Abstract
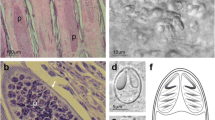
The myxosporean infection and its phylogenetic analysis were investigated in tilapia (Oreochromis niloticus) with average weight (70 ± 5 g) and crayfish (Procambarus clarkii) with average weight (20 ± 6 g). The prevalence of myxosporean infection was 100% for tilapia gills and internal organs without any specific clinical signs. Gross examination of affected tilapia showed enlargement of posterior kidney with macroscopic white nodules in kidneys and gills while crayfish gills showed black spots. Microscopic examination of the fresh preparation of kidneys of tilapia showed spores of Myxosporea. Moreover, the hepatopancreas fresh samples of crayfish demonstrated myxosporean spores. The gene sequencing of 18S gene from tilapia kidneys followed by phylogenetic analysis revealed that the isolated parasite had the highest sequence similarity to Myxobolus sp. ram SR-2018 [MK203074.1] giving 89.77% identity and was named Myxobolus sp. Egy1. In crayfish, histopathological examination showed the developmental stages of Myxosporea in the lumen and stroma of gill lamellae with degeneration of lining epithelium. In addition, different developmental stages of Myxosporea were scattered in the lining epithelium and lumen of hepatopancreas with vacuolation and multifocal deformation. In tilapia, histopathology revealed severe degenerative changes in the gill lamellae and renal tubules with presence of different developmental stages of Myxosporea. These findings support the high prevalence of myxosporean infection, particularly Myxobolus, in tilapia that induced histopathological damage in gills, and internal organs. Interestingly, we detected myxosporean spores in gills and hepatopancreas of crayfish which is considered a first report that needs further phylogenetic and epidemiological investigations.








Similar content being viewed by others
Data availability
The data are available upon request.
References
Abd El-Lateif RS, Torra DE, El Sherry YM (2023) Infestation of external ciliated protozoan in red swamp crayfish (Procambarus clarkii). J Adv Vet Res 13(2):197–206
Abd El-Moaty SM, Sharaf HM, Khalil AEM et al (2016) Survey on the parasites infested crayfish Procambarus clarkii, Girard, 1852 (Crustacea, Cambaridae) in Egypt. J Biosci App Res 2(6):395–400. https://doi.org/10.21608/jbaar.2016.108531
Abdel-Baki AAS, Abdel-Haleem HM, Sakran T et al (2015a) Two Myxobolus spp. infecting the kidney of Nile tilapia (Oreochromis niloticus) in the River Nile at Beni Suef governorate Egypt and the associated renal changes. Parasitol Res 114:1107–1112
Abdel-Baki AA, Zayed E, Sakran T et al (2015b) New record of Myxobolus brachysporus and M israelensis in the tilapia (Oreochromis niloticus) collected from the Nile River. Egypt Saudi J Bisol Sci 22(5):539–42. https://doi.org/10.1016/j.sjbs.2015.01.003
Abdel-Gaber R, Abdel-Ghaffar F, Maher S et al (2017) Morphological re-description and phylogenetic relationship of five myxosporean species of the family Myxobolidae infecting Nile tilapia. Dis Aquat Org 124(3):201–214. https://doi.org/10.3354/dao03118
Abdel-Ghaffar F, Morsy K, EL-Ganainy S et al (2015) Twelve myxosporean species of the family Myxobolidae infecting freshwater fishes of the River Nile, Egypt, with the description of four novel species. Parasitol Res. 114:2985–2998. https://doi.org/10.1007/s00436-015-4501-4
Abd-ELrahman SM, Gareh A, Mohamed HI et al (2023) Prevalence and morphological investigation of parasitic infection in freshwater fish (Nile tilapia) from Upper Egypt. Animals (basel) 13(6):1088. https://doi.org/10.3390/ani13061088
