Abstract
The current trial was designed to evaluate the positive impacts of different feed additives singly or in combination on the growth performance, nutritional parameters, intestinal histology, and gene expression of some growth and fat metabolism-related genes in the liver tissue of Nile tilapia. The experimented fish were allocated into eight groups in a glass aquarium (10 fish/aquarium in triplicate) with Pediococcus acidilactici, nanozeolites, and/or medium-chain fatty acid additives. The studied treatments were control (T0), nanozeolite (T1), Pediococcus (T2), medium-chain fatty acid (T3), nanozeolite + Pediococcus (T4), nanozeolite + medium-chain fatty acid (T5), Pediococcus + medium-chain fatty acid (T6), and nanozeolite + Pediococcus + medium-chain fatty acid (T7). The results of the growth and nutritional parameters (i.e., final body weight, total weight gain, feed intake, specific growth rate, feed conversion ratio, and protein efficiency ratio) of tilapia-fed diets supplemented with Pediococcus, nanozeolites, and medium-chain fatty acids improved, but the combination of these additives was significantly more effective. Moreover, expression of growth hormone receptor 1 gene was upregulated (P ≤ 0.05) in T7 fish when compared with T0, other groups showing intermediate values. Expression of insulin-like growth factor-1 was upregulated (P ≤ 0.05) in T5, T6, and T7 fish when compared with the other groups. The expression of the fatty acid-binding protein was higher in T6 and T7 fish (P ≤ 0.05) when compared with T0, T1, and T3 fish. In conclusion, combined additives had significant effects on improving growth and regulating growth-related genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture has gained ground as a means of producing fish for human consumption over the past few decades. It has been a crucial component of the food supply in many parts of the world and has been rising dramatically as the demand for seafood rises (Shija et al. 2023). Aquaculture practices have intensified in response to the rising demand for fish protein; however, growing performance is being limited by nutritional and health issues. To overcome this problem, antibiotics have long been used to prevent, control, and treat disease, as well as growth stimulants to improve the performance of animals. However, antibiotic use is currently completely or partially restricted in the European Union and other countries due to the prevalence of antibiotic cross-resistance. So, a variety of substitutes, such as probiotics, enzymes, and organic acids, are used singly or in combination to improve the immune state, growth efficiency, and output of farm animals (Simó-Mirabet et al. 2017).
In aquaculture, feed additives are commonly used to improve growth, reduce mortality, and improve fish health. Among those additives that can be used is natural zeolite, which is a hydrated, crystalline ground cation of alkaline crystalline aluminosilicates with distinctive ion exchange properties formed by volcanic eruptions (Eya et al. 2008). It consists of three-dimensional frameworks that are bonded by shared oxygen atoms (Papaioannou et al. 2005). Zeolite is used in a variety of applications, including industry, agriculture, environmental protection, and even medicine. Zeolite has been proven to increase growth performance, conversion nutrition, mortality reduction, and toxin removal in a variety of animals when used as a feed addition (Papaioannou et al. 2005; Obradovic et al. 2006). The scientific response to this point is that nanozeolite is indeed used a lot to get rid of toxins, especially mycotoxins, but it is used in very large quantities to perform this function, as scientists used it in research (5–10-g/kg diet) (Hassaan et al. 2020), and this is a very large quantity and completely uneconomical for use as an adsorbent for toxins. As for the percentage used in other research, it is a very small percentage and is commonly used as a dietary supplement to improve growth performance and reduce stress on fish development (2-mg/kg diet) (Bashar et al. 2021). Zeolite was utilized to enhance the chemical composition and quality of fish bodies (Zenhom et al. 2020). That can improve animals’ growth performance, intestinal bacterial balance, and antioxidant capacity. It has been used in poultry for this purpose (Qu et al. 2019).
A probiotic bacterium derived from lactic acid bacteria is called Pediococcus acidilactici. It is a living, harmless bacterium that either directly or indirectly aids in protecting the host animal from harmful bacterial illnesses, and it also has a beneficial effect when ingested in sufficient proportions (Guarner and Schaafsma 1998; FAO and WHO 2001). Pediococcus acidilactici has a positive effect through a variety of mechanisms. Adhesion site competition and antimicrobial resistance, rivalry with pathogens, creation of pathogen-inhibiting chemicals, improved immune response, and resistance to disease are just a few examples (Gómez and Balcázar 2008; Kesarcodi-Watson et al. 2008; Ferguson et al. 2010). It also aids in the digestion of nutrients in the intestine, as it creates a number of organic acids and metabolized products such as volatile fatty acids and lactic acid (Gibson 1999).
Taurine and medium-chain fatty acids are frequently used in aquafeeds as feed and metabolic enhancers (Tran et al. 2018; Abdel-Tawwab 2016). By fermenting carbohydrates with anaerobic bacteria in the organism’s digestive system, medium-chain fatty acids are produced (Bedford and Gong 2018). The medium-chain fatty acids generated primarily aid the beneficial microbiota in digesting and absorbing nutrients through the villi of the intestine, while is indirectly reducing the activity of harmful bacteria (Hoseinifar et al. 2017). As a result, medium-chain fatty acids can significantly improve growth performance, feed efficiency, antioxidant activity, and immunological responses (Zhou et al. 2019; Mirghaed et al. 2019; Dawood et al. 2020). Diets supplemented with Pediococcus, nanozeolites, and medium fatty acids are different in their modes of action, but we hypothesis that the combination of these additives may have a better effect. However, Abd Elshafy et al. (2023) reported the positive effects of nanozeolite, Pediococcus, and medium-chain fatty acids as feed additives on Nile tilapia fish without affecting their health but did not study their effects on the growth performance and gene expression of the growth-related genes. Consequently, the purpose of this study was to ascertain the impact of nanozeolite in comparison with Pediococcus acidilactici and/or medium-chain fatty acids on improving growth performance and the expression of growth-related genes in Nile tilapia fish.
Materials and methods
Sources of feed additives
Nanozeolite and Pediococcus acidilactici (P. acidilactici) and medium-chain fatty acids were obtained from Nano Tech Egypt for Photo-Electronics, City of 6 October, Al Giza, Egypt and EGAVET, Giza, Egypt, respectively. Pediococcus acidilactici was obtained as a commercial product (Bactocell®); each 1 g contains 1 × 109 colony-forming unit (CFU).
Experimental design
In the current experiment, 240 mono sex male Nile tilapia (Oreochromis niloticus) fish (7 ± 1 g/fish) were obtained from a local farm and used to evaluate the effect of nanozeolite, probiotics, and/or medium-chain fatty acids on growth performance, histopathology of the intestinal tissue, some gene expression related to growth in the liver tissue, and nutritional parameters of Nile tilapia. Fish were fed on a formulated control diet (Table 1) for 4 weeks as an acclimatizing period. All management was performed according to Shimeno et al. (1993). Glass aquariums measuring 80 × 40 × 40 cm, each containing 10 fish, were used in triplicate. Following was the design of the experimental feeding:
-
1-
T0: control group fed the basal diet without feed additives.
-
2-
T1: nanozeolite group at a rate of 2-mg/kg diet (adapted according to Bashar et al. 2021).
-
3-
T2: Pediococcus group at a rate of 2-g/kg diet (Fadl et al. 2013).
-
4-
T3: medium-chain fatty acid group used according to the produced company recommendation at a rate of 3.5-g/kg diet.
-
5-
T4: nanozeolite + Pediococcus group.
-
6-
T5: nanozeolite + medium-chain fatty acid group.
-
7-
T6: Pediococcus + medium-chain fatty acid group.
-
8-
T7: nanozeolite + Pediococcus + medium-chain fatty acid group.
