Abstract
The aim of this study is to identify the hidden etiologies of the black chromo-shifting transient phenomenon affecting Nile tilapia, Oreochromis niloticus, farmed in RAS-controlled ponds together with the assessment of the immunological reaction against the chronic irritating effects of the invasive agent. A total of 100 Nile Tilapia were collected from a private farm at Kafrelsheikh Province, located on the northern side of the Egyptian Nile Delta. The clinical history of the affected fish farm showed an unknown chromo-shifting phenomenon where tilapias were exhibiting severe black skin coloration, which gradually disappeared after removal from the tank’s water. A comprehensive gross examination of the collected fish; including parasitological examination of skin and gill scraps, was performed. Blood biochemical testing was performed on the infested blackish O. niloticus and control non-infested fish. The current study showed that the monogenean parasite, Gyrodactylus cichlidarum, was the abundant parasite detected in the infested fish leading to abnormal black discoloration of skin and disruption of the immune system represented by significant increase of cortisol levels, lysozyme activity and different liver enzymes compared to the control. Treatment trials have been applied with moderate degrees of success, where the monogenean count was sharply decreased, and the normal skin color was remarkably restored, at the end of day 14 post-treatment. A triple treatment plan was initiated through 7 days’ application of 0.7 g/m3 copper sulfates pentahydrate preceded by 1.5 ml/m3 hydrogen peroxide 40% solution for the same period. One day after the end of the initial treatment, a maintenance dosage of 0.095 ml/m3 of glutaraldehyde (15%)/quaternary ammonium compounds (QACs) mixture was administered for 3 days. As a supportive/immune-stimulant regimen, a weekly dosage of vitamin C (0.45 g g/m3) and Saccharomyces cerevisiae (0.45 g/m3) was added into the tank’s water to improve the general fish health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture is the fastest-growing animal production sector, representing a valuable source of protein for human nutrition (Carbone and Faggio 2016). Nile tilapia is characterized as fast-growing, highly resistant to diseases, low trophic level feeder, and low production costing aquaculture species (Costa-Pierce and Rakocy 1997; Haygood and Jha 2018).
Until recently, recirculating aquaculture systems have become more familiar and economical due to the enhanced unit processes (e.g. removing solids, oxygenation, biological nitrification, and dissolved gas management). Furthermore, recirculating systems are sustainably designed to utilize lower input of new water. Additionally, better solids removal techniques, such as bead and screen filters, have replaced the sand filters. At present, the granular plastic bead filters are extensively developed, enhanced, and commercially available. Also, screen filters that operate rotating drums or discs are also widely employed. These filters separate solids, consume little energy during water passage via them, and discard very little amount of water to waste during cleaning (Ali 2013).
Intensive aquaculture systems have raised the incidence of various fish diseases, from which ectoparasitic infestations presented negative impacts on health and sustainability of farmed species (Blaylock and Bullard 2014; Eissa et al. 2021). The most prevalent ectoparasites reported in farmed fish and Nile tilapia included Gyrodactylus sp., Trichodina spp, Dactylogyrus spp., Chilodonella sp., Argulus sp., Ergasilus sp., and Learnea sp. (Iyaji and Eyo 2008).
Currently, over 400 Gyrodactylus spp. have been identified to infest cultured fish (Harris et al. 2004). G. cichlidarum was first collected and identified from Galilaeus galilaeus in Ghana, Africa (Paperna 1968). Later, G. cichlidarum was morphologically characterized from other gyrodactylids formerly defined by Paperna (1968) and Mousavi et al. (2013). Gyrodactylus infestation negatively impacted the health and welfare of the farmed fish (García-Vásquez et al. 2007; Zhi et al. 2020). G. cichlidarum is the most common ectoparasitic infestation in juvenile Nile tilapia, and it can induce huge mortalities in intensively farmed fish globally. In Mexico, G. cichlidarum was declared the main reason for the high mortalities of farmed tilapia (Mousavi et al. 2013). Co-infection was commonly documented in several disease outbreaks such as Gyrodactylus spp. and Cichlidogyrus spp., which were accompanied by alterations in the fish immune response (Pedersen and Fenton 2007; Zago et al. 2014).
