Abstract
Future growth of Atlantic salmon (Salmo salar L.) in Norway is tied to finding solutions for major ecological challenges connected to salmon lice, escapees, and nutrient emissions from sea cages. At the same time, nutrient-rich sludge from salmon production comprises a valuable resource for the cultivation of lower trophic species using an integrated multi-trophic aquaculture (IMTA) approach. This study aimed to quantify the sedimentation of aquaculture sludge under sea cages of an Atlantic salmon aquaculture site and to qualify the composition of this sludge. Additionally, the study evaluated the hypothetical use of sludge from sea-based aquaculture as a feed source for polychaetes Hediste diversicolor. Using sediment traps, sludge samples were collected under two different Atlantic salmon sea cages, at two different depths, and three different sampling dates. Subsequently, they were quantified, and their composition was assessed with regards to carbon (C), nitrogen (N), phosphorus (P), lipid, fatty acid (FA), protein, amino acid (AA), and ash content as well as elemental ratios and composition of FAs and AAs. The quantity of collected sludge was significantly different between sea cages, with a strong positive correlation between feed input and collected sludge (R2 = 0.98, p < 0.05). Sampling depth did not affect the quantity of collected sludge in the sediment traps (2215 ± 480 mg DW day−1), and no significant difference in sedimented sludge as a proportion of theoretically produced sludge (12.94 ± 2.16%) was found when comparing the different cages and sampling depths. Furthermore, the composition of collected sludge was similar at all sampling points. The overall nutritional value was lower compared to sludge from land-based aquaculture; regardless, sludge from sea-based salmon production can in theory be considered a potential feed resource to be used for the production of polychaetes H. diversicolor.
Similar content being viewed by others
Introduction
Global aquaculture production is increasingly gaining significance, given that the production has doubled in the last 20 years and quadrupled in the last 30 years (FAO 2022). Norway is the world’s largest producer of Atlantic salmon (Salmo salar L.), with a total production volume of 1.52 million tonnes in 2023 (Shahbandeh 2020; Fiskeridirektoratet 2024a). A production of this magnitude entails ecological challenges connected to salmon lice, escapees, and nutrient emissions from sea cages (Lekang et al. 2016; Olaussen 2018). These nutrient emissions are directly linked to the use of feed as up to 62% of carbon (C), 57% of nitrogen (N), and 76% of phosphorus (P) contained in the salmon feed are not utilized by the fish and thus released into the environment in the form of feed loss, feces production, excretion, and respiration (Wang et al. 2012, 2013). Based on feed consumption of 1.94 million tonnes reported in 2023 (Fiskeridirektoratet 2024b), and an approximate content of 49% C, 6% N, and 1.5% P in feed (Olsen et al. 2008), the calculated nutrient release from Norwegian salmon production in 2023 amounted to 588,000 tonnes C, 66,000 tonnes N, and 22,000 tonnes P. With a projected fourfold production by 2050, these numbers will correspondingly increase in the years to come (Olafsen et al. 2012).
Waste generated by salmon farms can be categorized into three groups: particulate organic matter (POM), dissolved organic matter (DOM), and dissolved inorganic matter (DIM) (Sæther et al. 2013). POM consists of particulate organic carbon (POC), particulate organic nitrogen (PON), and particulate organic phosphorus (POP) that originate from feed waste and feces and can serve as a nutrient source for organisms in water masses and benthic environments (Troell et al. 2009). DOM comprises small molecules and particles that are resuspended from uneaten feed and feces; it is made up of dissolved organic carbon (DOC), dissolved organic nitrogen (DON), and dissolved organic phosphorus (DOP) (Fredriksen et al. 2011). DOM represents a minor fraction of the overall waste but is composed of stable substances with a prolonged turnover time that enter the microbial food web (Sæther et al. 2013). DIM consists of nutrients released into the water by fish through excretion and respiration. These nutrients are utilized by phytoplankton in the euphotic zone and macroalgae in the littoral zone (Olsen and Olsen 2008, Husa et al. 2014).
While the ecological effects of DOM and DIM are difficult to quantify, a monitoring program, following the Norwegian standard 9410, has been developed to quantify the effects of POM originating from salmon aquaculture on benthic habitats (Standard Norge 2016; Lovdata 2023). This standard consists of different types of analyses which include the B- and C-investigations. The B-investigation is a mandated trend monitoring for marine fish farming facilities in Norway which is carried out at regular intervals on site. It assesses seabed conditions beneath aquaculture facilities, utilizing a handheld grab for a qualitative evaluation based on three main categories: the presence of fauna, chemical condition, and sensory condition. The frequency of the B-investigation varies based on prior trend monitoring results, with poor conditions prompting more frequent assessments (Fiskeridirektoratet 2023a). In 2021, 91% of the B-investigations performed at aquaculture sites scored either “good” or “very good” (BarentsWatch 2023). The C-investigation is a comprehensive soft-bottom survey designed to evaluate the impact of aquaculture facilities on the adjacent seafloor. It extends outwards from the aquaculture site and surrounding waters, measuring sediment chemistry, composition, and benthic fauna. The investigation aims to identify the origin of organic material, determining whether it comes from the aquaculture facility or other sources in the vicinity (Fiskeridirektoratet 2023b). In 2015–2016, ca. 90% of surveyed aquaculture sites scored either “good” or “high” in the C-investigation (Fiskeridirektoratet 2016). Results from C-investigations and literature show that different polychaete species are often found in high abundance beneath sea cages as they thrive in the organically enriched marine environments created by uneaten feed, fish feces, and other organic matter that accumulates in the sediment (Tomassetti and Porrello 2005; Bannister et al. 2014; Valdemarsen et al. 2015). Using an integrated multi-trophic aquaculture (IMTA) approach which follows concepts of circular bioeconomy, previous studies have given promising results for the application of polychaetes for recycling aquaculture sludge from salmon aquaculture, both from sea cages (Nederlof et al. 2019, 2020) and land-based production (Wang et al. 2019a; Anglade et al. 2023b). Hereby, the species Hediste diversicolor has been focused on in several publications since it has not only been demonstrated to efficiently utilize nutrients contained in aquaculture sludge but could also serve as a potential feed resource to be used in aquafeeds (Fidalgo e Costa et al. 2000; Bischoff et al. 2009; Wang et al. 2019b, 2019a).