Fish were fed 6, 5, and 4% of their body weight daily for 1–2, 3–6, and 7–12 weeks, respectively, (dry matter feed/fish). Fish were given identical portions of feed twice daily, at 9:00 a.m. and 13:00 p.m. During the experimental period, the aquarium water was partially changed with dechlorinated water every day, where oxygen, salinity, pH, and temperature were adjusted at 5.8–6.1 ppm, 1.1–2‰, 7.4–8.1, and 24 °C, respectively. Animal ethics for this project were obtained from the Faculty of Agriculture, Alexandria University, Egypt (Approval No. MDPHD 0201707). The experiment was carried out in the Faculty of Agriculture, Alexandria University (Egypt).
Growth performance and feed
For 12 weeks, all fish in the different experimental groups and diets were weighed individually at the beginning and every 2 weeks in order to determine the weight gain and amount of utilized feed. The body weight gain (BWG) was calculated for each 2-week period. The entire BWG was then determined. In a similar manner, feed intake (FI), average daily gain (ADG), feed conversion ratio (FCR), specific growth rate (SGR), and protein efficiency ratio (PER) were determined for each period using equations in the studied parameters. Additionally, the total FI, ADG, FCR, SGR, and PER were computed. Meanwhile, the chemical composition of feed (Table 1) was measured in accordance with AOAC (2000) at 12 weeks.
Studied parameters
Body weight (BW): Weighing of the fish took place at the start of the experiment (W0) and then every 2 weeks for the next 12 weeks. The live body weight change was taken as measure for growth.
Weight gain (G) = (Final body weight − Initial body weight).
Average daily gain (ADG) was calculated by the following equation:
where W0 and W1 are the initial and final body weight per gram and T is the number of days in the feeding experimental period according to Castell and Tiews (1980).
Feed intake (FI): The diets were provided regularly every day at 9:00 a.m. and 13:00 p.m. and the 2-week feed intake was calculated by difference between the weight of offered feed and remained part, then divided by the number of the fish in each aquarium, and totalized to be per 2 weeks.
Feed conversion ratio (FCR) was calculated by dividing total feed intake per aquarium by the total body weight gain per the same aquarium (Lambert et al. 1936).
Specific growth rate (SGR) = SGR = (lnWf − lnWi × 100)/t (lnWf = the natural logarithm of the final weight, lnWi = the natural logarithm of the initial weight, and t = time (days) between lnWf and lnWi).
Protein intake = Feed intake × Protein% of the feed used/100.
Protein efficiency ratio (PER) = Weight gain/Protein intake was calculated according to McDonald et al. (1987).
Gut index (GI%): Liver and gut that were dissected out of five fish per aquarium were used, weighted individually. Gut index can be calculated (as g 100/ g body weight) (Igejongbo and Esther 2022) as follows:
Histopathology and intestinal morphometry
Five euthanized fish were collected from each replicate (15 fish/group) at 12 weeks to collect intestines from freshly dead fish. The intestinal samples were fixed in 10% neutral buffered formalin for at least 24 h. The traditional paraffin embedding method was applied to the fixed tissue samples. Subsequently, 5-µm-thick paraffin blocks were sectioned and stained for histological analysis using hematoxylin and eosin (H&E) (Bancroft and Gamble 2008). Also, intestinal morphometry, including villus length (from the tip of villus-to-villus crypt junction), villus width (width of each villus was measured in the median region, and spans the two epithelial monolayers plus the lamina propria), and goblet cell number, were determined. From villi of the same size, goblet cells were counted after being stained with PAS.
Gene expression
For the total RNA extraction, liver specimens were collected on liquid nitrogen and then kept at − 80 °C. Liver samples were first homogenized in phosphate-buffered saline (PBS); then, the extraction protocol was done using TRIzol according to the manufacturer’s instructions. The cDNA was synthesized complementary to the mRNA using reverse transcriptase, according to the illustration. Procedures (Thermo Scientific-RevertAid–First-Strand cDNA Synthesis Kit).
The hepatic relative expression of some growth-related genes, such as growth hormone receptor 1 (GHR1) and insulin-like growth factor-1 (IGF1), and fat metabolism-related genes, fat metabolism (FA) transporter genes (fatty acid-binding protein (FABP) and fatty acid translocase (CD36)), was done using gene-specific primers (Con et al. 2019; El-Naggar et al. 2021; El-Kassas et al. 2020; Zhang et al. 2020). The gene amplification was done in Piko-real PCR using a Sybr PCR mix. The reaction mix included 20 μl total volume, 10 μl of SensiFast™ SYBR master mix, 2 μl of cDNA, and 0.5 μM of each primer. The amplification conditions were as follows: pre-denaturation at 95 °C for 10 m, then 40 cycles of 95 °C for 10 s, and annealing at 60 °C for 30 s. The results were normalized against EF-1α Ct values as a housekeeping gene and the control group. The different gene relative expression was shown as fold changes, calculated by the 2−△△Ct according to Livak and Schmittgen (2001).
Statistical analysis
Experimental design
This experiment was carried out through a factorial design including three main factors (nanozeolite, probiotic, and fatty acid) with two levels of each factor (present or absent), all possible two-way interactions, and a three-way interaction as a total of eight experimental groups (23). Each group contained 30 fish that were divided into three equal replicates, with ten fish per replicate, summing 240 fish in the whole experiment (8 groups × 3 replicates × 10 fish).
Model of analysis
where
- Yij:
-
is the observation,
- Ti:
-
is the treatment (8 levels), and
- eij:
-
is the experimental error.
Statistical analysis has been carried out using jamovi (ver 2.3.9, 2022).
Results
Body weight
The impact of nanozeolite, Pediococcus, and/or medium-chain fatty acid supplementation in the diet of O. niloticus on the BW at different periods are presented in Table 2. BW at 2 weeks in the T5 group was greatly increased against the T0, T1, and T3 groups. Dietary supplementation of Pediococcus and nanozeolite (T4) improved the BW of O. niloticus against the T0 and T1 groups. In the meantime, the BW at 4 weeks revealed a distinction between the T5, T6, and T7 groups and the T0 group that was significant (P ≤ 0.05). Regarding the results of the BW at 6 and 8 weeks, there was no noteworthy difference between the various groups. Moreover, there was a valuable difference between the T4 and T1 groups at 10 weeks. However, the results of the BW at 12 weeks (final body weight), compared to the T2 and T5 groups, the T4 group showed a substantial increase. As opposed to that, the T5 group and the T7 group were greatly different from the T0 group.
Weight gain
The impact of nanozeolite, Pediococcus, and/or medium-chain fatty acid supplementation in the diet of O. niloticus on the G at different periods are presented in Table 3. The results of the G at 2 weeks in the T4 and T5 groups were greatly increased against the T0, T1, and T2 groups. Meanwhile, the G at 4 weeks showed that the T5, T6, and T7 groups greatly improved against the T0 group. As opposed to that, there was an insignificant (P ≤ 0.05) difference between the T1, T2, T3, and T4 groups. Regarding the results of the G at 6 and 8 weeks, there was an insignificant (P ≤ 0.05) variance between the different groups. There was a crucial increase in the G of the T4 group against the T1 group at 10 weeks. However, the results of the G at 12 weeks showed a crucial increase in the G of the T4 group against the G of the T2 and T6 groups. On the other side, the result of the T7 group significantly (P ≤ 0.05) increased against the T0 group.
Average daily gain
The impact of nanozeolite, Pediococcus, and/or medium-chain fatty acid supplementation in the diet of O. niloticus on the ADG at different periods are presented in Table 3. The results of the ADG at 2 weeks in the T4 and T5 were significantly (P ≤ 0.05) increased against the T0, T1, and T2. On the other hand, the ADG of the T5, T6, and T7 groups greatly improved against the T0 at 4 weeks. At 6 and 8 weeks, there was an insignificant difference between the different groups.
Meanwhile, T4 showed improvement against T1 at 10 weeks. However, ADG results at 12 weeks showed a significant increase in the T4 against the T2 and T6. As opposed to that, the T7 greatly improved ADG against the T0.