The current study aims to identify the etiology behind blackish discoloration in RAS-controlled Nile tilapia farming systems, as well as implement a successful treatment strategy to efficiently restore the normal skin color together with long-term enhancement of tilapia’s immune system.
Materials and methods
Fish farming data
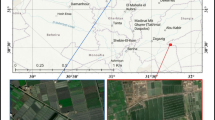
The affected fish farm is located at the center of Kafrelsheikh province. This coastal province located between the basin of the Mediterranean and Lake Burulus produces more than 50% of the entire Egyptian Nile tilapia harvest. Intensive RAS system–controlled farm utilizing 4-m diameter circular rearing tanks was studied. RAS was composed of mechanical filter, sand filter, and biological filter. The tank water column is 1.5 m on average. The water source is a 60-m-deep artesian well. Tanks were supplied with oxygen injectors (30 L/min) keeping the dissolved oxygen (DO) level at 5 mg /L ± 1 during the daylight. Stocking density was averaging 320 tilapia/m3 with the sum of 5000 tilapia/16 m3 sized tank. Nile tilapia’s weight was 350 g on average. Tilapias were fed 30% protein floating pellets (Aller Aqua Limited, Egypt). The farmer was complaining about swift black skin discoloration of tilapias a few minutes after they get out of tank water.
Fish sampling
During the mid-summer of 2022, a total of 100 moribund Nile tilapias suffering from black skin discoloration were collected from RAS controlled fish farm at Kafrelsheikh province, Egypt. Collected tilapias were transported alive to the Aquatic Animal Medicine and Management Laboratory(AAMML), Faculty of Veterinary Medicine, Cairo University, for clinical and laboratory investigations.
Water physico-chemical analysis
Using a Multi-probe HQ40D meter (HACH LDO; PHC301 & CDC41, Germany), dissolved oxygen (DO), temperature, and pH were recorded. Salinity was measured by a handheld refractometer (ATAGO CO., LTD. Japan). Moreover, water samples were gathered and subjected to additional testing for nitrate, nitrite, and unionized ammonia (NH3) using ready-to-use ammonia kits (Hanna, USA).
Clinical and parasitological examinations
Fish were laid down on the left side in a dissecting tray then a thorough gross examination was performed, including examination of skin scrapes, gill scrapes and muscle specimens under the light microscope at 40 × –400 × as described by Attia et al. (2021a, b). Gross and necropsy findings were recorded, and any detected parasites were carefully picked by pipetting and transported for further identification and imaging.
Determination of liver enzymes, glucose, and cortisol levels
Blood samples were collected from five uninfected fish and 20 infected fish. Blood was collected from the caudal vessels under anesthesia using benzocaine (50 mg/L−1), both with and without an anticoagulant. For obtaining the serum, blood was centrifuged for 15 min at 1500 rpm then sera were kept frozen at − 20 °C till analysed.
Serum glucose and aspartate aminotransferase (AST) concentrations were determined using A JASCO V-730 spectrophotometer (JASCO, Tokyo, Japan) and commercial kits (Spectrum-diagnostics, Egypt). Cortisol was measured according to e Silva et al. (2020).
Assessment of serum lysozyme activity
Serum lysozyme activity was measured following the method of Dotta et al. (2014), using the lysis of Micrococcus lysodeikticus (Sigma, USA), with certain modifications. In brief, 0.75 mL of M. lysodeikticus solution, pH 6.2, 0.2 mg/mL PBS, and 0.25 mL of serum were mixed. The reaction was carried out at room temperature and was measured at the absorbance of 450 nm after 0 and 20 min using a spectrophotometer (BM Co. Germany). The serum lysozyme concentrations were calculated using a calibration curve made from lyophilized chicken egg-white lysozyme (Sigma, USA).