To date, several studies have evaluated nutrient flows and dispersal from sea-based Atlantic salmon aquaculture; however, little knowledge exists on quantity and quality of particulate matter that sediments right below the sea cages. The presented study aimed to quantify sedimentation of aquaculture sludge directly under sea cages and assess the composition of this sludge. The potential application of this sludge from sea-based salmon aquaculture was evaluated as a hypothetical feed source for polychaetes in an IMTA context. Hereby, the hypotheses were that (1) different salmon cages at the same aquaculture site will have a different quantity of sedimented sludge, depending on feed input; (2) the sludge quantity that sediments to the seafloor will be lower than directly beneath the cages; (3) the composition of sludge will not be affected by cage location at the farm or sampling depth; and (4) the composition of sludge that sediments from sea-based salmon aquaculture could allow for it to potentially be used as a resource for cultivation of polychaetes H. diversicolor.
Material and methods
Collection of sludge
Sediment traps were used for the collection of aquaculture sludge from salmon cages and at a reference site in August 2022. Sludge collection was conducted at two salmon sea cages (hereafter referred to as “cage 1” and “cage 2”, respectively) at the aquaculture site “Lamøya” (site no. 12993, Måsøval AS), situated outside Sistranda in Trøndelag county, Norway (63°43ʹ59.0″N, 8°51ʹ17.9″E). Sludge from the aquaculture site was collected at three samplings points (n = 3), for 48 h at each sampling. As a reference site, a location approximately 3.5 km away and beyond the influence of any aquaculture sites was chosen (63°45ʹ6.3″N and 8°55ʹ15.9″E). Sample collection at the reference site ran for 7 days to accumulate sufficient material for analyses. The water depth at all sampling sites was approximately 50 m. The total cage depth of the salmon cages was 39 m. According to data obtained from Måsøval AS, at the time of sludge sampling, cage 1 held 105,372 ± 300 fish with an average weight of 896 ± 28 g, giving a total biomass of 94.42 ± 2.67 tonnes, whereas cage 2 held 75,012 ± 331 fish with an average weight of 853 ± 40 g, giving a total biomass of 63.99 ± 2.76 tonnes. The feed used during the sampling period was RAPID HP 500 50A (7 mm, EWOS AS); salmon in cage 1 were fed 642 ± 206 kg DW day−1; and those in cage 2 received 647 ± 144 kg DW day−1 in the 2 days prior to the samplings.
The sediment traps consisted of four PVC tubes, with a removable cup at the bottom of each tube. At each salmon cage, six traps were attached to the floating collar of the cage; three traps were placed right beneath the cage at 39 m depth (“top (T)”), and three traps were placed close to the seafloor at 44 m depth (“bottom (B)”). At the reference site, three sets of traps that were attached to buoys were deployed at the same depths as those at the salmon cages (39 m and 44 m). After the sampling period, the sediment traps were retrieved and transported to the feed barge. There, the traps were left for 5 min to allow for the sample material to settle on the bottom of the trap. Excess seawater was removed from the PVC tubes using a pump that was equipped with a 300-µm filter on the inlet and a 200-µm filter on the outlet to avoid any accidental pumping of sample material. Approximately 100 mL seawater containing the sampling material was left in the sampling tube. The cups holding the rest of the seawater and the sample were then detached from the sediment trap. The content of four tubes that make up one sediment trap was transferred into sampling bottles and frozen at − 20 °C. In preparation for further analyses, samples were centrifuged for a total of 15 min at 5000 rpm, using a Sorwall RC-5C Plus centrifuge for 5 min and a Heraeus Labofuge 400R (both Thermo Fisher Scientific, USA) for an additional 2 × 5 min. Subsequently, samples were freeze-dried, and the dry weight was recorded (balance: XA204DR, Mettler Toledo, Switzerland).
From each salmon cage, the samples from the three sediment traps at the same depth were pooled after drying, which gives the four different sampling groups: cage 1 — top (“C1T”), cage 1 — bottom (“C1B”), cage 2 — top (“C2T”), and cage 2 — bottom (“C2B”). From the reference site, samples from the three top traps and the three bottom traps, respectively, were mathematically pooled for quantification of sample material. For chemical analyses, samples from top and bottom traps at the reference site were pooled.
Chemical analyses
Salmon feed samples (n = 3) that were obtained from the producer, samples from the sediment traps at the salmon farm (four sampling groups; each n = 3) and the reference site (n = 3) were analyzed for their C, N, and P contents, elemental ratios, and ash content. Salmon feed (n = 2) and sludge samples from the salmon farm (four sampling groups, each n = 3) were further analyzed for total lipid content, fatty acid (FA) content and composition, amino acid (AA) content and composition, and protein content. Ash content was measured by combustion of samples in a muffle furnace at 450 °C for 5 h. C and N were examined via gas chromatography in an organic elemental analyzer (Vario EL Cube, Elementar Analysensysteme GmbH, Germany) with acetonitrile as the reference standard. P was oxidized using potassium peroxydisulfate, as outlined in the methodology by Koroleff (1976). Subsequently, quantitative analysis of phosphate content was conducted photometrically using an autoanalyzer (Flow Solution IV, O.I Analytical) following NS-EN ISO 6878. The elemental ratios of C:N, C:P, and N:P were calculated based on the respective C, N, and P contents (mg g−1 DW).
Lipids were extracted with chloroform (CHCl3) and methanol (CH3OH) (2:1 v/v) following Folch et al. (1957) and subsequently gravimetrically quantified. Following lipid extraction, FAs were hydrolyzed and esterified to fatty acid methyl esters with methanol and then analyzed by means of gas chromatography (7890B GC, Agilent Technologies, USA) equipped with helium carrier gas, a WCOT fused-silica capillary column coated with CP-wax 52CB (Holger CP7713) and a flame ionization detector (FID). AAs were analyzed according to Šližytė et al. (2017). Samples were hydrolyzed at 110 °C in 6 M HCL containing 0.4% mercaptoethanol for 24 h, followed by filtration using Whatman glass microfiber filters (grade GF/C, 47 mm). The pH was subsequently adjusted to 2.2, and the samples were then separated through a high-performance liquid chromatography (HPLC) system (Agilent Infinity 1260, Agilent Technologies, USA), which was coupled to an online post-column derivatization module (Pinnacle PCX, Pickering Laboratories, USA). Protein content was calculated by summation of water-free AAs.