Feed conversion ratio
The impact of nanozeolite, Pediococcus, and/or medium-chain fatty acid supplementation in the diet of O. niloticus on the FCR at different periods are presented in Table 4. The results of the FCR at 2 weeks showed an insignificant difference between the various groups. Meanwhile, the FCR at 4 weeks showed significant improvement in the T5, T6, and T7 against the FCR of the T0. Regarding the results of the FCR at 6, 8, and 10 weeks, there was an insignificant difference between various groups. Meanwhile, the results of the FCR at 12 weeks showed a significant improvement in the T4 against the T0, T3, and T6. As opposed to that, T1, T2, T5, and T7 did not show significant differences.
Specific growth rate
The impact of nanozeolite, Pediococcus, and/or medium-chain fatty acid supplementation in the diet of O. niloticus on the SGR at different periods are presented in Table 4. The results of the SGR at 2 weeks in the T4 and T5 were significantly increased against the T0, T1, and T2. The SGR at 4 weeks showed that the T5, T6, and T7 significantly improved against the T0. Regarding the results of the SGR at 6 and 8 weeks, there was an insignificant difference between the different groups. Meanwhile, T4 showed a significant (P ≤ 0.05) improvement against T1 at 10 weeks. Regarding the results of the SGR at 12 weeks, T4 showed a significant (P ≤ 0.05) increase against T2 and T6. On the other hand, the SGR of the T5 and T7 significantly increased against the SGR of the T0. Moreover, there was an insignificant difference between the other treatments at 12 weeks.
Feed intake
The impact of nanozeolite, Pediococcus, and/or medium-chain fatty acid supplementation in the diet of O. niloticus on the FI at different periods are presented in Table 5. The results of the FI at 2 and 4 weeks showed no considerable variation between the various groups. Meanwhile, the FI at 6 weeks showed that the T5, T6, and T7 significantly improved against the T0 group. The FI at 8 weeks showed that the T7 greatly improved against the T0. As opposed to that, there was an insignificant difference between the other treatments at 8 weeks. There was an insignificant variation between the different treatments at 10 weeks. The results of the FI at 12 weeks showed a significant (P ≤ 0.05) increase in the T3, T4, T5, and T7 against the T0. As opposed to that, there was an insignificant (P ≤ 0.05) difference between the T1, T2, and T6 groups.
Protein efficiency ratio
The impact of nanozeolite, Pediococcus, and/or medium-chain fatty acid supplementation in the diet of O. niloticus on the PER at different periods are shown in Table 5. The result of the PER at 2 weeks in the T5 was greatly improved against the results of the T0, T1, and T2. As opposed to that, there was an insignificant difference between the T3, T6, and T7 groups. Meanwhile, the PER at 4 weeks showed that the T5, T6, and T7 greatly increased against the T0. Moreover, T1, T2, T3, and T4 showed insignificant variation. Regarding the results of the PER at 6, 8, and 10 weeks, there was no difference between groups that was statistically significant. Regarding the results of PER at 12 weeks, T4 greatly improved against the T2, T3, and T6. As opposed to that, there was an insignificant variation between the T1, T5, and T7.
Gut index
The impact of nanozeolite, Pediococcus, and/or medium-chain fatty acid supplementation in the diet of O. niloticus on the gut index were noticed in Table 6 at the experimental end. The gut index showed a significant variation between the T1 and T5. Moreover, there was an insignificant (P ≤ 0.05) variation between the other treatments.
Histopathology and intestinal morphometry
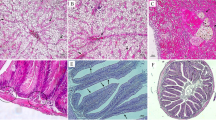
The histological examination of the intestinal tissue of the O. niloticus is illustrated in Fig. 1. The intestines of the T0, T1, T2, T3, T4, T5, T6, and T7 showed normal intestinal villi.
Representative photomicrograph of the intestine of Nile tilapia fish experimental groups after 90 days of the experiment stained with H&E (X200, scale bar = 100 µ), where A, B, C, D, E, F, G, and H are for the control group, nanogroup, probiotic group, medium-chain fatty acid group, nanopulse probiotic group, medium-chain fatty acid pulse probiotic group, nanopulse medium-chain fatty acid group, and nanopulse medium-chain fatty acid pulse probiotic group, respectively. All pictures show normal histo-architecture with characteristic intestinal villi (V)
Moreover, significant interactive effects of dietary nanozeolite, Pediococcus, and/or medium-chain fatty acid supplementation were noticed in villus length and width and goblet cells of Nile tilapia (Table 6). There was an improvement in the villus length of the T7 against the T4. Moreover, the villus length showed a significant difference in the T1, T2, and T3 against the T0. Meanwhile, the villus width showed a significant variation between the T0, T1, and T6 against the T4. As opposed to that, there was an insignificant difference between the different groups. Goblet cells showed a significant difference between the T7 group against the T4, T5, and T6. Dietary supplementation with the T2 and T3 groups showed improvement in the goblet cells of O. niloticus when compared with the T0 group.
Gene expression
Significant interactive effects of dietary nanozeolite, Pediococcus, and/or medium-chain fatty acid supplementation were noticed on the hepatic expression of the GHR1, IGF1, FABP, and CD36 genes of O. niloticus as shown in Fig. 2. Regarding the growth-related genes GHR1 and IGF1, the dietary supplementation of nanozeolite (T1) and Pediococcus (T2) alone did not modulate GHR1 and IGF1 expression (P > 0.05). While medium-chain fatty acid (T2) significantly upregulated both gene expressions (P ≤ 0.05). The expression levels of GHR1 and IGF1 were significantly higher in the mixed groups, including nano + medium-chain fatty acid group (T5), the medium-chain fatty acid + probiotic group (T6), and the nano + medium-chain fatty acid + probiotic (T8) group (P ≤ 0.05). Moreover, the mix of the three supplements, nano + medium-chain fatty acid + probiotic (T8) group, showed the highest expression level (P ≤ 0.05).
The measured fat metabolism-related genes (FABP and CD36) were variably modulated by the different supplements alone or in combination. Whereas, nanozeolite (T1) or fatty acid (T3) groups alone did not enhance FABP levels against the T0 (P > 0.05). While, Pediococcus (T2) alone upregulated FABP levels against the T0. As opposed to that, the dietary mixture of two or all supplements significantly enhanced FABP expression compared to the non-supplemented group or the other groups, which took only one treatment (P ≤ 0.05). Moreover, the T5 and T7 expressed the highest FABP levels against all other groups (P ≤ 0.05).
Regarding CD36 relative expression, the T1 group and T2 group slightly enhanced the CD36 level (P > 0.05), while medium-chain fatty acid supplementation alone (T3) or mixed with other treatments (T5, T6, and T7) significantly enhanced the expression level. The expression level was relatively similar in the T5 and T7. Additionally, the fish group supplemented with nano + probiotics (T4) showed the highest CD36 mRNA level against other groups (P ≤ 0.05).
