Abstract
Curcumin, a polyphenol, targets multiple signaling molecules and shows activity at the cellular level, supporting its various health benefits. Thereafter, the present study examines the effects of different doses of dietary curcumin on growth indices, feed efficiency, serum metabolites, redox (oxidation) status, immunity, histological assessment, and antioxidant-related genes in red tilapia (Oreochromis sp, Oreochromis aureus x O. mossambicus). A total of 1200 red tilapia with an average weight of 19.1±0.03 g were distributed in 12 cement ponds (3×3.5×1m). Each treatment was assigned three pounds (n=100 replicates). For 60 days, fish were fed baseline diets containing 0, 0.4, 0.6, and 0.8 g of curcumin/kg of diet. The treated fish showed increased body weight and SGR (specific growth rate) compared to the T0 group (P<0.05). Curcumin in the diet significantly improved weight gain, percentages, and FCR (feed conversion ratio), with a dose-dependent effect (P < 0.05). Curcumin supplementation showed no influence on feed intake, fish mortality, or survival rate (P >0.05). The curcumin-treated fish groups improved tissue structure in hepatocytes, pancreatic lumens, hepatic blood sinusoids, and intestine tissue layers, particularly the mucosal layer. The T0.8 group had reduced liver enzymes (AST and ALT) and greater total protein and albumin levels (P<0.05). The T0.6 and T0.8 groups showed significantly lower MDA levels than the other groups (P < 0.05). Curcumin groups demonstrated significantly higher antioxidant indices (CAT, GPx, and SOD) compared to the basal diet (P<0.05). Curcumin administration resulted in significant improvements in IgM and lysosome levels (P<0.05). All supplemented groups had considerably (P<0.05) higher levels of SOD, CAT, and GPx mRNA than the control group. Consequently, administering 0.6–0.8 g/kg of curcumin to red tilapia (Oreochromis sp.) diets may improve the fish’s growth, health, tissue composition, and antioxidant response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, fish culture is expanding in new directions, intensifying and diversifying. Red tilapia, a hybrid species born from the union of Oreochromis niloticus and Oreochromis mossambicus, is rapidly gaining popularity in the aquaculture industry (Edwards et al. 2019). Its multifaceted appeal stems from a combination of desirable traits. Red tilapia, valued for its rich nutritional profile and delectable taste, holds a prominent position in the Egyptian economy. Egyptian consumers increasingly favor this fish, with per capita consumption rising from 8.5 kg/year in 1996 to a remarkable 15.4 kg/year by 2008 (FAO 2012). Egypt’s dominant role in Tilapia farming is well-established, ranking first in Africa and second globally, just behind China (Kaleem and Sabi 2021). To improve productivity, several synthetic growth promoters, antibiotics, and hormones have been used to enhance the growth, feed efficiency, and health status (Saeed et al. 2019; Khafaga et al. 2019; Ashour et al. 2020; El-Saadony et al. 2021; Hendam et al. 2023). In recent years, consumers have become increasingly concerned with the quality and safety of farmed fish, demanding products free from pollutants, antibiotics, and carcinogens. This growing awareness has shifted the focus of fish rearing strategies, placing greater emphasis on food hygiene alongside traditional concerns like growth performance. Phytochemicals, a diverse group of plant-derived compounds, offer a wealth of health benefits. They are commonly found in fruits, vegetables, beans, cereals, and plant-based beverages like tea and wine (Alagawany et al. 2021). These natural treasures play a crucial role in promoting optimal health and well-being due to their powerful antioxidant, anti-microbial anti-inflammatory, and immunomodulatory effects (Chakraborty et al. 2014). The natural remedies have been used for centuries in human medicine, providing a safe, affordable, and gentler alternative to synthetic drugs, with fewer side effects. Curcumin, a biomolecule found in turmeric, has emerged as a potential dietary supplement for fish, offering a natural way to improve their health and growth (Jastaniah et al. 2024). Previously, it has been shown that curcumin supplementation can lead to improved growth performance in various fish species, including increased body weight, feed conversion efficiency, and survival rates (Yonar et al. 2019). Previous studies have proven that curcumin is an antioxidant agent that works to improve and preserve the tissue structures of aquatic animals and increase their health status (Eissa et al. 2023). Curcumin contains a wide spectrum of pharmacological activities, including antioxidant, antibacterial, antimicrobial, and immunomodulatory actions (Li et al. 2022). However, little is known about curcumin’s beneficial effects on fish species, specifically the impact of varying amounts of curcumin on red tilapia growth performance and health condition. Many authors have documented that curcumin possesses significant biological properties, such as growth promotion (Fagnon et al. 2020), hepatoprotective (Mohamed et al. 2020), antioxidant, anti-bacterial, and immunomodulatory (Alagawany et al. 2021) for fish. As a result, it is critical to develop growth-promoting, immunostimulant, and antioxidant-rich diets to increase tilapia health, production, and sustainability (Eissa et al. 2024). Although there is some previous knowledge on the enhancing effect of curcumin in improving the health status and growth of fish, the potential beneficial effects of curcumin integrated into the diets of red tilapia remain to be examined. Based on the biological activities of curcumin, we hypothesized that the addition of curcumin in the diets of red tilapia would improve growth and health status via boosting the antioxidant features and immune markers. This study sought to assess the effects of various dietary curcumin concentrations on growth performance, feed utilization, histo-morphological structure, redox status, and antioxidant-related genes in red tilapia.