Adriano EA, Arana S, Carriero MM, Naldoni J, Ceccarelli PS, Maia AA (2009) Light, electron microscopy and histopathology of Myxobolus salminus n sp a parasite of Salminus brasiliensis from the Brazilian Pantanal. Veterinary Parasitol 165(2):25–29. https://doi.org/10.1016/j.vetpar.2009.07.001
Alama-Bermejo G, Holzer AS, Bartholomew JL (2019) Myxozoan Adhesion and Virulence: Ceratonova shasta on the Move. Microorganisms 7:397. https://doi.org/10.3390/microorganisms7100397
Ali MA, AL-Rasheid KA, Sakran T et al (2002) Some species of the genus Myxobolus (Myxozoa: Myxosporea) infecting freshwater fish of the River Nile, Egypt, and the impact on their hosts. Parasitol Res 88(1):9–15. https://doi.org/10.1007/s004360100449. (PMID: 11826871)
Alvarez-Pellitero P, Sitjà-Bobadilla A (1993) Pathology of Myxosporea in marine fish culture. Dis Aquat Org 17:229–232
American Fisheries Society (2014) Guidelines for the Use of Fishes in Research. Bethesda, Maryland USA
Bell TA, Lightner DV (1988) A handbook of normal penaeid shrimp. World Aquaculture Society, Baton Rouge, LA
de Auró OA, Ocampo CL (1998) Histopathological characterization of tilapia (Oreochromis sp) response to a mixed infection by myxosporean parasites. A Natural Case Vet Mex 29(2):213–216
dos Santos CAL, Howgate P (2011) Fish borne zoonotic parasites and aquaculture: a review. Aquaculture 318:253–261. https://doi.org/10.1016/j.aquaculture.2011.05.046
Eissa AE, Abu Mourad MK, Borhan T (2006) A contribution on Myxosoma infection in cultured Oreochromis niloticus in Lower Egypt. Nature Sci 4(4):40–46
Eissa AE, Attia MM, Elgendy MY et al (2021) Streptococcus, Centrocestus formosanus and Myxobolus tilapiae concurrent infections in farmed Nile tilapia (Oreochromis niloticus). Microb Pathog 158:105084. https://doi.org/10.1016/j.micpath.2021.105084
El-Asely AM, Abd El-Gawad EA, Soror EI et al (2015) Studies on some parasitic diseases in Oreochromis niloticus fish hatchery with emphasis to life stages. J Adv Vet Res 5(3):99–108
El-Mansy A, Abdel-Ghaffar F (2003) Tilapian proliferative kidney disease (TPKD) and a diagnostic evidence for the presence of myxosporean parasites. J Egypt Ger Soc Zool 40D:139–159
El-Matbouli M, Hoffmann RW (1998) Light and electron microscopic studies on the chronological development of Myxobolus cerebralis to the actinosporean stage in Tubifex tubifex. Int J Parasitol 28:195–217. https://doi.org/10.1016/S0020-7519(97)00176-8
El-Sayed AAM, Al-Damhougy KhA, Zaakouk SA, Amer MA (2015) Biometric relationships of the invasive crayfish Procambarus clarkii to the Egyptian freshwater drainage canals. Egypt Acad J Biolog Sci 7(1):53–65
Emam WM, Khalil MT (1995) Population dynamics and stock assessment of the newly introduced crayfish, P. clarkii in the River Nile, Egypt. Proc. Zool. Soc. A.R. Egypt 26:131–143
Feist SW, Morris DJ, Alama-Bermejo G, Holzer AS (2015) Cellular processes in myxozoans. In: Okamura B, Gruhl A, Bartholomew J (eds) Myxozoan evolution, ecology and development. Springer, Cham. https://doi.org/10.1007/978-3-319-14753-6_8
Fiala I, Bartošová-Sojková P, Whipps CM (2015) Classification and phylogenetics of myxozoa. In: Okamura B, Gruhl A, Bartholomew J (eds) Myxozoan evolution, ecology and development. Springer, Cham. https://doi.org/10.1007/978-3-319-14753-6_5
Folefack GPL, Tiwa AET, Dongmo BF, Nguegnang LM et al (2019) Morphotaxonomy and histopathology of three species of Myxobolus Bütschli, 1882 parasites of Enteromius martorelli Roman, 1971 from the Anga River in Cameroon. Int J Biol Chem Sci 13(3):1705–1719. https://doi.org/10.4314/ijbcs.v13i3.40
Gridley MF (1960) Manual of histologic and special staining technique. MacGraw-Hill Book Company 28–29:82–83