Discussion
The growth performance outcomes of the current experiment of different treatments either singly or in mix improved with regard to the T0 (control), but the combination of the studied additives was more effective (Tables 2 and 3). These outcomes are consistent with the results of Maas et al. (2021), who stated that probiotics improved performance of the growth significantly. This improvement might be attributable to P. acidilactici’s capacity to preserve the intestine’s structural integrity, which increases the capacity of the intestine to absorb nutrients. P. acidilactici is regarded as having additional nutritional value because it contains a lot of protein and vitamin B complex. This interpretation resembled that which El-Banna and Atallah (2009) stressed, who stated that the feed additive addition to diets of fish improves immune state of fish, survival, and weight and weight gain of the body with an improvement in the economic and productive effectiveness of fish farms. P. acidilactici is a probiotic that has beneficial effects on the balance and function of the gut flora; it also strengthens the immune system and increases the animal performances (Jin et al. 2000; Coppola and Gil-Turnes 2004; Salama et al. 2020). Protease enzymes are produced in the fish digestive system by probiotic bacteria like Bacillus, which can boost feed digestibility (Wardika and Sudaryono 2014). Additionally, probiotic bacteria have a part to play in enhancing fish health since they can low the population of harmful bacteria (Cruz et al. 2012). The association between specific digestive enzyme activity in tilapia and the improvement in fish growth with the addition of probiotics is explicable. According to Sumon et al. (2018), probiotics that contain lactic acid bacteria that have colonized the fish’s gut can aid in increasing the production of natural digestive enzymes such as amylases, lipases, and proteases. In addition, probiotics can also offer growth factors, including fatty acids, amino acids, and vitamins, that improve nutrient absorption (Sumon et al. 2018). Others have already noted an increase in the beneficial bacterial population in the gut of the fish group that had probiotics added (Giri et al. 2018). According to Merrifield et al. (2010), an increase in the bacterial population in fish guts can aid in better nutrient absorption and metabolism, which in turn increases the immune system in the fish body and increases disease resistance. However, probiotics’ positive effects on health have been attributed to a variety of factors, including preserving the health of the digestive system (by supporting the gut, enhancing GIT microflora, and assisting in digestion), enhancing the immune system (Jha et al. 2020), having an antioxidant effect (Yang et al. 2019; Feng and Wang 2020; Sánchez Macarro et al. 2021), decreasing inflammatory responses (Azad et al. 2018; Galdeano et al. 2019; Jha et al. 2020; Zommiti et al. 2020), and serving as a source of vitamins, particularly vitamins B complex and K, which nourish the host and support metabolism (Liu et al. 2019a; Yoshii et al. 2019).
Similarly, nanozeolite improved growth performance in this study. The concomitant enhancements in apparent whole tract digestibility of nutrient and/or a potential increase in gut function and general health status may have been caused by the increases in growth rate and feed efficiency of fish-fed diets supplemented with zeolite in tilapia (Nssar 2019). The results presented in Tables 2, and 3 correspond to previous studies that found dietary clays have a beneficial impact on growth performance in common carp and rainbow trout, respectively (Obradovic et al. 2006; Eya et al. 2008; Danabas 2009; Nssar 2019). Similar to this, Kanyılmaz et al. (2015) found that adding zeolite to Nile tilapia in the diets improved feed utilization and performance against controls. These effects could be brought on by the ability of the natural clay to lower feed and nutrient levels when used as a feed additive. This might be partially attributed to improvements in intestinal structure and digestion processes, such as elevated enzyme activity of pepsin, alkaline phosphatase, trypsin, and amylase (Sheikhzadeh et al. 2017; Hamidian et al. 2018). The results of Kaya et al. (2022) and Zenhom et al. (2020) are consistent with the increase in Nile tilapia performance and efficiency of feed shown in this study. Application of zeolite to cultured fish diets resulted in improved growth performance (Abbas et al. 2021; Nssar 2019). Zeolite improves Nile tilapia growth (El-Gendy et al. 2015).
Similar results were obtained by using medium-chain fatty acids alone. Magouz et al. (2020a, b) reported the positive effect of AQUAGEST and medium-chain fatty acid on the growth performance of common carp and Nile tilapia, respectively. Moreover, Ullah et al. (2022) reported the greater effect of lauric acid on the growth of juvenile black sea bream. Diets rich in medium-chain fatty acids are preferable to other types of feed because they can be passively taken by animals where they are quickly digested (Odle 1997). This positive effect on growth is attributed to the positive effect of medium-chain fatty acids on metabolism (Van Wymelbeke et al. 1998). Medium-chain fatty acids increase the somatotropic axis’ anabolic activity (Simó-Mirabet et al. 2017). Moreover, Nishi et al. (2005) reported that medium-chain fatty acids lead to ghrelin acetylation; this acetylation decreases ghrelin activity. However, this point was confirmed by the results of feed intake in the medium-chain fatty acid group. The gastric mucosa secretes ghrelin, which contributes to increased feed intake (Nakazato et al. 2001). However, Zhou et al. (2023) reported that medium-chain fatty acids had no significant effects on the growth of white shrimp. The different fish species could be the cause of this variation. Ng and Koh (2011) reported that fish development was enhanced by the addition of organic acid to the diet, but the kind of organic acid, the content of the diet, the species of fish, and age and farming conditions all have a direct role in this.
On the other side, the combination treatments increased performance parameters and improved FCR (Table 4). To our knowledge, there are not enough publications about combinations of different treatments in the present trial. However, Choi et al. (2023) found that the performance of juvenile olive flounder improved with four functional feed additive supplements. Simó-Mirabet et al. (2017) reported the beneficial impact of a sodium salt medium-chain fatty acid mixed with a probiotic based on Bacillus in Sparus aurata. However, alongside lactic acid, short-chain fatty acids (SCFAs), which include acetic acid, propionic acid, and butyric acid, are produced by probiotics when they interact with fiber. These SCFAs alter the GIT’s pH to prevent pathogenic microorganisms from growing, boost calcium and iron intake, and play a part in maintaining homeostasis in terms of energy. SCFAs are thought to be the primary energy source for colonocytes, while leftovers, particularly propionate, are used by other organs. Additionally, SCFAs control the oxidation and fatty acid synthesis as well as lipolysis. By enhancing oxidation and inhibiting synthesis and catabolism of fat, SCFAs reduce plasma levels of free fatty acids and body weight (Cerdó et al. 2019). According to Sa’ad et al. (2010), propionate increases the tissue’s sensitivity to insulin while reducing free fatty acid levels in the blood, liver, and food consumption. Additionally, SCFAs stimulate G-protein-coupled receptors in the epithelium of the gut and cells of the immune system such as GPR41, GPR43, and GPR109A. This stimulation led to secretion of hormones of the gut such as GLP-1 and PYY. The gut hormones (GLP-1) led to decrease oxidative stress and aggregation of platelet (Nogal et al. 2021).
When contrasted with the study’s control group, probiotics, nanozeolites, and medium-chain fatty acid treatment alone or in mix showed significant enhanced growth rate and feed utilization (Table 5). We looked at morphometric parameters that were enhanced, FCR that was significantly lower, and growth performance that was significantly greater. The current study is in line with earlier research that found Labeo rohita (Verma et al. 2016), rabbitfish (Dawood et al. 2019), and tilapia (Elsabagh et al. 2018), all of which demonstrated enhanced growth performance and feed consumption when fed a probiotic-rich diet. (1) By creating growth-promoting elements like co-factors, amino and fatty acids, and vitamins (Balami et al. 2022), which could then increase rate of growth and utilization of feed in the probiotic-tested groups. Vitamins such as vitamin B complex and vitamin C (Rodrigues et al. 2011; Rossi et al. 2005). Amino acids are essential and non-essential such as tryptophan, isoleucine, histidine, leucine, and lysine and tyrosine, glutamate, and alanine, respectively. The fermentation process releases these amino acids. These biologically active substances may be crucial for aquatic species’ ability to absorb, assimilate, and grow their food. (2) Amylases, proteases, and lipases, among other extracellular enzymes, have been found to be released by P. pentosaceus in prawns (Adel et al. 2017; Wanna et al. 2021).
However, the findings of the growth performance were supported by the results of the gut histology (Fig. 1). Moreover, to assess the health and wellbeing of the gut, the histology of the intestine had been determined (Banan Khojasteh 2012). Histological observations showed that, compared to the control group, probiotic, nanozeolites, and/or medium-chain fatty acid administration significantly improved a number of characteristics of intestinal histoarchitecture, including villus height and goblet cell numbers, without any pathological changes in the intestine. These results are in line with those of Zheng et al. (2018), Won et al. (2020), and Ashouri et al. (2020), who similarly noted an increase in crypt depth as well as width of villi and visible villus surface. The increase in intestinal surface area associated with probiotic, nanozeolites, and/or medium-chain fatty acid treatments may increase absorption surface, which is linked to an improvement in brush border integrity. Theses enhance absorption of nutrient and consumption of feed and in turn stimulate performance and survival (Ashouri et al. 2020; Hoseinifar et al. 2017). Moreover, Han et al. (2020) and Ullah et al. (2022) reported the significant impact of medium-chain fatty acid on the villus height and goblet cell number of weaned piglets and black sea bream, respectively. Abbas et al. (2021) found the positive impact of Yemeni Zeolite on the performance of Nile tilapia exposed to lead toxicity without adverse effect on the intestine. On the other side, Simó-Mirabet et al. (2017) found a significant improvement in the number of goblet cells in the villi of Sparus aurata when fed sodium salt medium-chain fatty acid + probiotic based on Bacillus in their diet.