Material and methods
Experimental design and diet preparation
Red tilapia was obtained from the Fish Research Center, Al-Arish University, Egypt. Water parameters were checked daily throughout the experimental period. After the acclimation period, a total of 1200 healthy red tilapia with an average body weight of 19.1±0.03 g were randomly distributed in cement ponds (3×3.5×1 m) with a natural photoperiod during the entire study period. The fish were divided into four groups with each group consist of 3 replicates, in cement ponds (100 fish/ pond). The fish were fed basal diets containing 0, 0.4, 0.6, and 0.8 g/kg diet for 60 days. These doses were selected based on the study of Amer et al. (2022). The fish were given experimental diets up to apparent satiety three times a day at 8:00, 12:00, and 16:00. The diet was supplied based on body weight, where the fish were fed 6% of their body weight during the experimental period. Every day, 20% of the water in the ponds was changed. The chemical composition of the diets used in this experiment is presented in Table 1.
Water quality measurements
During the experimental period, the water variables were assessed daily using A Hanna HI-9147 automated probe following the method of APHA (2012). The water temperature, salinity, pH, dissolved oxygen, and NH4+ were 26.87±0.12 °C, 2.03±0.03, ppt, 7.80±0.00, 5.93±0.02 mg/L and 0.34±0.00, respectively.
Growth performance assays
After 60 days of feeding trial, all fish groups were weighed to assess the growth indices and feed utilization following equations:
Where W2 is the final weight (g), W1 is the initial weight (g), and T is the trial period (day)
The W1 and W2 were initial and final body weights (g), and d expressed the days of experiment. Feed intake (g/fish): The amounts of feed expended during the investigational period/fish (g). FCR (feed conversion ratio) was calculated according to the weight gain and the feed intake (g feed/ g gain).
Histological studies
At the conclusion of the experiment, the hepatopancreas and intestine specimens were fixed in 10% neutral buffered formalin for 24 h. Tissue sections were then processed by passing them through ethyl alcohols and xylene, infiltrating them with paraplast wax (melting point 56°C), embedding them in blocks, cutting them with a rotary microtome (Leica, UK) to a thickness of 4 μm, obtaining ribbons, staining the prepared slides with hematoxylin and eosin dyes, dehydrating, clearing, and finally photographing them. Bar graphs were generated to show measurements of intestinal villi following the method outlined by Genten et al. (2009) and Bancroft and Gamble (2008).
Blood sample analysis
For serum collection, fish (n=3) from each tank were anesthetized with 100 mg/L tricaine methane sulfonate (MS-222). Blood samples were collected from the caudal artery using heparinized tubes. The tubes were kept at room temperature for 1 h to allow for serum separation, and after which the specimens were centrifuged at 3500 g for 15 min at 4 °C. The fish serum was then aliquoted and stored at −20 pending biochemical examinations.
