema K, Lucini L, Rocchetti G, Delmas D, Daglia M, Capanoglu E (2020) Interaction of dietary polyphenols and gut microbiota: microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Frontiers 1(2):109–133
Chedea VS, Palade LM, Marin DE, Pelmus RS, Habeanu M, Rotar MC, Taranu I (2018) Intestinal absorption and antioxidant activity of grape pomace polyphenols. Nutrients 10(5):588
Choy YY, Quifer-Rada P, Holstege DM, Frese SA, Calvert CC, Mills DA, Waterhouse AL (2014) Phenolic metabolites and substantial microbiome changes in pig feces by ingesting grape seed proanthocyanidins. Food Funct 5(9):2298–2308
Costa MM, Alfaia CM, Lopes PA, Pestana JM, Prates JA (2022) Grape By-Products as Feedstuff for Pig and Poultry Production. Animals 12(17):2239
Costantini D, Marasco V, Møller AP (2011) A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J Comp Physiol B 181:447–456
Dawood MA, Habotta OA, Elsabagh M, Azra MN, Van Doan H, Kari ZA, Sewilam H (2022) Fruit processing by-products in the aquafeed industry: a feasible strategy for aquaculture sustainability. Rev Aquac 14(4):1945–1965
Decker K, Keppler D (1974) Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol 71:77–106
Feigl V, Ujaczki É, Vaszita E, Molnár M (2017) Influence of red mud on soil microbial communities: application and comprehensive evaluation of the Biolog EcoPlate approach as a tool in soil microbiological studies. Sci Total Environ 595:903–911
Fernley HN, Walker PG (1965) Kinetic behaviour of calf-intestinal alkaline phosphatase with 4-methylumbelliferyl phosphate. Biochem J 97(1):95–103
Fiesel A, Gessner DK, Most E, Eder K (2014) Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet Res 10(1):1–11
Filipe D, Gonçalves M, Fernandes H, Oliva-Teles A, Peres H, Belo I, Salgado JM (2023) Shelf-life performance of fish feed supplemented with bioactive extracts from fermented olive mill and winery by-products. Foods 12(2):305
Gai F, Ortoffi M, Giancotti V, Medana C, Peiretti PG (2015) Effect of red grape pomace extract on the shelf life of refrigerated rainbow trout (Oncorhynchus mykiss) minced muscle. J Aquat Food Prod Technol 24(5):468–480
Gaur V, Mathur A (2017) Evaluation of antioxidant profile of Labeo rohita in stress condition after exposure to phenolic compounds. Int J Sci Res Publ 7(6):2250–3153
Gazeau F, Alliouane S, Bock C, Bramanti L, López Correa M, Gentile M, Ziveri P (2014) Impact of ocean acidification and warming on the Mediterranean mussel (Mytilus galloprovincialis). Front Mar Sci 1:62
Gorissen M, Flik G0 (2016) The endocrinology of the stress response in fish. In: Schreck CB, Tort L, Farrell AP, Brauner CJ (Eds) Fish Physiology: Biology of Stress in Fish. Elsevier, pp 75–111
Graça MA, Bärlocher F, Gessner MO (2005) Methods to study litter decomposition: a practical guide. Springer, Dordrecht, The Netherlands
Halliwell B (2012) Free radicals and antioxidants: updating a personal view. Nutr Rev 70(5):257–265
Hamre K, Kolås K, Sandnes K (2010) Protection of fish feed, made directly from marine raw materials, with natural antioxidants. Food Chem 119(1):270–278
Harikrishnan R, Devi G, Van Doan H, Balasundaram C, Esteban MÁ, Abdel-Tawwab M (2021) Impact of grape pomace flour (GPF) on immunity and immune-antioxidant-anti-inflammatory genes expression in Labeo rohita against Flavobacterium columnaris. Fish Shellfish Immunol 111:69–82
Hernández A, García BG, Jordán MJ, Hernández MD (2014) Natural antioxidants in extruded fish feed: protection at different storage temperatures. Anim Feed Sci Technol 195:112–119
Hossen MN, Das M, Sumi KR, Hasan MT (2013) Effect of storage time on fish feed stored at room temperature and low temperature. Prog Agric 22(1–2):115–122
Kop A, Gamsız K, Korkut AY, Sayğı H (2019) The effects of different storage temperatures and durations on peroxide values of fish feed ingredients. Turk J Agric-Food Sci Technol 7:43–49
Laiz-Carrión R, Sangiao-Alvarellos S, Guzmán JM, del Río MPM, Soengas JL, Mancera JM (2005) Growth performance of gilthead sea bream Sparus aurata in different osmotic conditions: implications for osmoregulation and energy metabolism. Aquaculture 250(3–4):849–861
Leyva-López N, Lizárraga-Velázquez CE, Hernández C, Sánchez-Gutiérrez EY (2020) Exploitation of agro-industrial waste as potential source of bioactive compounds for aquaculture. Foods 9(7):843