Through a number of signaling pathways, the GH and IGF1 genes are necessary for controlling fish development and cell activity (Solberg et al. 2012). The results of the performance and histopathology were supported by the gene expression results in the liver tissue of the Nile tilapia (Fig. 2). A study by Eissa et al. (2022) found that when Prime™ (commercial probiotic) was administered as a water addition, the expression levels of genes that related to growth (GH and IGF1) were considerably 2–3 times greater than in the control group. Moreover, Amphiprion ocellaris, a probiotic used to supplement the water of clownfish, caused a threefold rise in the expression of IGF1 and IGF2 genes, which was associated with greater performance compared to the non-supplemented group (Avella et al. 2010). Moreover, Ahmadifar et al. (2020) reported that P. acidilactici MA 18/5 M caused the growth-related gene in zebrafish to be upregulated. According to our findings, Safari et al. (2021) reported similar findings in common carp that fed malic acid and expressed more growth-related genes than non-fed. Similar outcomes were achieved by Abdel-Tawwab et al. (2021) in Nile tilapia juveniles fed sodium butyrate nanoparticles. However, the combination treatment upregulated growth gene expression. These outcomes are in consistent with those of Choi et al. (2023), who reported significant upregulation of the growth-related gene of Juvenile Olive Flounder when supplemented with four feed additives. On the other hand, the expression of FABP and CD36 genes in the tissue of the liver of Nile tilapia was upregulated against the control group. These outcomes align with those of Tawfik et al. (2022). Additionally, Ismail et al. (2019) reported the same results in Nile tilapia supplemented with probiotics. Liu et al. (2019b) reported that normal growth and lipid metabolism were benefited by the predominant fatty acid in O. niloticus. Following the inclusion of moringa leaves in the feed, growth hormone receptor, IGF, lipoprotein lipase, and fatty acid synthase mRNA levels increased relatively, which promoted performance and induced hypolipidemic response (El-Kassas et al. 2020).
Conclusion
Overall, it was observed that there were many different beneficial effects resulting from the different dietary additives used in this trial. Pediococcus, nanozeolites, and medium-chain fatty acid, singly or in combination, induced positive effects on growth performance, growth rates, and gene expression of the growth and fat metabolism-related genes with normal intestinal architecture. However, combined additives (i.e., three treatments) had significant effects on improving growth and regulating growth-related genes. This conclusion was confirmed by the positive effects of combined treatment on the final body weight, total weight gain, feed intake, SGR, FCR, and PER as growth parameters. In addition, upregulation of the GHR1 and IGF1 genes. Singly, the three additions demonstrated intriguing results, offering a potentially effective means of enhancing aquaculture growth.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Abbas WT, Ali SE, Melegy AA, Gamil AA (2021) Fish diet supplemented with Yemeni Zeolite improves growth performance and reduces lead toxicity in Nile tilapia (Oreochromis niloticus). Aquac Res 52(12):6678–6688. https://doi.org/10.1111/are.15537
Abd Elshafy MB, Abd EL-Monem AIM, Khattab IM, Fadl SE, Abou Khadiga G (2023) Nutritional impact of nano zeolite, probiotic, and fatty acids as feed additives on health status of Nile tilapia (Oreochromis niloticus). Sci Rep 13(1):22740. https://doi.org/10.1038/s41598-023-50034-2
Abdel-Tawwab M, Shukry M, Farrag FA, El-Shafai NM, Dawood MA, Abdel-Latif HM (2021) Dietary sodium butyrate nanoparticles enhanced growth, digestive enzyme activities, intestinal histomorphometry, and transcription of growth-related genes in Nile tilapia juveniles. Aquac 536:736467. https://doi.org/10.1016/j.aquaculture.2021.736467
Abdel-Tawwab M (2016) Feed supplementation to freshwater fish: experimental approaches. LAP Lambert Academic Publishing. scholar.google.com
Adel M, Yeganeh S, Dawood MAO, Safari R, Radhakrishnan S (2017) Effects of Pediococcus pentosaceus supplementation on growth performance, intestinal microflora and disease resistance of white shrimp Litopenaeus Vannamei. Aquac Nutr 23(6):1401–1409. https://doi.org/10.1111/anu.12515
Ahmadifar E, Dawood MA, Moghadam MS, Shahrestanaki AH, Van Doan H, Saad AH, Aboubakr M, Abdelhiee EY, Fadl SE (2020) The effect of Pediococcus acidilactici MA 18/5M on immune responses and mRNA levels of growth, antioxidant and immune-related genes in zebrafish (Danio rerio). Aquac Rep 17:100374. https://doi.org/10.1016/j.aqrep.2020.100374
AOAC (2000) Official Methods of Analysis. 17th Edition. The Association of Official Analytical Chemists, Gaithersburg, MD, USA. scholar.google.com
Ashouri G, Soofiani NM, Hoseinifar SH, Jalali SAH, Morshedi V, Valinassab T, Bagheri D, Doan HV, Mozanzadeh MT, Carnevali O (2020) Influence of dietary sodium alginate and Pediococcus acidilactici on liver antioxidant status, intestinal lysozyme gene expression, histomorphology, microbiota, and digestive enzymes activity, in Asian sea bass (Lates calcarifer) juveniles. Aquac 518:734638. https://doi.org/10.1016/j.aquaculture.2019.734638
Avella MA, Gioacchini G, Decamp O, Makridis P, Bracciatelli C, Carnevali O (2010) Application of multi-species of Bacillus in sea bream larvi culture. Aquac 305(1–4):12–19. https://doi.org/10.1016/j.aquaculture.2010.03.029
Azad MAK, Sarker M, Wan D (2018) Immunomodulatory effects of probiotics on cytokine profiles. BioMed Res Int 8063647. https://doi.org/10.1155/2018/8063647
Balami S, Paudel K, Shrestha N (2022) A review: use of probiotics in striped catfish larvae culture. Int J Fish Aquat Stud 10:41–49 (http://www.fisheriesjournal.com/)
Banan Khojasteh SM (2012) The morphology of the post-gastric alimentary canal in teleost fishes: a brief review. Int J Aquatic Sci 3(2):71–88
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques, 5th ed. Churchill Livingstone, Edinburgh (UK 800 p). scholar.google.com
Bashar A, Hasan NA, Haque MM, Rohani M, Hossain M (2021) Effects of dietary silica nanoparticle on growth performance, protein digestibility, hematology, digestive morphology, and muscle composition of Nile tilapia, Oreochromis niloticus. Front Mar Sci 1100. https://doi.org/10.3389/fmars.2021.706179
Bedford A, Gong J (2018) Implications of butyrate and its derivatives for gut health and animal production. Anim Nutr 4(2):151–159. https://doi.org/10.1016/j.aninu.2017.08.010
Castell JD, Tiews K (1980) Report of the EIFAC. IUNS and ICES working group on the standardization of methodology in fish nutrition research. Hamburg, Fedral Republic of Germany, EIFAC Technology 36: 24. scholar.google.com
Cerdó T, García-Santos JA, Bermúdez M, Campoy C (2019) The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients 11(3):635. https://doi.org/10.3390/nu11030635