Serum metabolites analysis
Serum metabolites of fish in treated and non-treated groups were assessed. Protein fraction including total protein (TP) and albumin (ALB) levels in serum were measured spectrophotometrically, while globulin (GLO) levels were determined by the total protein/albumin subscription. The standard protocol of Reitman and Frankel (1957) was used to assess the liver enzyme activity in fish blood serum, including ALP (alkaline phosphatase), AST (aspartate aminotransferase), and ALT (alanine aminotransferase). The antioxidant-related variables such as superoxide dismutase (SOD) and catalase (CAT) were assessed following the protocol as described by McCord and Fridovich (1969) and Aebi (1984), respectively. Malondialdehyde (MDA) was identified as a biomarker of lipid peroxidation using a thiobarbituric acid reaction according to the procedure of Ohkawa et al. (1979). The activity of glutathione peroxidase (GPx) was determined using the methyl catechol reaction (Paglia and Valentine 1967). Hemolymph lysozyme activity was assessed using the turbidimetric approach reported by Engstad et al. (1992). Immunoglobulins M (IgM) in fish serum were assessed following the method of Magnadóttir (1998). All tests were conducted using a spectrophotometer (Spectro UV-Vis Auto, UV-2602; USA), and the commercial kits were obtained from Biodiagnostic Company (Giza, Egypt) and utilized in accordance with the manufacturer’s guidelines.
Gene expression
Total RNA was isolated from 50 mg of liver tissue from each experimental group using the RNA purification kit (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. The purity of the extracted RNA was assessed by measuring the absorbance ratio at 260 nm and 280 nm using a Nanodrop Lite spectrophotometer (Thermo Scientific, USA). Subsequently, cDNA was synthesized from 1 μg of RNA using the SuperScript III First-Strand Synthesis System (Invitrogen, USA) and Oligo-dT primers, following the manufacturer’s protocol. The cDNA samples were then stored at −20°C until further use. The product size, slope, efficiency, and NCBI GenBank accession numbers of the genes analyzed are provided in Table 2. Primers of Nile tilapia (Oreochromis niloticus) were used to amplify those of red tilapia (Oreochromis sp.) according to Eissa et al. (2024). Beta-actin (β-actin) was employed as a reference gene to normalize the mRNA expression levels of these genes. To quantify the relative changes in gene transcription, the mRNA expression levels of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx)-associated genes were analyzed using qPCR (Table 2). The experiment employed the SensiFast SYBR Lo-Rox kit (Bioline, London, UK). The thermal cycle conditions for the reaction were adjusted as follows: 10 min at 95 °C, 40 cycles at 95 °C for 15 s, 30 min at 60 °C, and finally 5 min at 85 °C. The transcription levels were standardized to β-actin gene according to the 2−ΔΔCT method (Schmittgen and Livak 2008).
Statistical analysis
The statistical analyses were carried out using the SPSS statistics package version 16. One-way analysis of variance (ANOVA) was used to statistically evaluate the data. Duncan’s multiple range test (Duncan 1955) was used to determine the significant difference between means at the 95% confidence level.
Results
Effects on growth performance
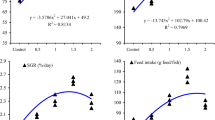
All treated fish groups had a greater final body weight compared to the T0 group (P<0.05), with the maximum increase seen with the addition of 0.8 g/kg of diet (Table 3). The weight gain and its percentages were significantly improved by the inclusion of curcumin in the diet, and this improvement was dose-dependent (P<0.05). Curcumin supplementation did not affect feed intake, fish final number, or survival rate (P>0.05). The specific growth rate (SGR) was considerably increased, while the FCR (feed conversion ratio) was substantially decreased with curcumin supplementation (P<0.05).
Histological findings
In the hepatopancreas samples, the histological structures of the hepatic cells in the hepatic cords and the endothelium surrounding the central hepatic vein were improved. Additionally, the pancreatic ducts showed improvements in pancreatic lumens, hepatocytes, hepatic cords, and blood sinusoids as shown in all curcumin-supplemented groups. There was a decrease in melano-macrophages in the T0.6 (Fig. 1C), T0.8 (Fig. 1D), and T0.4 (Fig. 1B) groups compared to the control group T0 (Fig. 1A).