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hallett SL, Diamant A (2001) Ultrastructure and small-subunit ribosomal DNA sequence of Henneguya lesteri n. sp. (Myxosporea), a parasite of sand whiting Sillago analis (Sillaginidae) from the coast of Queensland. Australia Dis Aquat Organ 46:197–212. https://doi.org/10.3354/dao046197
Hamdi SA, Abd El-Monem S (2006) Processing, products and marketing of the red swamp crawfish, Procambarus clarkii (Crustacea, Decapoda). The Egyp J Experimental Biol (zoology) 2:93–98
Hamdi SAH, Zaghloul KH (2006) Evaluation of the crawfish, Procambarus clarkii as a cheaper source of human diet in comaprison with two marine shrimps in Egypt J Egypt. Ger Soc Zool 50(D):153-174
Hardiono SA, Yanuhar U (2021) The profile of myxobolus infection in the gill tissue of common carp (Cyprinus carpio L.) strain punten from concrete ponds. IOP Conf Ser: Earth Environ Sci 674:012017. https://doi.org/10.1088/1755-1315/674/1/012017
Huner JV, Barr JE (1991) Red swamp crayfish, biology and exploitation, 3rd edn. Louisiana State University, Baton Rouge, Louisiana, USA
Kent ML, Khattra J, Hervio DML et al (1998) Ribosomal DNA sequence analysis of isolates of the pkx myxosporean and their relationship to members of the genus Sphaerospora. J Aquat Anim Health. https://doi.org/10.1577/1548-8667(1998)010%3c0012:RDSAOI%3e2.0.CO;2
Lom J, Dyková I (1992) Protozoan parasites of fishes. In: Developments in Aquaculture and Fisheries Science, vol 26. Elsevier, Amsterdam, p 315
Lom J, Dyková I (2006) Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol 53:1–36. https://doi.org/10.14411/fp.2006.001
Manrique WG, Claudiano GS, Figueiredo MAP, Petrillo TG, Moraes JRE, Moraes FR (2012) Myxosporidiosis in intensively-reared Piaractus mesopotamicus: histopathological diagnosis by means of Ziehl-Neelsen staining. Pesq Vet Bras 32:1133–1137
Martone CB, Spivak E, Busconi L, Folco EJE et al (1999) A cysteine protease from myxosporean degrades host myofibrils in vitro. Comp Biochem Physiol B Biochem Mol Biol 123:267–272
Matter AF, Abbass AA, Abd El Gawad EA et al (2013) Studies on myxosporidiosis in some fresh water fishes. Benha Veterinary Medical Journal 25(2):316–325
Milanin T, Eiras JC, Arana S et al (2010) Phylogeny, ultrastructure, histopathology and prevalence of Myxobolus oliveirai sp. nov, a parasite of Brycon hilarii (Characidae) in the Pantanal wetland. Brazil Memorias do Instituto Oswaldo Cruz 105(6):762–769. https://doi.org/10.1590/s0074-02762010000600006
Mohammed N, Rabie S, Hussein A et al (2012) Infestation of Oreochromis niloticus and Tilapia zilli fresh-water fishes with myxosporean parasites, Qena Province. Egypt Egyptian Acad J Biol Sci, B Zool 4(1):235–246. https://doi.org/10.21608/eajbsz.2012.14305
Molnar K (2007) Site preference of myxozoans in the kidneys of Hungarian fishes. Dis Aquat Organ 78:45–53
Nissa K, Kaur H (2020) First record of the genus Hennegoides Lom, Tonguthai and Dyková, 1991 from Punjab (India) infecting the catfish, Sperata seenghala (Sykes, 1839). International journal for parasitology. Parasites and Wildlife 14:7–12. https://doi.org/10.1016/j.ijppaw.2020.11.009
Noga EJ (2010) Fish disease: diagnosis and treatment. Blackwell Publishing, Iowa
Osman GY, Abd El Wahab TM, Mohamed AH, Mazen TA A (2015) The relationship between the bioaccumulation of heavy metals in Clarias gariepinus tissues and endoparasitic helminths at Kafr El Sheikh Governorate, Egypt. Egypt J Chem Environ Health 1:1003-1016. https://doi.org/10.21608/ejceh.2015.253993
Radwan M, Shehata S, Abdelhadi Y et al (2021) Histopathological, haematological and biochemical indices of Clarias gariepinus (Burchell, 1822) parasitized by endoparasitic fauna in fish farm of the northeastern Egypt. Turk J Fish Aquat Sci 21:465–478. https://doi.org/10.4194/1303-2712-v21_9_05