Choi W, Moniruzzaman M, Hamidoghli A, Bae J, Lee S, Lee S, Min T, Bai SC (2023) Effect of four functional feed additives on growth, serum biochemistry, antioxidant capacity, gene expressions, histomorphology, digestive enzyme activities and disease resistance in juvenile olive flounder Paralichthys Olivaceus. Antioxid 12(8):1494. https://doi.org/10.3390/antiox12081494
Con P, Nitzan T, Slosman T, Harpaz S, Cnaani A (2019) Peptide transporters in the primary gastrointestinal tract of pre-feeding Mozambique tilapia larva. Front Physiol 10:808. https://doi.org/10.3389/fphys.2019.00808
Coppola MDM, Gil-Turnes C (2004) Probiotics and Immune Response. Ciência Rural 34:1297–1303. https://doi.org/10.1590/S0103-84782004000400056
Cruz PM, Ibanez AL, Hermosillo OAM, Saad HCR (2012) Use of probiotic in aquaculture. ISRN Microbiol 2012:1–13. https://doi.org/10.5402/2012/1916845
Danabas D (2009) Effects of different rates of zeolite (clinoptilolite) on some water parameters and growth and body composition of rainbow trout (Oncorhynchus mykiss Walbaum, 1792). Department of Fisheries, Institute of Naturel and Applied Sciences University of Cukurova 82. scholar.google.com
Dawood MA, Mohsen M, El-dakar A, Abdelraouf E, Moustafa E, Ahmed H (2019) Effectiveness of exogenous digestive enzymes supplementation on the performance of rabbitfish (Siganus rivulatus). Slov Vet Res 56(22-Suppl):409–419. https://doi.org/10.26873/SVR-779-2019
Dawood MA, Eweedah NM, Elbialy ZI, Abdelhamid AI (2020) Dietary sodium butyrate ameliorated the blood stress biomarkers, heat shock proteins, and immune response of Nile tilapia (Oreochromis niloticus) exposed to heat stress. J Therm Biol 88:102500. https://doi.org/10.1016/j.jtherbio.2019.102500
Eissa ESH, Baghdady ES, Gaafar AY, El-Badawi AA, Bazina WK, Abd Al-Kareem OM, Abd El-Hamed NN (2022) Assessing the influence of dietary Pediococcus acidilactici probiotic supplementation in the feed of European sea bass (Dicentrarchus labrax L.) (Linnaeus, 1758) on farm water quality, growth, feed utilization, survival rate, body composition, blood biochemical parameters, and intestinal histology. Aquac Nutr 2022:1–11. https://doi.org/10.1155/2022/5841220
S El-Banna S Atallah 2009 Study the role of feed additives in prevention of fish diseases incidence in Oreochromis niloticus and common carp fish and its economic importance J Arabian Aquac Soc 4 2 121 140 www.pdffactory.co
El-Gendy MO, Gouda AH, Shehab El-Din MT (2015) Effect of zeolite on feeding rates and growth performance for Nile tilapia (Oreochromis niloticus). Int J Sci Res Agri Sci 2:018–024
El-Kassas S, Abdo SE, Abosheashaa W, Mohamed R, Moustafa EM, Helal MA, El-Naggar K (2020) Growth performance, serum lipid profile, intestinal morphometry, and growth and lipid indicator gene expression analysis of mono-sex Nile tilapia fed Moringa oleifera leaf powder. Aquac Rep 18:100422. https://doi.org/10.1016/j.aqrep.2020.100422
El-Naggar K, Mohamed R, El-katcha MI, Abdo SE, Soltan MA (2021) Plant ingredient diet supplemented with lecithin as fish meal and fish oil alternative affects growth performance, serum biochemical, lipid metabolism and growth-related gene expression in Nile tilapia. Aquac Res 52(12):6308–6321. https://doi.org/10.1111/are.15494
Elsabagh M, Mohamed R, Moustafa EM, Hamza A, Farrag F, Decamp O, Dawood MAO, Eltholth M (2018) Assessing the impact of Bacillus strains mixture probiotic on water quality, growth performance, blood profile and intestinal morphology of Nile tilapia Oreochromis Niloticus. Aquac Nutr 24(6):1613–1622. https://doi.org/10.1111/anu.12797
Eya JC, Parsons A, Haile I, Jagidi P (2008) Effects of dietary zeolites (bentonite and mordenite) on the performance juvenile rainbow trout Onchorhynchus myskis. AJBAS 2(4):961–967
Fadl SE, Elsabaghb M, El- Habashic NM, Shehab El-Dind MT (2013) Some studies on Pediococcus acidilactici as a feed additive in tilapia finger lings. EJNF 16(2):351–364
FAO (Food and Agriculture Organization of the United Nations) and WHO (World Health Organization) (2001) Evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Report of a Joint FAO/WHO October. scholar.google.com
Feng T, Wang J (2020) Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: a systematic review. Gut Microbes 12(1):1801944. https://doi.org/10.1080/19490976.2020.1801944
Ferguson RMW, Merrifield DL, Harper GM, Rawling MD, Mustafa S, Picchietti S, Balcázar JL, Davies SJ (2010) The effect of Pediococcus acidilactici on the gut microbiota and immune status of on-growing red tilapia (Oreochromis niloticus). J Appl Microbiol 109(3):851–862. https://doi.org/10.1111/j.1365-2672.2010.04713.x
Galdeano CM, Cazorla SI, Dumit JML, Vélez E, Perdigón G (2019) Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab 74(2):115–124. https://doi.org/10.1159/000496426
Gibson GR (1999) Dietary modulation of human gut microflora using the prebiotics oligofructose and inulin. J Nutr 129:1438–1441
Giri SS, Yun S, Jun JW, Kim HJ, Kim SG, Kang JW, Kim SW, Han SJ, Sukumaran V, Park SC (2018) Therapeutic effect of intestinal autochthonous Lactobacillus reuteri P16 against waterborne lead toxicity in Cyprinus carpio. Front Immunol 9:1824. https://doi.org/10.3389/fimmu.2018.01824
Gómez GD, Balcázar JL (2008) A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol Medical Microbiol 52:145–154. https://doi.org/10.1111/j.1574-695X.2007.00343.x
Guarner F, Schaafsma GJ (1998) Probiotics. Int J Food Microbiol 39:237–238
Hamidian G, Zirak K, Sheikhzadeh N, Khani Oushani A, Shabanzadeh S, Divband B (2018) Intestinal histology and stereology in rainbow trout (Oncorhynchus mykiss) administrated with nanochitosan/zeolite and chitosan/zeolite composites. Aquac Res 49(5):1803–1815. https://doi.org/10.1111/are.13634
Han Y, Zhan T, Zhao Q, Tang C, Zhang K, Han Y, Zhang J (2020) Effects of mixed organic acid and medium-chain fatty acid as antibiotic alternatives on the performance, serum immunity, and intestinal health of weaned piglets orally challenged with Escherichia coli K88. Anim Feed Sci Technol 269:114617. https://doi.org/10.1016/j.anifeedsci.2020.114617
Hassaan MS, Nssar KM, Mohammady EY, Amin A, Tayel SI, El-Haroun ER (2020) Nano-zeolite efficiency to mitigate the aflatoxin B1 (AFB1) toxicity: effects on growth, digestive enzymes, antioxidant, DNA damage and bioaccumulation of AFB1 residues in Nile tilapia (Oreochromis niloticus). Aquac 523:735123. https://doi.org/10.1016/j.aquaculture.2020.735123
Hoseinifar SH, Sun YZ, Caipang CM (2017) Short-chain fatty acid as feed supplements for sustainable aquaculture: an updated view. Aquac Res 48(4):1380–1391. https://doi.org/10.1111/are.13239
Igejongbo TF, Esther O (2022) Gut content and viscerosomatic index analysis of family Clariidae in the riverine area of south western Nigeria. East African Scholars J Agri Life Sci 5(3):53–59. https://doi.org/10.36349/easjals.2022.v05i03.001
Ismail T, Nassef ED, Hegazi ES, Bakr AN, Moustaf EM, Abdo W, Elbialy Z (2019) The modulatory effect of dietary betaine on intestinal absorptive capacity, lipogenesis and expression of lipid metabolism-and growth-related genes in Nile tilapia fed on soybean meal-based diet. Slov Vet Res 56(Suppl 22):25–38. https://doi.org/10.26873/SVR-741-2019