A–D The hepatopancreas micrograph for four experimental groups of red tilapia; T0 (A), T0.4 (B), T0.6 (C), and T0.8 (D). The pancreatic (P), hepatic cords (HC), hepatic sinusoids (S), inflammatory melano-macrophage cells (asterisks), and hepatic Kupffer cells (red arrows) were noticed, H&E, and scale bar 50 μm
Regarding the intestinal samples, the histological structures of the columnar epithelial cells lining the villi with a mucosa layer were improved. The length and width of the villus (Fig. 2) were increased in the T0.6 group followed by the T0.8 group. There was an enhancement in the architecture general of the intestine which increased the surface area for absorption, due to the presence of curcumin in the diet of the T0.6 (Fig. 3B, C), T0.8 (Fig. 3D), and T0.4 (Fig. 3B) groups, which compared to the control group T0 (Fig. 3A).
A and B The intestinal parameters for four experimental groups of red tilapia; villus length (A) and villus width (B). The fish groups were represented as control group (T0.0 group), T1 (T0.4 group), T2 (T0.6 group), and T3 (T0.8 group). Each bar explained as means ± standard errors, n=3 for each fish group, the different subscribe letters (a, b) for significantly (P<0.05) in villus length and not significant in villus width (P>0.05)
Blood parameters
Effects on serum metabolites
AST and ALT levels were significantly decreased by the addition of dietary curcumin (P < 0.05; Table 4). The T0.08 group had the lowest values for AST and ALT compared to the other groups (P < 0.05). ALP levels were similar in the T0.04 and control groups (P > 0.05), while both the T0.6 and T0.8 groups had lower ALP values compared to the other groups (P < 0.05). Although the addition of curcumin significantly improved protein fraction (total protein, globulin, and albumin), the T0.8 group had the highest values for total protein and albumin compared to the other groups (P < 0.05).
Effects on serum oxidative stress
In terms of oxidative stress, both the T0.6 and T0.8 groups significantly decreased MDA levels compared to the other groups (P < 0.05; Table 4). MDA levels were similar in the T0.4 and control groups (P > 0.05). All antioxidant-related indices (CAT, GPx, and SOD) were higher in the curcumin groups compared to the basal diet (P < 0.05). The best results for SOD, GPx, and CAT were observed in the T0.8 group (P < 0.05).
Effects on serum immune response
In terms of the immune response, curcumin supplementation considerably improved IgM levels (P < 0.05; Table 4). The addition of 0.8 g of curcumin had the greatest effect on increasing IgM levels in fish serum (P < 0.05). Lysosome activity in fish serum was significantly enhanced by the addition of 0.6 and 0.8 g of curcumin per kg of diet (P < 0.05). The T0.4 group also increased lysosome activity compared to the control diet (P < 0.05).
Antioxidative-related genes
The current results revealed that the expression of antioxidative-related genes, namely superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), in the hepatic tissue of red tilapia was significantly modulated by dietary curcumin supplementation (P<0.05). Compared to the control group, the expression of these genes was significantly enhanced (P<0.05) with increasing curcumin levels up to 0.80 g kg−1 of diet (Fig. 4A–C). Fish groups fed with 0.80 g kg−1 curcumin-incorporated diet demonstrated the highest values (P<0.05) for SOD, CAT, and GPx compared to those in the control group, followed by fish groups fed in the T0.6 group, and then the T0.4 group, respectively (Fig. 4A–C). Overall, curcumin addition boosted the expression of antioxidative-related genes in fish hepatic tissues (P<0.05).
Discussion
With the growing concern for stress in aquatic animal aquaculture, there is an urgent need for innovative approaches to ensure both desired production and optimal animal welfare (Radwan et al. 2023). Nutraceuticals have recently been used as growth promoters in aquatic fish production, with curcumin being one of the most applied phytochemicals for this purpose. In this study, we observed that the addition of curcumin to the diets of red tilapia fish significantly improved growth performance, feed efficiency, and health status. This improvement was attributed to the ability of curcumin to enhance antioxidant capacity, boost immunity, and alter serum metabolites in red tilapia. Dietary curcumin dramatically improved the growth indices and blood antioxidative profiles. Growth metrics were considerably improved in all curcumin-supplemented groups when compared to untreated group. These findings are supported by Eissa et al. (2022), who discovered that adding curcumin to Oreochromis niloticus diets led to a considerable increase in growth performance in tilapia fish. Additionally, Mahmoud et al. (2017) discovered that varying doses of curcumin significantly enhanced growth performance and feed consumption in tilapia fish as informed in the current research.