Quantification of sludge collection and theoretical sludge production
The collected sample material at the two different depths at each of the salmon cages and the reference site was quantified, and the amount of sludge that sedimented at each depth at the aquaculture site was calculated by the extrapolation of the trap area to the total cage area.
where msedimented sludge (kg DW day−1) is the calculated mass of sedimented sludge for the whole cage, Acage (m2) is the cage area, Atraps (m2) is the combined area of all three sediment traps at each depth, and mcollected sludge (kg DW day−1) is the mass of collected sludge that was recorded in our experiment. The four PVC tubes that made up one sediment trap had an inner diameter of 6.50 cm each, giving a total area of 132.73 × 10−4 m2 per trap. The total collection area Atraps, consisting of three traps at each depth, was 398.20 × 10−4 m2. The total salmon cage area is defined by the circumference of the cages of 120 m, giving a cage area Acage of 1145.92 m2. The mass of collected sludge mcollected sludge was calculated by deducting the mass of sample material (kg DW day−1) collected at the reference site from the mass of sludge (kg DW day−1) collected under the salmon cages for each sediment trap to adjust for naturally occurring POM.
The theoretical production of sludge (kg DW day−1) was quantified based on Aas and Åsgård (2017), where the assumption is made that 87% of supplied salmon feed (DW) is ingested, meaning 13% remain uneaten. The ingested feed has an apparent digestibility of 70%, while 30% of ingested feed will be defecated. Based on these numbers, the total theoretical sludge production, which is made up of uneaten feed and feces, will be 39.1% of supplied feed (kg DW day−1). The sedimented proportion was calculated by dividing the mass of theoretically produced sludge (kg DW day−1) by the mass of sedimented sludge (kg DW day−1).
Statistical analysis
Statistical analyses were carried out using Sigmaplot for Windows Version 14.0 (Systat Software, Inc., USA). Minitab® 21.1 (Minitab, LLC) was used for principal component analysis (PCA) of FA and AA compositions of sludge samples collected at the salmon farm.
The normal distribution of data was assessed using Shapiro–Wilk tests, and the homogeneity of variance was examined using Brown-Forsythe tests. For the comparison of two groups, Welch’s t-test was employed. In cases where the data did not follow a normal distribution, log transformation was applied or a non-parametric test, the Mann–Whitney Rank Sum Test for two-group comparisons, was used. Results from sludge sample qualification, namely, ash, lipid, FA, protein, AA, C, N, and P contents, as well as elemental ratios, percentages of saturated FAs (SAFAs), monounsaturated FAs (MUFAs), polyunsaturated FAs (PUFAs), and percentages of essential AAs (EAA) and non-essential AAs (NEAAs) were compared at the two different depths at the same cage (C1T vs. C1B and C2T vs. C2B) and the same depth at the two different cages (C1T vs. C2T and C1B vs. C2B). Additionally, C, N, and P contents of samples at the reference site were compared with those from the salmon cages. Results from qualification of fish feed were compared with sludge samples using one-way analysis of variance (ANOVA) followed by pairwise multiple comparisons using the Holm-Sidak method. As for sample quantification, C1T vs. C2T and C1B vs. C2B were analyzed for significant differences in the percentage of sludge that was collected. Furthermore, linear regression analysis was conducted to display the relationship between salmon feed supplied and sludge collected under the salmon cages.
All statistical analyses were carried out at the 95% confidence level (p < 0.05).
Results
Quantification of sedimented sludge
Feed input and the correlated theoretical production of sludge at the two different salmon cages varied between sampling dates (Table 1). A similar mass of sludge (kg DW day−1) was collected right under the salmon cages (top) and on the seafloor (bottom) on all sampling dates at both cages. There was no significant difference in the sedimented proportion (%) when comparing different depths at the same cages and the same depths at different cages (C1T vs. C1B and C2T vs. C2B) (Welch’s t-test, p ≥ 0.05). Linear regression analysis found a strong positive correlation (R2 = 0.98, p < 0.05) between the feed supplied to the salmon and the sludge that was collected by the sediment traps (Fig. 1).
Biochemical composition of reference site samples, sludge samples from the salmon farm, and fish feed
C content (mg g−1 DW) of samples collected under the salmon cages was similar at the same depth at different cages (C1T vs. C2T and C1B vs C2B) and different depths at the same cage (Welch’s t-test, p ≥ 0.05; Table 2). Furthermore, all samples from the salmon cages had a significantly higher C content than samples collected at the reference site (one-way ANOVA, p < 0.05). The C content of fish feed was significantly higher than that of the sludge samples (one-way ANOVA, p < 0.05). N content (mg g−1 DW) of sludge samples was similar at different depths and different cages (Welch’s t-test, p ≥ 0.05), and not different from the reference samples (one-way ANOVA, p ≥ 0.05). Fish feed had a significantly higher N content than sludge samples (one-way ANOVA, p < 0.05). No difference in P content (mg g−1 DW) of samples collected under the salmon cages was found between the different sampling groups (Welch’s t-test, p ≥ 0.05). However, sludge samples had a significantly higher P content than reference samples (one-way ANOVA, p < 0.05), with sludge samples containing 13–18 times as much P as samples at the reference site. The P content of fish feed was not significantly different from that of sludge samples (one-way ANOVA, p ≥ 0.05). The C:N ratio of sludge samples was similar at different depths and different cages (Welch’s t-test, p ≥ 0.05) and significantly higher than that of samples collected at the reference site (one-way ANOVA, p < 0.05). A significantly lower C:N ratio of fish feed compared to sludge samples was found (one-way ANOVA, p < 0.05). There was no significant difference in C:P ratio of sludge samples when comparing sampling groups (Welch’s t-test, p ≥ 0.05), and all sludge samples had a significantly lower C:P ratio than reference samples (one-way ANOVA, p < 0.05). The C:P ratio of fish feed was significantly higher than that of sludge samples (one-way ANOVA, p < 0.05). The N:P ratio was similar for all sludge samples (Welch’s t-test, p ≥ 0.05), while the N:P ratio of reference samples was significantly higher (one-way ANOVA, p < 0.05). Fish feed had an N:P ratio that was significantly higher than that of sludge samples (one-way ANOVA, p < 0.05).
Ash content (mg g−1 DW) of sludge samples was similar across sampling groups and not significantly different from samples at the reference site (Welch’s t-test, p ≥ 0.05; Table 3). The ash content of fish feed was significantly lower than that of sludge samples (one-way ANOVA, p < 0.05). No difference between sludge samples at different depths or different cages was found for the content of protein and lipid (mg g−1 DW), while fish feed had a significantly higher protein and lipid content than sludge samples. The content of unidentified material (which includes carbohydrates) (mg g−1 DW) was not significantly different between sludge samples (Welch’s t-test, p ≥ 0.05) and when comparing sludge samples with fish feed (one-way ANOVA, p ≥ 0.05).