Jauncey K, Ross B (1982) A guide to tilapia feeds and feeding. Institute of Aquaculture. Scotland: University of Stirling (No. Va0769). scholar.google.com
Jha R, Das R, Oak S, Mishra P (2020) Probiotics (direct-fed microbials) in poultry nutrition and their effects on nutrient utilization, growth and laying performance, and gut health: a systematic review. Anim 10(10):1863. https://doi.org/10.3390/ani10101863
Jin LZ, Ho YW, Abdullah N, Jalaludin S (2000) Digestive and bacterial enzyme activities in broilers fed diets supplemented with Lactobacillus cultures. Poult Sci 79(6):886–891. https://doi.org/10.1093/ps/79.6.886
Kanyılmaz M, Tekelioğlu N, Sevgili H, Uysal R, Aksoy A (2015) Effects of dietary zeolite (clinoptilolite) levels on growth performance, feed utilization and waste excretions by gilthead sea bream juveniles (Sparus aurata). Anim Feed Sci Technol 200:66–75. https://doi.org/10.1016/j.anifeedsci.2014.09.023
Kaya D, Genc E, Palić D, Genc MA, Todorović N, Sevgili H, Vasiljevic M, Kanyılmaz M, Guroy D (2022) Effect of dietary modified zeolite (clinoptilolite) on growth performance of gilthead sea bream (Sparus aurata) in the recirculating aquaculture system. Aquac Res 53(4):1284–1292. https://doi.org/10.1111/are.15662
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L (2008) Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquac 274:1–14. https://doi.org/10.1016/j.aquaculture.2007.11.019
Lambert WV, Ellis NR, Block WH, Titus HW (1936) The role of nutrition in genetics. Am Res Soc Anim Prod Proc 29:236–236
Liu Y, Van Bennekom EO, Zhang Y, Abee T, Smid EJ (2019a) a) Long-chain vitamin K2 production in Lactococcus lactis is influenced by temperature, carbon source, aeration and mode of energy metabolism. Microb Cell Factories 18(1):1–14. https://doi.org/10.1186/s12934-019-1179-9
Liu Y, Wen JJ, Ning LJ, Jiao JG, Qiao F, Chen LQ, Zhang ML, Du ZY (2019b) b) Comparison of effects of dietary-specific fatty acid on growth and lipid metabolism in Nile tilapia. Aquac Nutr 25(4):862–872. https://doi.org/10.1111/anu.12906
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Maas RM, Verdegem MC, Debnath S, Marchal L, Schrama JW (2021) Effect of enzymes (phytase and xylanase), probiotics (B. amyloliquefaciens) and their combination on growth performance and nutrient utilisation in Nile tilapia. Aquac 533:736226. https://doi.org/10.1016/j.aquaculture.2020.736226
Magouz FI, Dawood MA, Salem MF, El-Ghandour M, Van Doan H, Mohamed AA (2020a) a) The role of a digestive enhancer in improving the growth performance, digestive enzymes activity, and health condition of Nile tilapia (Oreochromis niloticus) reared under suboptimal temperature. Aquac 526:735388. https://doi.org/10.1016/j.aquaculture.2020.735388
Magouz FI, Essa M, Mansour M, Paray BA, Van Doan H, Dawood MA (2020b) b) Supplementation of AQUAGEST as a source of medium-chain fatty acid and taurine improved the growth performance, intestinal histomorphology, and immune response of common carp () fed low fish meal diets. Annal Anim Sci 20(4):1453–1469. https://doi.org/10.2478/aoas-2020-0046
McDonald P, Edwards RA, Greenhelgh JFD (1987) Animal nutrition text. English Language Book Socity/Longman. scholar.google.com
Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RT, Bøgwald J, Castex M, Ringø E (2010) The current status and future focus of probiotic and prebiotic applications for salmonids. Aquac 302(1–2):1–18. https://doi.org/10.1016/j.aquaculture.2010.02.007
Mirghaed AT, Yarahmadi P, Soltani M, Paknejad H, Hoseini SM (2019) Dietary sodium butyrate (Butirex® C4) supplementation modulates intestinal transcriptomic responses and augments disease resistance of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 92:621–628. https://doi.org/10.1016/j.fsi.2019.06.046
Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S (2001) A role for ghrelin in the central regulation of feeding. Nature 409(6817):194–198. https://doi.org/10.1038/35051587
Ng WK, Koh CB (2011) Application of organic acid in aquafeeds: impacts on fish growth, nutrient utilization and disease resistance. Standards for acidifiers, principles for the Use of organic acid in animal nutrition. Nottingham University Press, Nottingham, United Kingdom, 49–58. scholar.google.com
Nishi Y, Hiejima H, Hosoda H, Kaiya H, Mori K, Fukue Y, Yanase T, Nawata H, Kangawa K, Kojima M (2005) Ingested medium-chain fatty acid are directly utilized for the acyl modification of ghrelin. Endocrinology 146(5):2255–2264. https://doi.org/10.1210/en.2004-0695
Nogal A, Valdes AM, Menni C (2021) The role of short-chain fatty acid in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microb 13(1):1–24. https://doi.org/10.1080/19490976.2021.1897212
Nssar KM (2019) Effects of dietary natural clay zeolite supplementation on growth performance, hematological parameters and body composition of Nile tilapia, Oreochromis niloticus L. fingerlings. Annal Agri Sci 57(3):681–686. https://doi.org/10.21608/assjm.2019.98125
Obradovic S, Adamovic M, Vukasinovic M, Jovanovic R, Levic J (2006) The application effects of natural zeolite in feed and water on production results of Oncorhynchus Mykiss (Walbaum). Rom Biotechnol Lett 11(6):3005
Odle J (1997) New insights into the utilization of medium-chain triglycerides by the neonate: observations from a piglet model. J Nutr 127(6):1061–1067
Papaioannou D, Katsoulos PD, Panousis N, Karatzias H (2005) The role of natural and synthetic zeolites as feed additives on the prevention and/or the treatment of certain farm animal diseases: a review. Microporous Mesoporous Mater 84(1–3):161–170. https://doi.org/10.1016/j.micromeso.2005.05.030
Qu H, Cheng Y, Chen Y, Li J, Zhao Y, Zhou Y (2019) Effects of dietary zeolite supplementation as an antibiotic alternative on growth performance, intestinal integrity, and cecal antibiotic resistance genes abundances of broilers. Anim 9(11):909. https://doi.org/10.3390/ani9110909
Rodrigues D, Santos CH, Rocha-Santos TA, Gomes AM, Goodfellow BJ, Freitas AC (2011) Metabolic profiling of potential probiotic or synbiotic cheeses by nuclear magnetic resonance (NMR) spectroscopy. J Agric Food Chem 59(9):4955–4961. https://doi.org/10.1021/jf104605r
Rossi M, Corradini C, Amaretti A, Nicolini M, Pompei A, Zanoni S, Matteuzzi D (2005) Fermentation of fructooligosaccharides and inulin by bifidobacteria: a comparative study of pure and fecal cultures. Appl Environ Microbiol 71(10):6150–6158. https://doi.org/10.1128/AEM.71.10.6150-6158.2005
Sa’ad H, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K (2010) Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta Mol Cell Biol Lipids 11:1175–1183. https://doi.org/10.1016/j.bbalip.2010.07.007
Safari R, Hoseinifar SH, Dadar M (2021) Enrichment of common carp (Cyprinus carpio) diet with malic acid: effects on skin mucosal immunity, antioxidant defence and growth performance. Annal Anim Sci 21(2):561–573. https://doi.org/10.2478/aoas-2020-0092