Field treatment
The treatment plan was broken down into three major trials, as follows:
-
A.
Basic treatment: Copper sulfate pentahydrate 99% was applied at a dosage of 0.7 g/m3 every day for 7 days. The daily dosage of copper sulfate pentahydrate powder was moderately dissolved in water before being evenly distributed within the tank’s water at 12 PM. Before this regimen, hydrogen peroxide 40% solution was added to the tank’s water at a dosage of 1.5 mL/m3 in the early morning for the same period.
-
B.
Maintenance treatment: One day after the cessation of the initial treatment, a dosage of 0.095 mL/m3 of glutaraldehyde (15%)/quaternary ammonium compounds (QACs) mixture was applied for three successive days in the late afternoon.
-
C.
Supportive treatment: A supportive treatment program was adopted at the conclusion of the second week of treatment. As part of weekly regular practice, vitamin C (0.45 g g/m3) and Saccharomyces cerevisiae (0.45 g g/m3) were added to the tank’s water in the late afternoon to improve the aquatic biota and fish immune systems. At 14 days post-treatment, a parasitological examination was carried out utilizing gill and skin scraping. The number of fish that died and the levels of parasites found in the skin and gills of treated fish were recorded.
Statistics
Results were summarized as means ± standard error. Paired sample t-test was used to examine the degree of helminth infection based on mucous scraping of skin and gills at the start of the treatment trial (day 0) and 2 weeks post-treatment. An independent sample t-test was performed to compare means of serum parameters between infested and control groups. Analysis was done by PASW Statistics, Version 18.0 software (SPSS Inc., Chicago, IL, USA). Statistical significance was set at P ≤ 0.05.
Results
Water quality measurement
At 1 PM, the recorded DO was 3 ppm, temperature was 29 °C and pH was 8.7. The recorded salinity was 2 ppt. The recorded nitrate, nitrite, and unionized ammonia (NH3) levels were 1.8 ppm, 1.35 ppm and 0.5 ppm consecutively.
Clinical and parasitological examination
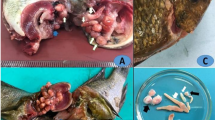
The main clinical signs observed on the affected tilapias were severe skin darkening shortly after removal from water (Fig. 1). Dissection of the moribund fish showed congested or pale liver, fatty spongy or even friable livers in some cases, enlarged spleen, and congested kidneys (Fig. 1). The fish tanks and filters were infested with high numbers of monogeneans. It is worthy to mention that the monogenean was detected in the RAS biological filter with a remarkably large number.
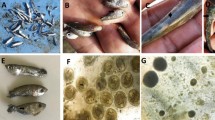
Parasitological examination showed heavy infestation with monogeneans, which were identified as G. cichlidarum based on morphometric analysis of different body regions. The morphometric analysis displayed a total body length of 330 µm (247–405 µm) and body width of 85 µm (65.89–100.9 µm). Haptor length was measured at 79 µm (69.89–90.98), the total length of the anchor was 69 µm (59.97–70.90), the total length of the marginal hook was 25 (24.5–28.5), the marginal hook sickle length was 8 µm (7.5–8.3), the marginal hook handle length was 3.5 (3.95–4.55), the total length of the dorsal bar was 23 µm (21.77–25.99); and median width of the dorsal bar was 1.65 µm (1.95–1.59) (Fig. 2).
Assessment of liver enzymes and cortisol level of infested fish
Table 1 presented serum glucose, cortisol, AST, and lysozyme concentrations in control and Monogenea-infested fish. Results indicated significant differences between both groups for all the measured parameters. Infested fish exhibited elevated cortisol, AST, and lysozyme levels more than the control (P < 0.0001). However, glucose concentrations displayed lower values in the infested group (P = 0.002) compared to the control group.