In the present study, the tissue structure of the hepatopancreas and intestine in red tilapia was shown to be significantly improved in the curcumin-diet fish (T0.6 and T0.8 groups) compared to the control group (T0). Because of curcumin’s antioxidant and hepatoprotective attributes, liver enzymes were found in lower amounts in the fish-fed curcumin compared to the control group (T0) in the current study. The liver enzymes ALT, AST, and ALP are indicators of liver health and are raised in the presence of liver dysfunction (Javed et al. 2016). Curcumin works by dilating blood vessels, increasing the blood flow and oxygen supply to the muscle tissues (Abdel-Tawwab et al. 2022; Alagawany et al. 2021). It also reduces cell inflammation and protects tissues of red tilapia. Much research has shown that curcumin and nanocurcumin in the diet enhanced the histological impacts in aquatic animals, which is consistent with our findings (Eissa et al. 2022; Xavier et al. 2022; Eissa et al. 2023). Curcumin has been identified as an anti-inflammatory compound (Calder 2013; Eissa et al. 2022), with protective effects against inflammatory diseases as well as inhibition of the NF-kB protein complex, which is involved in the synthesis of oxidative stress and inflammatory indices (Zhao et al. 2005). Curcumin is a polyphenolic substance isolated from turmeric that may act as a growth booster for beneficial gut bacteria (like prebiotics), while inhibiting the growth of harmful bacteria (Gessner et al. 2017). There have been numerous studies on the use of curcumin as a feed supplement in the diets of various fish species (Baldissera et al. 2018; Abdel-Tawwab et al. 2022).
In this study, the immunological components in the blood (lysozyme, IgM, albumin, globulin, and total protein) were shown to be higher in the curcumin-fed fish than in the control group. Antioxidant enzymes (SOD, CAT, and GPx) were significantly greater in curcumin-supplemented fish compared to the control group. Several research have shown that curcumin stimulates the immune system in fish, which is consistent with our findings (Xia et al. 2015; Elgendy et al. 2016; Li et al. 2022; Eissa et al. 2023). Improving the antioxidant status of aquatic animals is critical for promoting growth and feed utilization, indicating the overall good health status of fish. Data on the effects of curcumin incorporation on red tilapia growth and hepatic antioxidant genes (SOD, CAT, and GPx) expression are still scarce. In this research, we found a significant improvement in the antioxidant status at the molecular level in red tilapia-fed diets with curcumin. This can be attributed to the immuno-stimulating and antioxidative properties as well as the hepatoprotective role of curcumin in fish by targeting multiple signal molecules and exerting activity at the cellular level (Gupta et al. 2013; Yonar et al. 2019). These data are consistent with the study by Jiang et al. (2016) who observed an increase in the relative mRNA expression of SOD, CAT, and GPx in crucian carp Carassius auratus after curcumin supplementation in their diets. They also mentioned that curcumin enhanced the digestion, growth, and antioxidants responses of carp. Similar findings were reported in bluegill sunfish (Elabd et al. 2016) and yellow perch (Elabd et al. 2017). Moreover, El-Abd et al. (2021) indicated that the antioxidative status of Nile tilapia (Oreochromis niloticus) was noticeably enhanced by the supplementation of dietary curcumin. Further, Xavier et al. (2022) noted that the expression of antioxidant defense genes (GPx, CAT, and SOD) of gilthead seabream post-larvae was upregulated due to the incorporation of curcumin in their diet which help to improve their health status and ameliorate their total antioxidant capacity. On the other hand, Aksakal et al. (2021) also demonstrated that incorporating “royal jelly,” a nutrient-rich bee product with antioxidative and antibiotic effects (Melliou and Chinou 2005; Nagai et al. 2006), in the diets of zebrafish modulated the mRNA expression of SOD, CAT, and GPX, genes by exhibiting a significant increase in liver, kidney, and muscles tissues among experimental fish groups compared to control. Incorporating of curcumin at a level of 0.80 g kg−1 in fish diet can enhance the antioxidant status of red tilapia and improve the expression of its antioxidative-related genes. This supplementation presents a promising approach to enhance fish health, growth, and overall well-being. The benefits of curcumin include improved disease resistance, antioxidant capacity, and potential anti-carcinogenic effects, making it a valuable tool for sustainable aquaculture practices. Further research is needed to understand the optimal dosage and potential interactions with other dietary components, but curcumin holds significant potential for shaping the future of fish farming.