The total AA content (mg g−1 DW) of sludge samples was not significantly different at different depths or different cages (Welch’s t-test, p ≥ 0.05); fish feed, however, had a significantly higher AA content than all sludge samples (one-way ANOVA, p < 0.05; Table 3). The sludge samples at different depths or at different cages showed no significant differences for the relative content of ΣEAA (% of total AAs) (Welch’s t-test, p ≥ 0.05; Table 4). Furthermore, no difference was detected for ΣNEAA content (% of total AAs). A significantly lower proportion of EAA and a significantly higher percentage of NEAA were found when comparing fish feed with sludge samples (one-way ANOVA, p < 0.05). Leucine, phenylalanine, and lysine were the EAAs with the largest proportions in sludge samples. In fish feed, leucine, lysine, arginine, and phenylalanine were the most abundant EAAs. Of the NEAAs, glutamic acid + glutamine, alanine, serine, and aspartic acid + asparagine had the largest share of total AA, both in sludge samples and fish feed. PCA revealed that relative AA composition (% of total AAs) of sludge samples was not affected by the position at the farm (cage 1 or 2) or the water depth at which they were taken (Fig. 2). Differences in the variance of AA composition were smaller between the different sludge samples than when comparing sludge samples to fish feed. Together, PC1 and PC2 explained 67% of the variance in AA composition.
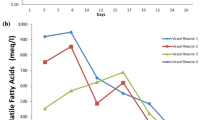
The total FA content (mg g−1 DW) of sludge samples taken at different depths and different cages was not significantly different from each other (Welch’s t-test, p ≥ 0.05; Table 3). Fish feed had a significantly higher FA content than sludge samples (one-way ANOVA, p < 0.05). No differences were detected in relative content of ΣSAFAs, ΣMUFAs, and ΣPUFAs (% of total FAs) when comparing sludge samples from different depths and different cages (Welch’s t-test, p ≥ 0.05; Table 5). The proportion of SAFAs in fish feed was significantly lower compared to sludge samples, while the proportion of PUFAs was significantly higher (one-way ANOVA, p < 0.05). The proportion of MUFAs contained in fish feed was not significantly different from that in sludge samples (one-way ANOVA, p ≥ 0.05). The FAs with the largest proportions in both sludge and fish feed were oleic acid (C18:1 n-9), linoleic acid (C18:2 n-6), palmitic acid (C16:0), and stearic acid (C18:0). There were no differences in percentages of arachidonic acid (ARA; C20:4 n-6), eicosapentaenoic acid (EPA, C20:5 n-3), and docosahexaenoic acid (DHA, C22:6 n-3) between the sludge samples from different depths and different cages. The percentage of EPA and DHA in fish feed was significantly higher than in sludge samples, while the proportion of ARA was not significantly different. PCA showed that relative FA composition (% of total FAs) of sludge samples was not affected by sampling depth. However, it was impacted by the position of the cages at the farm to some extent (Fig. 3). Differences in the variance of FA composition were smaller between the different sludge samples than when comparing sludge samples to fish feed. PC1 and PC2 combined explained 79% of the variance in the FA data set.
Discussion
Our results show that varying feed inputs at different cages at the aquaculture site “Lamøya” lead to different quantities of sludge collected in the sediment traps, with a strong correlation between feed input and collected sludge and with no difference in the proportion of sludge that sedimented, when comparing the two different cages. Contrary to our hypothesis, we found no difference between sludge quantities collected at 39 m and 44 m water depth, suggesting a limited effect of ocean current on sludge sedimentation between these sampling points. This argumentation is supported by the site report of the studied site, in which average ocean current speeds of 8.0 cm s−1 and 3.9 cm s−1 were recorded at 5 m and 15 m depths, respectively (Havbrukstjenesten 2013), showing a decrease in current speed with increasing depth.
The quantification of sludge collected in this study confirms that, overall, only a small fraction of the sludge that is theoretically produced in salmon aquaculture sediments directly beneath the sea cages. The sedimented proportion that was calculated based on sludge collected by sediment traps was 10–15% of the total theoretically produced sludge, implying that most of the sludge is spread in proximity of farms, with the dispersal being influenced by environmental conditions and ocean currents (Law and Hill 2019; Carvajalino-Fernandez et al. 2020). Simulations by Broch et al. (2017) found that aquaculture sludge from salmon cages sediments up to at least 500 m away from the farm and would hence not be registered in B-investigations which are carried out directly beneath the aquaculture site (Fiskeridirektoratet 2023a). Our findings of only low sedimentation directly beneath the sea cages are supported by the latest B- and C-investigations that were conducted at the studied site. The B-investigation classified the general condition under the farm as 1 — very good, with no sludge being registered in any of the 13 grab samples and the benthic ecosystem being evaluated as healthy, with a turnover rate sufficient enough for the seafloor not to be negatively impacted by sludge dispersed from the salmon cages (Åkerblå, 2021). Results from the most recent C-investigation gave a “moderate” score with the highest organic matter load found southeast of the farm and the dispersal following the main current direction (Havbrukstjenesten 2013; Åkerblå, 2020).
The quantity of sedimented sludge in this study may however have been affected by the presence of wild fish. Floating structures such as salmon farms have been described as fish aggregation devices (FADs), meaning wild fish aggregate around them to feed on uneaten feed (Dempster et al. 2009; Sanchez-Jerez et al. 2011). Biomass estimates of wild fish underneath sea cages range from 10 to 100 tonnes which would have a significant impact on the quantity of sludge that sediments to the sea floor as a large proportion of feed pellets would be consumed before they can sink down (Sæther et al. 2013).
Finally, some uncertainties in our quantification results due to the used method cannot be excluded. When calculating the theoretical mass of produced sludge, a proportion of 13% feed spill was used, following Aas and Åsgård (2017). However, other studies have reported considerably lower numbers of 3–5% (Reid 2007; Wang et al. 2012, 2013), suggesting a potential overestimation in the calculation of theoretically produced sludge in our study. Moreover, numbers of digestibility and defecation ratios vary. Wang and Olsen (2023) reported a defecation ratio of 18% as opposed to 30% which were used in this study based on numbers from Aas and Åsgård (2017). Additionally, the trap area was small compared to the total cage area which implicates that only small deviations in the mass of collected sludge would have a large effect on the calculated mass of sedimented sludge.