Field treatment
Dead fish were collected daily during the treatment strategy and discarded by sanitary burying. The cumulative mortalities were reduced from 36% to about 5% at the end of the treatment period (Table 2). Furthermore, after 2 weeks of therapy, a statistically significant reduction in the intensity of infestation Gyrodactylus count/microscopical field was observed when mucous skin and gill scraps from randomly selected treated fish were examined under the microscope (Table 2). Further, the regular skin color of Nile tilapias was gradually restored at the end of the treatment.
Discussion
In recirculating aquaculture systems (RAS), fish are kept in tanks at moderate to high densities. The wastewater discharged from RAS tanks is partly reused after being subjected to mechanical and biological filtrations for controlling solid and metabolic (nitrogen and phosphorous) wastes, respectively (Ebeling and Timmons 2012). Hence, the sludge and the surfaces of biological filters usually incorporate complex communities of microbes, protozoans, and nematodes (Sugita et al. 2005). Despite the positive impacts of the RAS farming system on the production potentials due to the bridging of biosecurity outflows associated with classical farming systems, yet few rare occasions of heavy parasitic invasions were reported among reared fishes under this system (Sergaliyev et al. 2017).
The current study presents a severe case of monogenean invasion to the skin of Nile tilapia farmed under RAS, which perfectly accords with data from previous worldwide studies published by Sergaliyev et al. (2017). In our case, the G. cichlidarum could have been brought to the fish tanks as early as Nile tilapia seeds were initially stocked, at the beginning of the rearing cycle. They might exist at the beginning as ova or minute larvae on the tilapia seeds’ skin or in hatchery water coming with the transportation of plastic bags containing the seeds during the acclimatization process. These monogenean stages have completed their cycle on fish, and some of them were stuck into the RAS filters in relatively large numbers. These might explain the detection of high numbers of G. cichlidarum throughout the examined RAS filters brought to the laboratory together with the affected tilapias. This hypothesis could be affirmed by similar assumptions concluded by Buchmann and Lindenstrøm (2002), who emphasized that monogeneans comprise both oviparous and viviparous organisms, which differ in their mechanisms of host-seeking strategies. For oviparous parasites, Buchmann (1997) had previously reported that the hatched free-swimming oncomiracidia larvae could actively seek their fish host over short distances in water. However, most gyrodactylids are viviparous organisms and depend on limited modes of transmission through direct or indirect contact between fish. In the current study, the opisthaptoral hard parts of the collected G. cichlidarum were highly identical to those of G. cichlidarum reported by Garca-Vásquez et al. (2007). Based on morphometric parameters, G. cichlidarum have comparable morphological parameters in terms of approximate sizes but differ in having a smaller haptor width, longer anchor shaft, and smaller anchor point.
In recent years, significant deteriorations occurred in aquaculture’s ecological environment, leading to increasing fish diseases. Blood indices are one of the most valuable methods of diagnosing numerous pathological and ecotoxicological lesions affecting the health status of fish (Mansour et al. 2023; Abu‑Elala et al. 2023). Furthermore, measurements of various enzyme activities in fish can be used for monitoring any changes in water quality (Shahsavani et al. 2010).
Alanine aminotransferase (AST), a physiological element linked to the liver of Nile Tilapia, was evaluated in the current study in two groups, comprising healthy fish and sick fish. A notable increase in the activity of hepatic damage marker aspartate transaminase (AST) was reported in O. niloticus fish infested with G. cichlidarum compared to the control fish. Aspartate aminotransferase (AST) is a non-plasma-specific enzyme localized within the organ tissue cells, such as the liver, kidneys, heart, muscles, and gills. AST was previously reported to elevate following infections with fish pathogens (Shahsavani et al. 2010; Julinta et al. 2019). Elevation of AST serum activity may reflect acute liver parenchymal necrosis (Shahsavani et al. 2010; Liu et al. 2010; Manoj et al. 2010). Moreover, hepatic enzyme activities were reported to increase in response to elevated cortisol levels (Pfalzgraff et al. 2022).