Conclusion
This study assessed the potential effects of four doses (0.40, 0.60, and 0.80 g/kg diet) of curcumin on red tilapia (Oreochromis sp.). The results indicated that dietary curcumin enhanced growth performance, feed utilization, histological structure, redox state, biochemical parameters, immune response, metabolic parameters, and antioxidant-related genes. These findings suggest that curcumin could serve as a beneficial dietary supplement for red tilapia, with recommended inclusion levels between 0.60 and 0.80 g/kg diet.
Data availability
All data regarding this study are presented in the paper.
References
Abdel-Tawwab M, Eissa EH, Tawfik WA, Abd Elnabi HE, Saadony S, Bazina WK, Ahmed RA (2022) Dietary curcumin nanoparticles promoted the performance, antioxidant activity, and humoral immunity, and modulated the hepatic and intestinal histology of Nile tilapia fingerlings. Fish Physiol Biochem 48(3):585–601
Aebi H (1984) Catalase in vitro. In Methods in enzymology. Elsevier 105:121–126
Aksakal E, Ekinci D, Supuran CT (2021) Dietary inclusion of royal jelly modulates gene expression and activity of oxidative stress enzymes in zebrafish. J Enzyme Inhib Med Chem 36(1):885–894
Alagawany M, Farag MR, Abdelnour SA, Dawood MA, Elnesr SS, Dhama K (2021) Curcumin and its different forms: a review on fish nutrition. Aquaculture 532:736030
Amer SA, El-Araby DA, Tartor H, Farahat M, Goda NIA, Farag MFM et al (2022) Long-term feeding with curcumin affects the growth, antioxidant capacity, immune status, tissue histoarchitecture, immune expression of proinflammatory cytokines, and apoptosis indicators in Nile tilapia, Oreochromis niloticus. Antioxidants 11(5):937
APHA (2012) Standard methods for the examination of water and waste water, 22nd edn. American Public Health Association, American Water Works Association, Water Environment Federation
Ashour EA, El-Hack MEA, Shafi ME, Alghamdi WY, Taha AE, Swelum AA et al (2020) Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture 10(10):457
Baldissera MD, Souza CF, Zeppenfeld CC, Descovi S, Machado VS, Santos RCV, Baldisserotto B (2018) Efficacy of dietary curcumin supplementation as bactericidal for silver catfish against Streptococcus agalactiae. Microb Pathog 116:237–240
Bancroft JD, Gamble M (eds) (2008) Theory and practice of histological techniques. Elsevier Health Sciences
Calder PC (2013) Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol 75(3):645–662
Chakraborty SB, Horn P, Hancz C (2014) Application of phytochemicals as growth-promoters and endocrine modulators in fish culture. Rev Aquac 6(1):1–19
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Edwards P, Zhang W, Belton B, Little DC (2019) Misunderstandings, myths and mantras in aquaculture: its contribution to world food supplies has been systematically over reported. Mar Policy 106:103547
Eissa EH, Alaidaroos BA, Jastaniah SD, Munir MB, Shafi ME, Abd El-Aziz YM et al (2023) Dietary effects of nano curcumin on growth performances, body composition, blood parameters and histopathological alternation in red tilapia (Oreochromis sp.) Challenged with Aspergillus flavus. Fishes 8(4):208
Eissa ESH, El-Sayed AFM, Ghanem SF, Dighiesh HS, Abd Elnabi HE, Hendam BM et al (2024) Dietary mannan-oligosaccharides enhance hematological and biochemical parameters, reproductive physiology, and gene expression of hybrid red tilapia (Oreochromis niloticus x O. mossambicus). Aquaculture 581:740453
Eissa ESH, Ezzo OH, Khalil HS, Tawfik WA, El-Badawi AA, Abd Elghany NA et al (2022) The effect of dietary nanocurcumin on the growth performance, body composition, haemato-biochemical parameters and histopathological scores of the Nile tilapia (Oreochromis niloticus) challenged with Aspergillus flavus. AquacRes. 53(17):6098–6111