When comparing the composition of sludge from sea-based salmon cages with that of land-based production, some differences were found. The ash content of sludge samples collected under the salmon farm was similar to that of sludge from land-based smolt production reported by Wang et al. (2019a), while Anglade et al. (2023b) reported a lower ash content for post-smolt sludge from brackish water production and a substantially lower ash content of smolt sludge collected from freshwater. Samples from a saline environment often exhibit a higher ash content due to a higher concentration of dissolved minerals and salts compared to freshwater. Since our study was conducted at open sea, the ash content of our samples could have further been elevated by shell, silt, and sand particles < 200 µm which were not removed during sample filtration (Nagao et al. 2001).
Values of C and P contents of sludge samples were substantially lower than those reported by Anglade et al. (2023a); however, when considering the differences in ash content and regarding the numbers as proportion of total organic matter (TOM), they fell within a comparable range. Furthermore, the P content of sludge was similar to that of salmon feces (Wang et al. 2013). N, protein, and AA contents were lower than that of sludge from land-based salmon production (Wang et al. 2019a; Anglade et al. 2023b, 2023a) which can be explained by the changes in feed requirements for salmon and the correlated change in feces composition. In early life stages, salmon depend on a higher proportion of protein to support rapid growth and development (Nordgarden et al. 2002). As the fish age and go through transition to seawater environments, lipid requirements increase as lipid storage is crucial for maintaining buoyancy and thermal regulation (Sargent et al. 2002). Accordingly, the feed composition is adapted in farmed salmon (EWOS 2022b, 2022a) and, therefore, sludge from earlier life stages may have a higher protein, AA, and N contents but a lower lipid and FA content. Lipid content of sludge collected in our study was similar or higher to that reported in previous studies (Wang et al. 2013, Wang et al. 2019a, Anglade 2023b), while the FA content was slightly lower. However, the lipid content of fish feed analyzed in this study was significantly lower than indicated by the producer (EWOS 2022a), suggesting that lipid and FA content of both fish feed and sludge may have been undervalued in our analyses.
Lipid and protein content of sludge are moreover likely to have been affected by fish health. Prior to our study, salmon at the farm were diagnosed with pancreatic disease (PD) (BarentsWatch 2022). Among other symptoms, PD caused by SAV2 leads to reduced appetite in salmon and can decrease both protein and lipid digestibility of feed (Røsæg et al. 2019) and, as a result, change the composition of feces and sludge. PCA revealed no effect of sampling location or depth on AA composition; however, a grouping of cages in the PCA plot was observed for FA composition. This may have either been influenced by the health status of the fish and the associated feed digestibility or a different proportion of feed spill in the cages.
As described previously, a potentially large proportion of feed pellets from salmon farms may be consumed by wild fish that aggregate around the cages (Dempster et al. 2009; Uglem et al. 2014). This does not only impact the quantity of sedimented sludge but also the composition. As reported by Wang et al. (2013), feed pellets have a higher nutritional value than salmon feces as they have a higher C, N, P, and lipid content. A smaller proportion of feed in sludge would explain the lower nutrient density of sludge collected in this trial compared to sludge from land-based aquaculture (Wang et al. 2019a; Anglade et al. 2023b, 2023a). Additionally, feed spill in land-based aquaculture has been reported to be higher compared to sea-based aquaculture (Aas and Åsgård 2017) which would also lead to a higher proportion of feed to feces in sludge and hence a higher nutritional value of sludge from land-based aquaculture.
Overall, there was no significant difference in ash, C, N, P, lipid, FA, protein, or AA content of sludge samples from different depths and different cages, suggesting that the composition of sludge that sedimented from salmon cages was independent of location at the farm or sampling depth within the scope of this study. However, more research is necessary to assess how seasonal variation (Wang and Olsen 2023) and factors such as salmon size and health status affect quality of sludge that sediments from salmon cages.
Although the majority of surveyed aquaculture sites scored highly in B- and C-investigations in the past (Fiskeridirektoratet 2016; BarentsWatch 2023), an increasing emphasis is placed on the environmental effects of high organic matter loading, especially with a continuously growing industry. Alongside other challenges such as diseases and escapees (Lekang et al. 2016; Olaussen 2018), a future scenario where salmon are cultivated in sea-based closed-containment systems becomes increasingly relevant. Closed-containment systems provide a more controlled environment, reducing the risk of disease transmission between farmed and wild fish. Furthermore, they provide a physical barrier that reduces the risk of fish escapes and can help mitigate the environmental impact of salmon farming by preventing the direct release of nutrients, chemicals, and waste into surrounding waters as all intake and effluent water is filtrated (Rosten et al. 2011; Nilsen 2019). Though sludge from sea-based aquaculture may have a lower nutritional value than sludge from land-based production, cultivation in closed-containment systems would result in immense quantities of collected sludge that adequate applications are required for. Sludge from land-based aquaculture is currently used for biogas production; however, the salt content of saline sludge from sea-based production can change the bacterial community during biogas production and thus decrease biogas yield (Gebauer 2004; Mirzoyan et al. 2010). Additionally, dewatering such large volumes of sludge would result in high energy consumption (Del Campo et al. 2010; Aas and Åsgård 2017) which makes it apparent that there is a need for alternative solutions.
Following an IMTA approach, nutrient-rich aquaculture sludge from salmon aquaculture can be utilized by lower trophic organisms such as polychaetes, as demonstrated by several studies (Nederlof et al. 2019; Wang et al. 2019a; Anglade et al. 2023b). Whether the composition of the sludge collected in this study could be suited for it to hypothetically be used as a diet for polychaetes H. diversicolor depends upon different factors. In general, the nutrient composition and elemental ratios of organisms like H. diversicolor serve as a reliable indicator of their dietary nutrient needs (Sterner and Schulz 1998; Wagner et al. 2013). Due to a low N content of sludge collected in this study, the C:N ratio was substantially higher than that of sludge used for cultivation of polychaetes in other studies (Wang et al. 2019a; Anglade et al. 2023a). Furthermore, the N:P ratio of sludge was half that of sludge from land-based salmon production, which may affect efficient utilization of P. As N, AA, and protein contents were identified to promote polychaete growth (Santos et al. 2016; Wang et al. 2019b), low contents in sludge collected in our study, when comparing to previous trials, may pose a challenge and lead to reduced growth in H. diversicolor when fed with sludge from sea-based aquaculture as a sole feed source. In previous studies, the lipid content of H. diversicolor was strongly positively correlated with the lipid content of their diet. Since the lipid content of sludge collected in this study was similar or higher than that of sludge used by Wang et al. (2019a) and Anglade et al. (2023b), it can be considered appropriate for cultivation of the species. Although Anglade et al. (2023b) reported no significant effect of a different ash content between smolt and post-smolt sludge on composition and growth on polychaetes, it should be considered whether the higher ash and associated salt content in sludge from sea-based aquaculture compared to sludge from land-based smolt and post-smolt production could affect digestibility in H. diversicolor.