Sera of the G. cichlidarum-infested fish revealed significantly higher cortisol concentrations than the control. Cortisol is a corticosteroid hormone and an effective stress marker in fish. When fish are subjected to stress, cortisol is released from the inter-renal cells in the head kidney to mobilize energy and cope with the stressors to restore homeostasis (Pfalzgraff et al. 2022; Roque d’orbcastel et al. 2021). The cortisol levels in the blood rise within minutes of the onset of stressors and drop within hours or days after cessation, so they reflect the current/recent environment (Ellis et al. 2012). Elevated plasma cortisol concentrations adversely impact aquaculture production traits, such as growth, food conversion, disease resistance, and reproduction (Roque d’orbcastel et al. 2021; Ellis et al. 2012).
Lysozyme activity was significantly elevated than normal in Gyrodactylus cichlidarum-infested fish due to the severe monogenea infestation. Lysozymes are critical leucocytic-derived mucolytic antimicrobial enzymes that play a significant role in fish’s innate immune system. The high increase in plasma lysozyme levels was related to infection and increased antibody titer in fish (Saurabh and Sahoo 2008; Attia et al. 2021a, b).
Kittilsen et al. (2009) stated that salmonid fish are known for producing eumelanin black pigment and that color polymorphism is displayed as characteristic black spots on the skin. Further, the more the fishes were melanised, the lower their physiological and behavioral reactions to stressors. Many studies have revealed the substantial effects of steroid hormones, such as corticosteroids, on behavioral and body traits through modulating the gene expressions and via other non-genomic receptors (Øverli et al. 2007; Koolhaas et al. 1999; Schoech et al. 2011). Krkošek et al. (2007) displayed the effect of exogenous cortisol in stimulating multiple behavioral attributes in the HR rainbow trout line. The above-mentioned worldwide reports coincide with our achieved elevated cortisol levels in black melanized Nile tilapias examined throughout the current study. Statistical data confirmed such an assumption through significant differences in cortisol levels between control and G. cichlidarum-affected Nile tilapias.
Additionally, steroids are significant immunity modulators (McEwen et al. 1997; Da Silva 1999), including those elements implicated in the defense against parasitic infestations (Klein 2004). Parasites may be a major selective force in determining the fitness of variable melanin morphs. One question that has not been investigated, is whether a positive relationship between parasite resistance and melanin pigmentation is present in the otherwise well-studied salmonid model system. These assumptions are in balanced accordance with sensitized local cellular (melano-macrophages) and chemical immune response posed by G. cichlidarum-infested Nile tilapias. Thus, we can conclude that the severe monogenean skin infestation, together with relatively large numbers of them in the biological filter, could have resulted in the expected luxurious excretion of metabolites which further triggered tilapia skin to induce a cascade of cellular recruitments to the affected site as well as floods of chemical mediators throughout the generalized blood circulation (McEwen et al. 1997; Da Silva 1999). Both cellular and chemical cascades are in an endless connection that assures the transient black coloration of skin while fish are in tank water (cortisol-mediated melanin bind macrophages activation) (Bertaglia et al. 2023).
Buchmann and Lindenstrøm (2002) concluded that Larval and adult stages could immediately pick suitable substrates and host factors, such as mucus which indicates the high sensitivity of monogeneans toward host components. No previous studies have relied on anatomical features alone in explaining host susceptibility. However, if the morphology and mechanisms of the parasite attachment would not conform to the architecture of the host epidermis, the parasite will dislodge (Cone and Wiles 1989). Hence, the opisthaptor is usually developed to fit its distinct habitat (Llewellyn and Owen 1960; Kearn and Macdonald 1976; Chisholm et al. 1998). After monogenean attachment to fish, an interaction occurs between the parasite’s epitopes and the host molecules. Further, the activity of host molecules could be affected by the surrounding conditions of the micro-environment, such as calcium and magnesium levels in water (Buchmann and Lindenstrøm 2002).