Ramadan N (1997) A study on some protozoa infesting the freshwater crayfish, Procambarus clarkii in Egypt. Egyp J Aquat Biol Fisheries 1(2):359–377. https://doi.org/10.21608/ejabf.1997.3395
Sitjà-Bobadilla A (2009) Can Myxosporean parasites compromise fish and amphibian reproduction? Proceed Royal Soc B 276:2861–2870. https://doi.org/10.1098/rspb.2009.0368
Soliman GN, EL-Assal F, Salah El-Deen M et al (1998) Habitat, distribution and behavior of the red swamp crayfish Procambarus clarkii (Girard, 1852) (Decapoda: Cambaridae) in the River Nile. Egypt Egypt J Zool 30:297–310
Soror E, Mahrous K, Eissa IAM et al (2012a) Epizotiological studies on proliferative kidney disease in tilapia (Oreochromis niloticus) and African catfish (Clarias gariepinus). Benha Vet Med J 23(1):150–158
Soror E, Mahrous K, Eissa IAM et al (2012b) Identification of the causative agents of proliferative kidney disease in Oreochromis niloticus and Clarias gariepinus using PCR with special reference to the associated. Benha Vet Med J 23(1):159–170
Stratton CE, Reisinger LS, Behringer DC, Reinke AW, Bojko J (2023) Alternosema astaquatica n. sp. (Microsporidia: Enterocytozoonida), a systemic parasite of the crayfish Faxonius virilis. J Invertebr Pathol 199:107948. https://doi.org/10.1016/j.jip.2023.107948
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30(12):2725-9. https://doi.org/10.1093/molbev/mst197
Xiao C, Desser SS (2000) The longevity of actinosporean spores from oligochaetes of Lake Sasajewun, Algonquin Park, Ontario, and their reaction to fish mucus. J Parasitol 86:193–195. https://doi.org/10.1645/0022-3395(2000)086[0193:tloasf]2.0.co;2
Acknowledgements
The authors acknowledge Prof. Mansour El Matbouli, University of Veterinary Medicine Vienna, Austria, for providing the support to conduct the present study and prof. Hatem Soliman, Aquatic Animal Medicine and Management, Faculty of Veterinary Medicine, Assiut University, Egypt, for his support in conducting the molecular part. Shimaa E. Ali was supported by Norwegian Agency for Development Cooperation (NORAD) project number (RAF-19/0051).
Funding
This work was supported by Norwegian Agency for Development Cooperation (NORAD) project number (RAF-19/0051).
Author information
Authors and Affiliations
Contributions
The study was conceptualized by ES and AEA. The laboratory work for direct and stained smears was conducted by ES and AEA. AA conducted histopathology. SA conducted the laboratory work and the phylogenetic study. The manuscript was drafted and revised by all authors.
Corresponding authors
Ethics declarations
Ethics approval
The experiment was carried out in accordance with “Guidelines for the Use of Fishes in Research” published by American Fisheries Society (2014). Moreover, the research work was done after completing Laboratory Animal Science Course for Research workers which satisfies the requirements of the Norwegian Ministry of Agriculture and Food’s definition of competence at “FELASA C” level. Additionally, the research work was strictly supervised by advisory committee with the approval of WorldFish Egypt country director.
Competing interests
The authors declare no competing interest.
Additional information
Handling Editor: Brian Austin
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soror, E.I., Amin, A.A., Ali, S.E. et al. Clinical, histopathological, and phylogenetic studies on Oreochromis niloticus and Procambarus clarkii affected with Myxosporea in Egypt. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01395-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01395-6