El-abd H, El-latif A, Shaheen A (2021) Effect of curcumin on growth performance and antioxidant stress status of Nile tilapia (Oreochromis niloticus). Iran J Fish Sci 20(5):1234–1246
Elabd H, Wang HP, Shaheen A, Yao H, Abbass A (2016) Astragalus membranaceus (AM) enhances growth performance and antioxidant stress profiles in bluegill sunfish (Lepomis macrochirus). Fish Physiol Biochem 42(3):955–966
Elabd H, Wang HP, Shaheen A, Yao H, Abbass A (2017) Antioxidative effects of some dietary supplements on yellow perch (Perca flavescens) exposed to different physical stressors. Aquac Rep 8:21–30
Elgendy MY, Hakim AS, Ibrahim B, Soliman WS, Ali SE (2016) Immunomodulatory effects of curcumin on Nile tilapia, Oreochromis niloticus and its antimicrobial properties against vibrio alginolyticus. J Fish Aquat Sci 11:206–215
El-Saadony MT, Abd El-Hack ME, Swelum AA, Al-Sultan SI, El-Ghareeb WR, Hussein EO et al (2021) Enhancing quality and safety of raw buffalo meat using the bioactive peptides of pea and red kidney bean under refrigeration conditions. Ital J Anim Sci 20(1):762–776
Engstad RE, Robertsen B, Frivold E (1992) Yeast glucan induces increase in lysozyme and complement-mediated haemolytic activity in Atlantic salmon blood. Fish Shellfish Immunol 2:287–297
Fagnon MS, Thorin C, Calvez S (2020) Meta-analysis of dietary supplementation effect of turmeric and curcumin on growth performance in fish. Rev Aquac 12(4):2268–2283
FAO (2012) Food and Agriculture Organization of the United Nations. 2012. The State of World Fisheries and Aquaculture 2012. Rome.
Genten F, Terwinghe E, Danguy A (2009) Atlas of fish histology. CRC Press
Gessner DK, Ringseis R, Eder K (2017) Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J Anim Physiol Anim Nutr 101(4):605–628
Gupta SC, Patchva S, Aggarwal BB (2013) Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J 15(1):195–218
Hendam BM, Munir MB, Eissa ME, El-Haroun E, van Doan H, Chung TH, Eissa ES (2023) Effects of water additive probiotic, Pediococcus acidilactici on growth performance, feed utilization, hematology, gene expression and disease resistance against Aspergillus flavus of Nile tilapia (Oreochromis niloticus). Anim Feed Sci Technol 303:115696
Jastaniah SD, Mansour AA, Al-Tarawni AH, El-Haroun E, Munir MB, Saghir SAM et al (2024) The effects of nano-curcumin on growth performance, feed utilization, blood biochemistry, disease resistance, and gene expression in European seabass (Dicentrarchus labrax) fingerlings. Aquac Rep 36:102034
Javed M, Ahmad I, Ahmad A, Usmani N, Ahmad M (2016) Studies on the alterations in haematological indices, micronuclei induction and pathological marker enzyme activities in Channa punctatus (spotted snakehead) perciformes, channidae exposed to thermal power plant effluent. Springerplus 5(1):761
Jiang J, Wu XY, Zhou XQ, Feng L, Liu Y, Jiang WD et al (2016) Effects of dietary curcumin supplementation on growth performance, intestinal digestive enzyme activities and antioxidant capacity of crucian carp Carassius auratus. Aquaculture. 463:174–180
Kaleem O, Sabi AS (2021) Overview of aquaculture systems in Egypt and Nigeria, prospects, potentials, and constraints. Aquac Fish 6(6):535–547
Khafaga AF, Abd El-Hack ME, Taha AE, Elnesr SS, Alagawany M (2019) The potential modulatory role of herbal additives against Cd toxicity in human, animal, and poultry: a review. Environ Sci Pollut Res 26:4588–4604
Li M, Kong Y, Wu X, Guo G, Sun L, Lai Y et al (2022) Effects of dietary curcumin on growth performance, lipopolysaccharide-induced immune responses, oxidative stress and cell apoptosis in snakehead fish (Channa argus). Aquac Rep 22:100981
Magnadóttir B (1998) Comparison of immunoglobulin (IgM) from four fish species. Icel Agric Sci 12:47–59