As a field trial to combat the actual etiology behind the black chromo-shift of tilapias’ skin and to stop the ongoing mortalities, a sequence of chemical parasiticidal medications have been efficiently applied to remarkably lower the intensity of the monogenean infestation, followed by a remarkable decline in mortalities. Successive daily application of copper sulfate for 1 week was efficient in eradicating a high percentage of the monogeneans (Ragab et al. 2022; Abu-Elala et al. 2013). Glutaraldehyde/QAC effectively eradicated the remaining low percentage of monogeneans with potential control of any probably concurrent bacterial or viral infections (Eissa et al. 2022; Ragab et al. 2022). As a restoration therapy for the exhausted dermal and systemic cell-mediated and humeral immune barriers, a supportive symbiotic (Saccharomyces cerevisiae) and immunostimulant (Vitamin C) (Eissa et al. 2022; Abu-Elala et al. 2013) was supplied into tank water with positive impacts on both fish health and skin normal color.
This infestation was considered not a case of direct parasitism but rather an indirect opportunistic colonization event triggered by several factors belonging to incompetent biosecurity at both the hatchery level and the target Nile tilapia RAS-controlled farm. The large populations of G. cichlidarum ova/larvae incidentally introduced to the fish tanks through the process of seed transfer from the hatchery to the affected farm tanks were not avoidable by the physical, mechanical and biological filters of the recirculating aquaculture system. Once the biological filter got saturated with huge numbers of the monogeneans, they freely swam through water to attach to the skin of reared Nile tilapias.
Conclusions
Eventually, a transient black skin chromo-shift was developed among the highly sensitized immune-responsive Nile tilapias that were chronically exposed to different stages of the monogenean and its metabolites throughout the production cycle. Fortunately, a triple parasiticidal/immune-enhancing treatment strategy was adopted to stop mortalities and restore the tilapias’ normal skin color with remarkable degrees of success.
Data availability
All data are included in the manuscript.
References
Abu-Elala N, Marzouk M, Moustafa M (2013) Use of different Saccharomyces cerevisiae biotic forms as immune-modulator and growth promoter for Oreochromis niloticus challenged with some fish pathogens. Int J Vet Sci 1(1):21–29
Abu-Elala NM, Khattabr MS, AbuBak HO, Helmy S, Hesham A, Younis NA, Dawood MAO, Basuini MEF (2023) Neem leaf powder (Azadirachta indica) mitigates oxidative stress and pathological alterations triggered by lead toxicity in Nile tilapia (Oreochromis niloticus). Sci Rep 13:9170
Ali SA (2013) Design and evaluate a drum screen filter driven by undershot waterwheel for aquaculture recirculating systems. Aquac Eng 54:38–44
Attia MM, Elgendy MY, Abdelsalam M, Hassan A, Prince A, Salaeh NM, Younis NA (2021a) Morpho-molecular identification of Heterophyes heterophyes encysted metacercariae and its immunological and histopathological effects on farmed Mugil cephalus in Egypt. Aquac Int 29(3):1393–1407
Attia MM, Abdelsalam M, Korany RMS, Mahdy OA (2021b) Characterization of digenetic trematodes infecting African catfish (Clarias gariepinus) based on integrated morphological, molecular, histopathological, and immunological examination. Parasitol Res 120(9):3149–3162. https://doi.org/10.1007/s00436-021-07257-x