Li HY, Hao ZB, Wang XL, Huang L, Li JP (2009a) Antioxidant activities of extracts and fractions from Lysimachia foenum-graecum Hance. Biores Technol 100(2):970–974
Li P, Mai K, Trushenski J, Wu G (2009b) New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids 37:43–53
Li X, Han T, Zheng S, Wu G (2022) Hepatic glucose metabolism and its disorders in fish. Recent advances in animal nutrition and metabolism. 207–236
López-Patiño MA, Skrzynska AK, Naderi F, Mancera JM, Míguez JM, Martos-Sitcha JA (2021) High stocking density and food deprivation increase brain monoaminergic activity in gilthead sea bream (Sparus aurata). Animals 11(6):1503
Lourenço SC, Moldão-Martins M, Alves VD (2019) Antioxidants of natural plant origins: from sources to food industry applications. Molecules 24(22):4132
Lygren B, Hemre GI (2001) Influence of dietary carbohydrate on antioxidant enzyme activities in liver of Atlantic salmon (Salmo salar L.). Aquacult Int 9:421–427
Martínez-Antequera FP, Molina-Roque L, de las Heras V, Mancera JM, Martos-Sitcha JA, Moyano FJ (2023) Feed supplementation with winery by-products improves the physiological status of juvenile Liza aurata during a short-term feeding trial and hypoxic challenge. Aquac Rep 31:101667
Montero D, Terova G, Rimoldi S, Betancor MB, Atalah E, Torrecillas S, Caballero MJ, Zamorano MJ, Izquierdo M (2015) Modulation of the expression of components of the stress response by dietary arachidonic acid in European sea bass (Dicentrarchus labrax) larvae. Lipids 50:1029–1041
Mousavi S, Sheikhzadeh N, Tayefi-Nasrabadi H, Alizadeh-Salteh S, Khani Oushani A, Firouzamandi M, Mardani K (2020) Administration of grape (Vitis vinifera) seed extract to rainbow trout (Oncorhynchus mykiss) modulates growth performance, some biochemical parameters, and antioxidant-relevant gene expression. Fish Physiol Biochem 46:777–786
Paukert CP, Lynch AJ, Whitney JE (2016) Effects of climate change on North American inland fishes: introduction to the special issue. Fisheries 41(7):329–330
Peña E, Badillo-Zapata D, Viana MT, Correa-Reyes G (2020) Use of grape pomace in formulated feed for the rainbow trout fry, Oncorhynchus mykiss (Walbaum, 1792). J World Aquac Soc 51(2):542–550
Perera E, Sanchez-Ruiz D, Sáez MI, Galafat A, Barany A, Fernández-Castro M, Martos-Sitcha JA (2020) Low dietary inclusion of nutraceuticals from microalgae improves feed efficiency and modifies intermediary metabolisms in gilthead sea bream (Sparus aurata). Sci Rep 10(1):18676
Pezzuto JM, Park EJ (2002) Autoxidation and antioxidants. In: Swarbrick J, Boylan JC (eds) Encyclopedia of pharmaceuticals technology, (1), 2nd edn. Marcel Dekker Inc., New York, pp 97–113
Plamada D, Vodnar DC (2021) Polyphenols—gut microbiota interrelationship: a transition to a new generation of prebiotics. Nutrients 14(1):137
Pulgar R, Mandakovic D, Salgado P, Venegas L, Ortiz D, Peña-Neira Á, Wacyk J (2021) Micro-encapsulated grape pomace extract (MGPE) as a feed additive improves growth performance, antioxidant capacity, and shifts the gut microbiome of rainbow trout. Aquaculture 544:737129
Requena T, Monagas M, Pozo-Bayón MA, Martín-Álvarez PJ, Bartolomé B, Del Campo R, Moreno-Arribas MV (2010) Perspectives of the potential implications of wine polyphenols on human oral and gut microbiota. Trends Food Sci Technol 21(7):332–344
Sagone AL Jr, Greenwald J, Kraut EH, Bianchine J, Singh DALJEET (1983) Glucose: a role as a free radical scavenger in biological systems. J Lab Clin Med 101(1):97–104
Sala MM, Arrieta JM, Boras JA, Duarte CM, Vaqué D (2010) The impact of ice melting on bacterioplankton in the Arctic Ocean. Polar Biol 33(12):1683–1694
Samaras A, Espírito Santo C, Papandroulakis N, Mitrizakis N, Pavlidis M, Höglund E, Pelgrim TNM, Zethof J, Spanings FAT, Vindas MA, Ebbesson LOE, Flik G, Gorissen M (2018) Allostatic load and stress physiology in European seabass (Dicentrarchus labrax L.) and gilthead seabream (Sparus aurata L.). Front Endocrinol (lausanne) 9:1–13
Sánchez-Alonso I, Solas MT, Borderías AJ (2007) Physical study of minced fish muscle with a white-grape by-product added as an ingredient. J Food Sci 72(2):E94–E101
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24(10):R453–R462