In summary, sludge from sea-based production collected in this study is in theory probable to be an appropriate diet with the practical application needing to be investigated in laboratory trials. An alternative to cultivation of H. diversicolor with sludge collected from sea-based salmon aquaculture would be a direct integration of naturally under salmon farm occurring polychaetes species such as Capitella sp. and Ophryotrocha craigsmithi, building on research by Kinoshita et al. (2008), Nederlof et al. (2019), and Nederlof et al. (2020). These species could be produced at high densities with a large biomass output (Tsutsumi et al. 2005), but, although initial efforts for a sea-based cultivation have been made, technical restraints persist and methods for cultivating and harvesting marine polychaetes beneath salmon farms have not been established (Jansen et al. 2019; Nederlof et al. 2019).
Conclusion
Our findings confirmed the hypothesis that the quantity of sedimented sludge will be different at different salmon cages at the same aquaculture site, with a strong positive correlation of feed input and sludge collection. The sedimented percentage of sludge as a proportion of theoretically produced sludge was similar for both cages and depths. Sampling depth did not affect the collected sludge quantity, suggesting a limited impact of ocean current underneath the sea cages at this specific location. With the exemption of FA composition, which seemed slightly affected by cage location, the composition of sedimented sludge was comparable across the salmon farm, with no significant difference of ash, C, N, P, lipid, FA, protein, or AA content between the different cages and sampling depths. The suitability of sludge collected in this study for cultivation of H. diversicolor using an IMTA approach was assessed theoretically, and although sludge collected from sea-based salmon production was found to have a lower nutritional value than that from land-based aquaculture, it could likely serve as a feed resource for production of polychaete biomass. Further confirmation in applied studies is, however, necessary.
Data availability
No datasets were generated or analysed during the current study.
References
Aas TS, Åsgård TE (2017) Estimated content of nutrients and energy in feed spill and faeces in Norwegian salmon culture. Nofima Rapportserie 19(2017):1–8
Åkerblå, (2020) C-undersøkelse NS9410:2016 for Lamøya. Frøya, Norway
Åkerblå, (2021) B-undersøkelse for lokalitet Lamøya NS9410:2016. Frøya, Norway
Anglade I, Kristensen BSB, Dahl TH, Hagemann A, Malzahn AM & Reitan KI (2023a) Upcycling of carbon, nitrogen, and phosphorus from aquaculture sludge using the polychaete Hediste diversicolor (OF Müller, 1776) (Annelida: Nereididae). Front Sustain Food Syst 7. https://doi.org/10.3389/fsufs.2023.1278586
Anglade I, Dahl TH, Kristensen BSB, Hagemann A, Malzahn AM, Reitan KI (2023b) Biochemical composition of Hediste diversicolor (OF Müller, 1776)(Annelida: Nereidae) reared on different types of aquaculture sludge. Front Mar Sci 10:899. https://doi.org/10.3389/fmars.2023.1197052
Bannister RJ, Valdemarsen T, Hansen PK, Holmer M, Ervik A (2014) Changes in benthic sediment conditions under an Atlantic salmon farm at a deep, well-flushed coastal site. Aquac Environ Interact 5:29–47. https://doi.org/10.3354/aei00092
BarentsWatch (2022) Fiskesykdom Lamøya Lokalitet 12993. https://www.barentswatch.no/fiskehelse/2022/32. Accessed 23.09.2022
BarentsWatch (2023) Emissions from fish farming plants. https://www.barentswatch.no/havbruk/miljoovervakning. Accessed 08.12.2023
Bischoff AA, Fink P, Waller U (2009) The fatty acid composition of Nereis diversicolor cultured in an integrated recirculated system: possible implications for aquaculture. Aquaculture 296:271–276. https://doi.org/10.1016/j.aquaculture.2009.09.002
Broch OJ, Daae RL, Ellingsen IH, Nepstad R, Bendiksen EÅ, Reed JL & Senneset G (2017) Spatiotemporal dispersal and deposition of fish farm wastes: a model study from central Norway. Front Mar Sci 4 https://doi.org/10.3389/fmars.2017.00199.
Carvajalino-Fernandez MA, Sævik PN, Johnsen IA, Albretsen J, Keeley NB (2020) Simulating particle organic matter dispersal beneath Atlantic salmon fish farms using different resuspension approaches. Mar Pollut Bull 161:111685. https://doi.org/10.1016/j.marpolbul.2020.111685
Del Campo LM, Ibarra P, Gutiérrez X, Takle H (2010) Utilization of sludge from recirculation aquaculture Nofima Norway. Nofima rapportserie 9(2010):1–63
Dempster T, Uglem I, Sanchez-Jerez P, Fernandez-Jover D, Bayle-Sempere J, Nilsen R, Bjørn PA (2009) Coastal salmon farms attract large and persistent aggregations of wild fish: an ecosystem effect. Mar Ecol Prog Ser 385:1–14. https://doi.org/10.3354/meps08050
EWOS (2022a) RAPID HP 500 50A. Bergen, Norway: EWOS AS
EWOS (2022b) EWOS CLEAR 4–100 Ekstrudert fullfôr til laks og ørret i ferskvann. Bergen, Norway: EWOS AS
FAO (2022) The state of world fisheries and aquaculture, 2022 Towards Blue Transformation Rome, Italy: FAO. https://doi.org/10.4060/cc0461en
Fidalgo e Costa P, Narciso L, Cancela da Fonseca L (2000) Growth, survival and fatty acid profile of Nereis diversicolor (OF Müller, 1776) fed on six different diets. Bull Mar Sci 67:337–343. https://doi.org/10.22092/ijfs.2019.118779
Fiskeridirektoratet (2016) Miljøovervåkning: C-undersøkelser - status for benthic fauna in the intermediate zone of impact (nEQR) 2015–2016. https://www.fiskeridir.no/Akvakultur/Drift-og-tilsyn/Overvaaker-miljoepaavirkningen/Cundersoekelser/_/attachment/download/71d73427-f0e7-4fb5-b7ddc1cf8cdec1ff:b378444c2a6f1f8673e82ee9c47a157d297bcb7b/Status%20for%20benthic%20fauna%20in%20the%20intermediate%20zone%20of%20impact%20%20-%20C-surveys.pdf. Accessed 08.12.2023