Bertaglia EA, Furtado WE, Silva E Souza ÂT, Fernandes MC, Pereira SA, Brasil EM, Mouriño JLP, Jerônimo GT, Martins ML (2023) Influence of seasonality and culture stage of farmed Nile Tilapia (Oreochromis niloticus) with monogenean parasitic infection. Animals: Open Access J MDPI 13(9):1525. https://doi.org/10.3390/ani13091525
Blaylock RB, Bullard SA (2014) Counter-insurgents of the blue revolution? Parasites and diseases affecting aquaculture and science. J Parasitol 100(6):743–755
Buchmann K (1997) Infection biology of gill parasitic monogeneans with special reference to the congeners Pseudodactylogyrus bini and P. anguillae (Monogenea: Platyhelminthes) from European eel. Ph.D. Thesis. Center for Forest, Landscape and Planning, University of Copenhagen, Copenhagen, p 141
Buchmann K, Lindenstrøm T (2002) Interactions between monogenean parasites and their fish hosts. Int J Parasitol 32(3):309–319
Carbone D, Faggio C (2016) Importance of prebiotics in aquaculture as immunostimulants. Effects on immune system of Sparus aurata and Dicentrarchus labrax. Fish Shellfish Immunol 54:172–178
Chisholm LA, Whittington ID, Schokaert E (1998) Morphology and development of the haptors among the monocotylidae (Monogenea). Hydrobiologia 383:251–261
Cone DK, Wiles M (1989) Ultrastructural study of attachment of Gyrodactylus colemanensis (Monogenea) to fins of fry of Salmo gairdneri. Proc Helminthol Soc 56:29–32
Costa-Pierce BA, Rakocy JE (1997) Tilapia aquaculture in the Americas. World Aquaculture Society, Baton Rouge, LA, USA, p 258
Da Silva JAP (1999) Sex hormones and glucocorticoids: interactions with the immune system. Ann N Y Acad Sci 876(1):102–118
Dotta G, de Andrade JIA, Gonçalves ELT, Brum A, Mattos JJ, Maraschin M, Martins ML (2014) Leukocyte phagocytosis and lysozyme activity in Nile tilapia fed supplemented diet with natural extracts of propolis and Aloe barbadensis. Fish Shellfish Immunol 39(2):280–284
Ebeling JM, Timmons MB (2012) Recirculating aquaculture systems. Aquac Prod Syst, Wiley-Blackwell, pp 245–277
Eissa AE, Attia MM, Abdelsalam M, Elgendy MY, Abou-Okada M, Ismail GA, Younis NA (2022) Investigating the etiologies behind emergent mass mortalities of farmed Liza carinata juveniles from coastal farms at Damietta. Egypt Sci Rep 12(1):16123
Eissa AE, Attia MM, Elgendy MY, Ismail GA, Sabry NM, Prince A, Mahmoud MA, El-Demerdash GO, Abdelsalam M, Derwa HI (2021) Streptococcus, Centrocestus formosanus and Myxobolus tilapiae concurrent infections in farmed Nile tilapia (Oreochromis niloticus). Microb Pathog 158:105084
Ellis T, Yildiz HY, López-Olmeda J, Spedicato MT, Tort L, Øverli Ø, Martins CI (2012) Cortisol and finfish welfare. Fish Physiol Biochem 38:163–188
e Silva MLR, Pereira RT, Arvigo AL, Zanuzzo FS, Barreto RE (2020) Effects of water flow on ventilation rate and plasma cortisol in Nile tilapia introduced into novel environment. Aquac Rep 18:100531
García-Vásquez A, Hansen H (1995) Shinn AA (2007) Revised description of Gyrodactylus cichlidarum Paperna, 1968 (Gyrodactylidae) from the Nile tilapia, Oreochromis niloticus niloticus (Cichlidae), and its synonymy with G. niloticus Cone, Arthur et Bondad-Reantaso. Folia Parasitol 54(2):129–140
Harris PD, Shinn AP, Cable J, Bakke TA (2004) Nominal species of the genus Gyrodactylus von Nordmann 1832 (Monogenea: Gyrodactylidae), with a list of principal host species. Syst Parasitol 59:1–27
Haygood AM, Jha R (2018) Strategies to modulate the intestinal microbiota of Tilapia (Oreochromis sp.) in aquaculture: a review. Rev Aquac 10(2):320–333