Schreck CB, Tort L (2016) The concept of stress in fish In: Schreck CB, Tort Lluis Farrell AP, Brauner CJ (Eds) Fish physiology: biology of stress in fish. Elsevier, pp 1–34
Siddhuraju P, Becker K (2003) Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (M. Oleifera Lam.). J Agric Food Chem 51:2144–2155
Souza CF, Baldissera MD, Descovi SN, Zeppenfeld CC, Verdi CM, Santos RC, Da Silva AS, Baldisserotto B (2019) Grape pomace flour alleviates Pseudomonas aeruginosa-induced hepatic oxidative stress in grass carp by improving antioxidant defense. Microb Pathog 129:271–276
Stévant P, Rebours C, Chapman A (2017) Seaweed aquaculture in Norway: recent industrial developments and future perspectives. Aquacult Int 25(4):1373–1390
Stewart HA, Aboagye DL, Ramee SW, Allen PJ (2019) Effects of acute thermal stress on acid–base regulation, haematology, ion-osmoregulation and aerobic metabolism in channel catfish (Ictalurus punctatus). Aquac Res 50(8):2133–2141
Teixeira A, Baenas N, Dominguez-Perles R, Barros A, Rosa E, Moreno DA, Garcia-Viguera C (2014) Natural bioactive compounds from winery by-products as health promoters: a review. Int J Mol Sci 15:15638–15678
Turcu RP, Olteanu M, Criste RD, Ropota M, Panaite TD, Soica C, Dragotoiu D (2018) The effect of using grape seeds meal as natural antioxidant in broiler diets enriched in fatty acids, on meat quality. J Hyg Eng Des 25:14–20
Viveros A, Chamorro S, Pizarro M, Arija I, Centeno C, Brenes A (2011) Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult Sci 90(3):566–578
Zhang P, Zhao T, Zhou L, Han G, Shen Y, Ke C (2019) Thermal tolerance traits of the undulated surf clam Paphia undulata based on heart rate and physiological energetics. Aquaculture 498:343–350
Zhang R, Kang X, Liu L, Wang X, Li H, Zhu J, Zhu H (2022) Gut microbiota modulation by plant polyphenols in koi carp (Cyprinus carpio L.). Fronti Microbiol 13:977292
Zhong L, Hu Y, Hu Y, Li J, Tian Y, Chen J, Xiao T (2020) Effects of dietary tea polyphenols on growth, immunity and lipid metabolism of juvenile black carp Mylopharyngodon piceus. Aquac Res 51(2):569–576
Acknowledgements
The authors want to thank Servicios Centrales de Investigación en Cultivos Marinos (SCI-CM, CASEM, University of Cádiz, Puerto Real, Cádiz, Spain) for providing experimental fish and for their excellent technical assistance.
Funding
Funding for open access publishing: Universidad de Almería/CBUA. This research was developed within the Project UBAGALAC, funded by the Junta de Andalucía (Proyectos Reto 2020, P20-00923). P. Simó-Mirabet is supported by a Postdoctoral Research Fellowship (Juan de la Cierva-Formación, Reference FJCI-2021–047759-I) from the Spanish Ministry of Science and Innovation.
Author information
Authors and Affiliations
Contributions
Conceptualization, F.J.M., J.A.M-S. and J.M.M.; methodology, P. S-M., F.P.M., V.H and M.R.; validation, F.J.M., F.P.M., J.A.M-S., P.S-M., V.H., M.R., and J.M.M..; formal analysis, P.S-M., F.P.M. and J.A.M-S.; resources, F.J.M and J.M.M.; data curation, F.J.M, F.P.M., J.A.M-S. and P.S-M.; writing—original draft preparation, F.P.M.; writ-ing—review and editing, F.J.M., P.S-M., J.A.M-S. and J.M.M; project administration, F.J.M and J.M.M.; funding acquisition, J.M.M.
Corresponding author
Ethics declarations
Ethical approval
All assay procedures were conducted in the authorized experimental facilities at the Central Research Services in Marine Cultures (SCI-CM, Operational Code REGA ES11028000312). In addition, all the above-described experiments agree with the Directives of the Spanish Government (RD53/2013) and the European Union Council (2010/63/EU) for the use of animals in research. The Ethical Committee from the Autonomous Andalusian Government approved the experiments under the reference number 15/09/2021/132.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Daniel Merrifield
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martínez-Antequera, F.P., Simó-Mirabet, P., de las Heras, V. et al. Grape pomace in diets for European sea bass: influence on oxidative status, intestinal microbiota, and fillet quality. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01540-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01540-1