Fiskeridirektoratet (2023a) Miljøovervåkning: B-undersøkelser. https://www.fiskeridir.no/Akvakultur/Drift-og-tilsyn/Overvaaker-miljoepaavirkningen/B-undersoekelser. Accessed 06.12.2023
Fiskeridirektoratet (2023b) Miljøovervåkning: C-undersøkelser. https://www.fiskeridir.no/Akvakultur/Drift-og-tilsyn/Overvaaker-miljoepaavirkningen/C-undersoekelser. Accessed 06.12.2023
Fiskeridirektoratet (2024a) Uttak av slaktet fisk fordelt på art 2017–2023 (produksjonsområde). https://www.fiskeridir.no/Akvakultur/Tall-og-analyse/Biomassestatistikk/Biomassestatistikk-etter-produksjonsomraade. Accessed 22.01.2024
Fiskeridirektoratet (2024b) Forbruk av fôr fordelt på art 2017–2023 (produksjonsområde). https://www.fiskeridir.no/Akvakultur/Tall-og-analyse/Biomassestatistikk/Biomassestatistikk-etter-produksjonsomraade. Accessed 22.01.2024
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Fredriksen S, Olsen Y, Husa V, Skjoldal R, Dale T, Christie H, Sjøtun K (2011) Vurdering av eutrofieringssituasjonen i kystområder, med særlig fokus på Hardangerfjorden og Boknafjorden
Gebauer R (2004) Mesophilic anaerobic treatment of sludge from saline fish farm effluents with biogas production. Bioresour Technol 93:155–167. https://doi.org/10.1016/j.biortech.2003.10.024
Havbrukstjenesten, (2013) Strømmåling Lamøya (12993), Frøya kommune - August 2007 og desember 2012. Frøya, Norway
Husa V, Kutti T, Ervik A, Sjøtun K, Hansen PK, Aure J (2014) Regional impact from fin-fish farming in an intensive production area (Hardangerfjord, Norway). Mar Biol Res 10:241–252. https://doi.org/10.1080/17451000.2013.810754
Jansen H, Hansen P, Brennan N, Dahlgren T, Fang J, Nederlof M, Strohmeier T, Sveier H, Strand Ø (2019) Enhancing opportunistic polychaete communities under fish farms: an alternative concept for integrated aquaculture. Aquac Environ Interact 11:331–336. https://doi.org/10.3354/aei00318
Kinoshita K, Tamaki S, Yoshioka M, Srithonguthai S, Kunihiro T, Hama D, Ohwada K, Tsutsumi H (2008) Bioremediation of organically enriched sediment deposited below fish farms with artificially mass-cultured colonies of a deposit-feeding polychaete Capitella sp. I Fish Sci 74:77–87. https://doi.org/10.1111/j.1444-2906.2007.01498.x
Koroleff F (1976) Determination of nutrients. In: Grasshof E, Kremling E (eds) Methods of seawater analysis. Verlag Chemie Weinhein, New York, USA, pp 117–181
Law BA, Hill PS (2019) Spatial and temporal variation in cumulative mass eroded and organic matter percentage in surface sediments near areas of active salmon aquaculture. Aquac Environ Interact 11:305–320. https://doi.org/10.3354/aei00315
Lekang OI, Salas-Bringas C, Bostock JC (2016) Challenges and emerging technical solutions in on-growing salmon farming. Aquacult Int 24:757–766. https://doi.org/10.1007/s10499-016-9994-z
Lovdata (2023) Forskrift om drift av akvakulturanlegg (akvakulturdriftsforskriften) § 35. Miljøovervåkning. https://lovdata.no/dokument/SF/forskrift/2008-06-17-822/KAPITTEL_3#%C2%A735. Accessed 06.12.2023
Mirzoyan N, Tal Y, Gross A (2010) Anaerobic digestion of sludge from intensive recirculating aquaculture systems: review. Aquaculture 306:1–6. https://doi.org/10.1016/j.aquaculture.2010.05.028
Nagao N, Toda T, Takahashi K, Hamasaki K, Kikuchi T, Taguchi S (2001) High Ash Content in Net-Plankton Samples from Shallow Coastal Water: Possible Source of Error in Dry Weight Measurement of Zooplankton Biomass. J Oceanogr 57:105–107. https://doi.org/10.1023/a:1016050728836
Nederlof MAJ, Jansen HM, Dahlgren TG, Fang J, Meier S, Strand Ø, Sveier H, Verdegem MCJ, Smaal A (2019) Application of polychaetes in (de)coupled integrated aquaculture: production of a high-quality marine resource. Aquac Environ Interact 11:221–237. https://doi.org/10.3354/aei00309
Nederlof MAJ, Fang J, Dahlgren TG, Rastrick SPS, Smaal AC, Strand Ø, Sveier H, Verdegem MCJ, Jansen HM (2020) Application of polychaetes in (de)coupled integrated aquaculture: an approach for fish waste bioremediation. Aquac Environ Interact 12:385–399. https://doi.org/10.3354/aei00371
Nilsen A (2019) Production of Atlantic salmon (Salmo salar) in closed confinement systems (CCS): salmon lice, growth rates, mortality and fish welfare. PhD Dissertation, Norwegian University of Life Sciences
Nordgarden U, Hemre G-I, Hansen T (2002) Growth and body composition of Atlantic salmon (Salmo salar L.) parr and smolt fed diets varying in protein and lipid contents. Aquaculture 207:65–78. https://doi.org/10.1016/S0044-8486(01)00750-5
Olafsen T, Winther U, Olsen Y, Skjermo J (2012) Value created from productive oceans in 2050. Report for The Royal Norwegian Society of Sciences and Letters (DKNVS) and Norwegian Academy of Technological Sciences (NTVA). Trondheim, Norway: DKNVS/NTVA
Olaussen JO (2018) Environmental problems and regulation in the aquaculture industry. Insights from Norway Mar Pol 98:158–163. https://doi.org/10.1016/j.marpol.2018.08.005
Olsen, Y & Olsen, L (2008) Environmental impact of aquaculture on coastal planktonic ecosystems. In: Tsukamoto, K, Kawanuira, T, Takeuchii, T, Beard, TD & Kaiser, MJ (eds.) Fisheries for global welfare and environment. 5th World Fisheries Congress 2008. Yokohama, Japan: TERRAPUB, 181–196
Olsen LM, Holmer M, Olsen Y (2008) Perspectives of nutrient emission from fish aquaculture in coastal waters. Literature review with evaluated state of knowledge. FHF project. https://doi.org/10.13140/RG.2.1.1273.8006.