Salama FA, Abdel-Rahman A, El Shehedy M (2020) Effect of adding Pediococcus acidilactici at low plant protein diets on growth performance of Nile tilapia (Oreochromis niloticus) fingerlings. Afr J Biol Sci 16(1):93–105
Sánchez Macarro M et al (2021) Antioxidant effect of a probiotic product on a model of oxidative stress induced by high-intensity and duration physical exercise. Antioxid 10(2):323. https://doi.org/10.3390/antiox10020323
Sheikhzadeh N, Kouchaki M, Mehregan M, Tayefi-Nasrabadi H, Divband B, Khataminan M, Oushani AK, Shabanzadeh S (2017) Influence of nanochitosan/zeolite composite on growth performance, digestive enzymes and serum biochemical parameters in rainbow trout (Oncorhynchus mykiss). Aquac Res 48(12):5955–5964. https://doi.org/10.1111/are.13418
Shija VM, Amoah K, Cai J (2023) Effect of Bacillus probiotics on the immunological responses of Nile tilapia (Oreochromis niloticus): a review. Fishes 8(7):366. https://doi.org/10.3390/fishes8070366
Shimeno S, Masumoto T, Hujita T, Mima T, Ueno S (1993) Alternative protein sources for fish meal in diets of young yellowtail. Bull Japan Soc Sci Fish 59(1):137–143
Simó-Mirabet P, Piazzon MC, Calduch-Giner JA, Ortiz Á, Puyalto M, Sitjà-Bobadilla A, Pérez-Sánchez J (2017) Sodium salt medium-chain fatty acid and Bacillus-based probiotic strategies to improve growth and intestinal health of gilthead sea bream (Sparus aurata). Peer J 5:e4001. https://doi.org/10.7717/peerj.4001/supp-1
Solberg MF, Kvamme BO, Nilsen F, Glover KA (2012) Effects of environmental stress on mRNA expression levels of seven genes related to oxidative stress and growth in Atlantic salmon Salmo salar L. of farmed, hybrid and wild origin. BMC Res Notes 5(1):1–16. https://doi.org/10.1186/1756-0500-5-672
Sumon MS, Ahmmed F, Khushi SS, Ahmmed MK, Rouf MA, Chisty MAH, Sarower MG (2018) Growth performance, digestive enzyme activity and immune response of Macrobrachium rosenbergii fed with probiotic Clostridium butyricum incorporated diets. J King Saud Univ Sci 30(1):21–28. https://doi.org/10.1016/j.jksus.2016.11.003
Tawfik W, Nassef E, Bakr A, Hegazi E, Ismail TA, Abdelazim AM, El-Nagar SH, Sabike I, Fadl SE, Sharoba AM (2022) Orange pulp in Nile tilapia (Oreochromis niloticus) diets: growth performance, biochemical parameters and gene expression for growth and fat metabolism. Aquac Rep 22:100970. https://doi.org/10.1016/j.aqrep.2021.100970
Tran NT, Li Z, Wang S, Zheng H, Aweya JJ, Wen X, Li S (2018) Progress and perspectives of short-chain fatty acid in aquaculture. Rev Aquac 12:283–298. https://doi.org/10.1111/raq.12317
Ullah S, Zhang J, Xu B, Tegomo AF, Sagada G, Zheng L, Wang L, Shao Q (2022) Effect of dietary supplementation of lauric acid on growth performance, antioxidative capacity, intestinal development and gut microbiota on black sea bream (Acanthopagrus schlegelii). PLoS ONE 17(1):e0262427. https://doi.org/10.1371/journal.pone.0262427
Van Wymelbeke V, Himaya A, Louis-Sylvestre J, Fantino M (1998) Influence of medium-chain and long-chain triacylglycerols on the control of food intake in men. AJCN 68(2):226–234. https://doi.org/10.1093/ajcn/68.2.226
Verma AK, Rani AB, Rathore G, Saharan N, Gora AH (2016) Growth, non-specific immunity and disease resistance of Labeo rohita against Aeromonas hydrophila in biofloc systems using different carbon sources. Aquac 457:61–67. https://doi.org/10.1016/j.aquaculture.2016.02.011
Wanna W, Surachat K, Kaitimonchai P, Phongdara A (2021) Evaluation of probiotic characteristics and whole genome analysis of Pediococcus pentosaceus MR001 for use as probiotic bacteria in shrimp aquaculture. Sci Rep 11(1):18334. https://doi.org/10.1038/s41598-021-96780-z
Wardika AS, Sudaryono A (2014) Pengaruh Bakteri Probiotik Pada Pakan Dengan Dosis Berbeda Terhadap Efisiensi Pemanfaatan Pakan, Pertumbuhan Dan Kelulushidupan Lele Dumbo (Clarias gariepinus). J Aquac Management Technol 3(4):9–17. http://ejournal-s1.undip.ac.id/index.php/jamt
Won S, Hamidoghli A, Choi W, Bae J, Jang WJ, Lee S, Bai SC (2020) Evaluation of potential probiotics Bacillus subtilis WB60, Pediococcus pentosaceus, and Lactococcus lactis on growth performance, immune response, gut histology and immune-related genes in whiteleg shrimp Litopenaeus Vannamei. Microorganisms 8(2):281. https://doi.org/10.3390/microorganisms8020281
Yang SJ, Lee JE, Lim SM, Kim YJ, Lee NK, Paik HD (2019) Antioxidant and immune-enhancing effects of probiotic Lactobacillus plantarum 200655 isolated from kimchi. Food Sci Biotechnol 28(2):491–499. https://doi.org/10.1007/s10068-018-0473-3
Yoshii K, Hosomi K, Sawane K, Kunisawa J (2019) Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front Nutr 6:48. https://doi.org/10.3389/fnut.2019.00048
Zenhom OA, Refaey MM, Ayyat AN, El-Sayed HGM (2020) Impact of natural zeolite on growth performance, feed efficiency, and carcass composition of Nile tilapia (Oreochromis niloticus, L.) cultured at different stocking densities. Abbassa Int J Aquac 3:191–210
Zhang X, Zhong H, Han Z, Tang Z, Xiao J et al (2020) Effects of waterborne exposure to 17β-estradiol on hepatic lipid metabolism genes in tilapia (Oreochromis niloticus). Aquac Rep 17:100382. https://doi.org/10.1016/j.aqrep.2020.100382
Zheng X, Duan Y, Dong H, Zhang J (2018) Effects of dietary Lactobacillus plantarum on growth performance, digestive enzymes and gut morphology of Litopenaeus vannamei. Probiotics Antimicro Prot 10:504–510. https://doi.org/10.1007/s12602-017-9300-z
Zhou JS, Guo P, Yu HB, Ji H, Lai ZW, Chen YA (2019) Growth performance, lipid metabolism, and health status of grass carp (Ctenopharyngodon idella) fed three different forms of sodium butyrate. Fish Physiol Biochem 45(1):287–298. https://doi.org/10.1007/s10695-018-0561-6
Zhou W, Xie Y, Xie M, Liang H, Li M, Zhou B, Ran C, Zhou Z (2023) The effect of dietary supplementation of medium-chain fatty acid products on gut and hepatopancreas health, and disease resistance in white shrimp (Litopenaeus vannamei). Aquac Rep 29:101481. https://doi.org/10.1016/j.aqrep.2023.101481
Zommiti M, Feuilloley MG, Connil N (2020) Update of probiotics in human world: a nonstop source of benefactions till the end of time. Microorganisms 8(12):1907. https://doi.org/10.3390/microorganisms8121907
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work whereas they designed, conducted the experiment, and wrote the manuscript.
Corresponding author
Ethics declarations
Guidelines
All methods were carried out in accordance with relevant guidelines and regulations.
ARRIVE guidelines
The authors confirm that the study was carried out in compliance with the ARRIVE guidelines.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Pierre Boudry
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elshafy, M.B.A., El-Monem, A.I.M.A., Khattab, I.M. et al. Dietary feed nanozeolite, Pediococcus, and medium-chain fatty acid enhanced growth performance and transcription of growth-related gene of Nile tilapia (Oreochromis niloticus). Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01448-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01448-w