Mahmoud HK, Al-Sagheer AA, Reda FM, Mahgoub SA, Ayyat MS (2017) Dietary curcumin supplement influence on growth, immunity, antioxidant status, and resistance to Aeromonas hydrophila in Oreochromis niloticus. Aquaculture 475:16–23
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244(22):6049–6055
Melliou E, Chinou I (2005) Chemistry and bioactivity of royal jelly from Greece. J Agric Food Chem 53(23):8987–8992
Mohamed AA, El-Houseiny W, El-Murr AE, Ebraheim LLM, Ahmed AI, El-Hakim YMA (2020) Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: role of curcumin supplemented diet. Ecotoxicol Environ Saf 188:109890
Nagai T, Inoue R, Suzuki N, Nagashima T (2006) Antioxidant properties of enzymatic hydrolysates from royal jelly. J Med Food 9(3):363–367
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Radwan M, Darweesh KF, Ghanem SF, Abdelhadi Y, Kareem ZH, Christianus A et al (2023) Regulatory roles of Pawpaw (Carica papaya) seed extract on growth performance, sexual maturity, and health status with resistance against bacteria and parasites in Nile tilapia (Oreochromis niloticus). Aquac Int 31(5):2475–2493
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63
Saeed M, Babazadeh D, Naveed M, Alagawany M, Abd El-Hack ME, Arain MA et al (2019) In ovo delivery of various biological supplements, vaccines and drugs in poultry: current knowledge. J Sci Food Agric 99(8):3727–3739
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3(6):1101–1108
Xavier MJ, Navarro-Guillén C, Lopes A, Colen R, Teodosio R, Mendes R et al (2022) Effects of dietary curcumin in growth performance, oxidative status and gut morphometry and function of gilthead seabream postlarvae. Aquac Rep 24:101128
Xia SL, Ge XP, Liu B, Xie J, Miao LH, Ren MC, Zhou QL, Zhang WX, Jiang XJ, Chen RL, Pan LK (2015) Effects of supplemented dietary curcumin on growth and non-specific immune responses in juvenile wuchang bream (Megalobrama amblycephala). Isr J Aquacult Bamidgeh 1174(2015):1–12
Yonar ME, Mişe Yonar S, İspir Ü, Ural MŞ (2019) Effects of curcumin on haematological values, immunity, antioxidant status and resistance of rainbow trout (Oncorhynchus mykiss) against Aeromonas salmonicida subsp. achromogenes. Fish Shellfish Immunol 89:83–90
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 4:283–333
Acknowledgements
All authors extend their appreciation to their universities.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by the Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Methodology, software, E.H.E., O.F.A.; conceptualization, visualization, methodology, E.H.E., O.F.A., W.F.A., A.H.Q., P.R., and S.E.S.; software, validation, formal analysis, P.R., S.A.A.; investigation, data curation, E.H.E., M.B.M., O.F.A., W.F.A., A.H.Q., M.E.H.E; histological processing, examination, and histological data interpretation, Y.M.A.El-A; writing original draft preparation, M.B.M., S.A.A., M.E.H.E.; writing review and editing, E.H.E., M.B.M., S.A.A., M.E.H.E.; supervision, E.H.E; project administration, E.H.E.; All authors have read and approved to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
The in vivo trial used fish subjected to the general protocol standards for the Care and Use of Laboratory Animals and approved by Arish University’s Institutional Animal Care and Use Committee (no Agri - 10 / 2023).
Consent to participate
Not applicable
Consent for publication
All authors review and approve the manuscript for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling editor: Amany Abbass
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eissa, ES.H., Awlya, O.F., Abusudah, W.F. et al. Curcumin’s effects on growth indices, histological scores, blood metabolites, redox state, immunity, and antioxidant-related genes of red tilapia (Oreochromis sp.). Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01500-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01500-9