Reid GK (2007) Nutrient impacts of farmed Atlantic salmon (Salmo salar) on pelagic ecosystems and implications for carrying capacity. Chapter one: nutrient releases from salmon aquaculture. WWF Salmon Aquaculture Dialogue "State of Information" Reports
Røsæg MV, Rimstad E, Guttvik A, Skjelstad B, Bendiksen EÅ, Garseth ÅH (2019) Effect of pancreas disease caused by SAV 2 on protein and fat digestion in Atlantic salmon. J Fish Dis 42:97–108. https://doi.org/10.1111/jfd.12914
Rosten TW, Ulgenes Y, Henriksen K, Terjesen BF, Biering E, Winther U (2011) Oppdrett av laks og ørret i lukkede anlegg-forprosjekt. Trondheim, Norway
Sæther B-S, Uglem I, Karlsen Ø (2013) Interaksjoner mellom havbruk og ville marine organismer - En kunnskapsoppsummering. Vedlegg til prosjektrapport ProCoEx prosjektnr 900772
Sanchez-Jerez P, Fernandez-Jover D, Uglem I, Arechavala-López P, Dempster T, Bayle-Sempere JT, Valle Pérez C, Izquierdo D, Bjørn P-A, Nilsen R (2011) Coastal fish farms as fish aggregation devices (FADs). Artificial reefs in fishery management. Florida, USA: CRC Press. Taylor & Francis Group, 187–208
Santos A, Granada L, Baptista T, Anjos C, Simões T, Tecelão C, Fidalgo e Costa P, Costa JL, Pombo A (2016) Effect of three diets on the growth and fatty acid profile of the common ragworm Hediste diversicolor (O.F. Müller, 1776). Aquaculture 465:37–42. https://doi.org/10.1016/j.aquaculture.2016.08.022
Sargent, J, Tocher, D & Bell, J (2002) The lipids. In: Halver, J & Hardy, R (eds.) Fish nutrition. California, USA: Academic Press
Shahbandeh, M (2020) Distribution of salmon production worldwide in 2018, by leading country. https://www.statista.com/statistics/1182142/leading-salmon-producers-worldwide/. Accessed 09.12.2023
Šližytė R, Opheim M, Storrø I, Sterten H (2017) Simple technologies for converting rest raw materials of Atlantic Salmon (Salmo salar) into high-quality, valuable, and tasty feed ingredients. J Aquat Food Prod Technol 26:604–619. https://doi.org/10.1080/10498850.2016.1247124
Standard Norge (2016) NS 9410:2016 Miljøovervåking av bunnpåvirkning fra marine akvakulturanlegg. https://online.standard.no/ns-9410-2016. Accessed 05.12.2023
Sterner RW, Schulz KL (1998) Zooplankton nutrition: recent progress and a reality check. Aquat Ecol 32:261–279. https://doi.org/10.1023/a:1009949400573
Tomassetti P, Porrello S (2005) Polychaetes as indicators of marine fish farm organic enrichment. Aquacult Int 13:109–128. https://doi.org/10.1007/s10499-004-9026-2
Troell M, Joyce A, Chopin T, Neori A, Buschmann AH, Fang J-G (2009) Ecological engineering in aquaculture - potential for integrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture 297:1–9. https://doi.org/10.1016/j.aquaculture.2009.09.010
Tsutsumi H, Kinoshita K, Srithongouthai S, Sato A, Nagata S, Inoue A, Yoshioka M, Ohwada K, Hama D (2005) Treatment of the organically enriched sediment below the fish farm with the biological activities of artificially mass-cultured colonies of a small deposit feeding polychaete. Capitella Sp i Benthos Res 60:25–38. https://doi.org/10.5179/benthos1996.60.1_25
Uglem I, Karlsen Ø, Sanchez-Jerez P, Sæther B (2014) Impacts of wild fishes attracted to open-cage salmonid farms in Norway. Aquac Environ Interact 6:91–103. https://doi.org/10.3354/aei00112
Valdemarsen T, Hansen PK, Ervik A, Bannister RJ (2015) Impact of deep-water fish farms on benthic macrofauna communities under different hydrodynamic conditions. Mar Pollut Bull 101:776–783. https://doi.org/10.1016/j.marpolbul.2015.09.036
Wagner ND, Hillebrand H, Wacker A, Frost PC (2013) Nutritional indicators and their uses in ecology. Ecol Lett 16:535–544. https://doi.org/10.1111/ele.12067
Wang C, Olsen Y (2023) Quantifying regional feed utilization, production and nutrient waste emission of Norwegian salmon cage aquaculture. Aquac Environ Interact 15:231–249. https://doi.org/10.3354/aei00463
Wang X, Olsen LM, Reitan KI, Olsen Y (2012) Discharge of nutrient wastes from salmon farms: environmental effects, and potential for integrated multi-trophic aquaculture. Aquac Environ Interact 2:267–283. https://doi.org/10.3354/aei00044
Wang X, Andresen K, Handå A, Jensen B, Reitan KI, Olsen Y (2013) Chemical composition and release rate of waste discharge from an Atlantic salmon farm with an evaluation of IMTA feasibility. Aquac Environ Interact 4:147–162. https://doi.org/10.3354/aei00079
Wang H, Seekamp I, Malzahn AM, Hagemann A, Carvajal AK, Slizyte R, Standal IB, Handå A, Reitan KI (2019a) Growth and nutritional composition of the polychaete Hediste diversicolor (OF Müller, 1776) cultivated on waste from land-based salmon smolt aquaculture. Aquaculture 502:232–241. https://doi.org/10.1016/j.aquaculture.2018.12.047
Wang H, Hagemann A, Reitan K, Ejlertsson J, Wollan H, Handå A, Malzahn AM (2019b) Potential of the polychaete Hediste diversicolor fed on aquaculture and biogas side streams as an aquaculture food source. Aquac Environ Interact 11:551–562. https://doi.org/10.3354/aei00331
Acknowledgements
We acknowledge funding by the RCN projects POLYCHAETE (#280836) and MIND-P (#268338) and would like to thank Måsøval AS for their assistance and enabling this study.
Funding
Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital) The authors received funding from the RCN projects POLYCHAETE (#280836) and MIND-P (#268338).
Author information
Authors and Affiliations
Contributions
IA: Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. TMK: Formal analysis, Investigation, Writing – review. KIR: Conceptualization, Investigation, Project administration, Funding acquisition, Supervision, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anglade, I., Krogli, T.M. & Reitan, K.I. Sludge from sea-based Atlantic salmon (Salmo salar L.) production: quantification, composition, and potential application in integrated multi-trophic aquaculture. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01485-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01485-5