Iyaji FO, Eyo JE (2008) Parasites and their freshwater fish host. Biol Res 6(1):328–338
Julinta RB, Abraham TJ, Roy A, Singha J, Boda S, Patil PK (2019) Dietary influences of oxytetracycline on the growth and serum biomarkers of Oreochromis niloticus (L.). Ecotoxicol Environ Saf 186:109752
Kearn GC, Macdonald S (1976) The chemical nature of host hatching factors in the monogenean skin parasites Entobdella soleae and Acanthocotyle lobianchi. Int J Parasitol 6:457–466
Kittilsen S, Schjolden J, Beitnes-Johansen I, Shaw JC, Pottinger TG, Sørensen C, Øverli Ø (2009) Melanin-based skin spots reflect stress responsiveness in salmonid fish. Horm Behav 56(3):292–298
Klein SL (2004) Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol 26(6–7):247–264
Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, Blokhuis HJ (1999) Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav 23(7):925–935
Krkosek M, Ford JS, Morton A, Lele S, Myers RA, Lewis MA (2007) Declining wild salmon populations in relation to parasites from farm salmon. Sci 318(5857):1772–1775
Liu B, Xie J, Ge X, Xu P, Wang A, He Y, Chen R (2010) Effects of anthraquinone extract from Rheum officinale Bail on the growth performance and physiological responses of Macrobrachium rosenbergii under high temperature stress. Fish Shellfish Immunol 29(1):49–57
Llewellyn J, Owen IL (1960) The attachment of the monogenean Discocotyle sagittata Leuckart to the gills of Salmo trutta L. Parasitology 50:51–59
Manoj CK, Nair CM, Patel MB, Salin KR (2010) Haematobiochemical and histopathological changes in Labeo rohita infected with Aeromonas hydrophila by immersion challenge. Fish Technol 47(2):151–160
Mansour AT, Eldessouki EA, Khalil RH, Diab AM, Selema TA, Younis NA, Abdel-Razek N (2023) In vitro and in vivo antifungal and immune stimulant activities of oregano and orange peel essential oils on Fusarium solani infection in whiteleg shrimp. Aquac Int 31:1959–1977
McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Weiss JM (1997) The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res Rev 23(1–2):79–133
Mousavi HE, Omidzahir S, Soltani M, Shayan P, Ebrahimzadeh E, Mousavi S, Hoseini M (2013) Morphometrical and molecular characterization of Gyrodactylus cichlidarum (Gyrodactylidae) from Astronotus ocellatus (Cichlidae) in Iran. Comp Clin Path 22:1093–1097
Øverli Ø, Sørensen C, Pulman KG, Pottinger TG, Korzan W, Summers CH, Nilsson GE (2007) Evolutionary background for stress-coping styles: relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci Biobehav Rev 31(3):396–412
Paperna I (1968) Monogenetic trematodes collected from fresh water fish in Ghana. Second report. Bamidgeh 20(2):88–99
Pedersen AB, Fenton A (2007) Emphasizing the ecology in parasite community ecology. Trends Ecol Evol 22(3):133–139
Pfalzgraff T, Lund I, Skov PV (2022) Prolonged cortisol elevation alters whole body and tissue metabolism in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol a: Mol Integr Physiol 263:111098
Ragab RH, Elgendy MY, Sabry NM, Sharaf MS, Attia MM, Korany RM, Eissa AE (2022) Mass kills in hatchery-reared European seabass (Dicentrarchus labrax) triggered by concomitant infections of Amyloodinium ocellatum and Vibrio alginolyticus. Int J Vet Sci 10(1):33–45
Roque d’obcastel E, Bettarel Y, Dellinger M, Sadoul B, Bouvier T, Amandé JM, Geffroy B (2021) Measuring cortisol in fish scales to study stress in wild tropical tuna. Environ Biol Fishes 104(6):725–732
Saurabh S, Sahoo PK (2008) Lysozyme: an important defence molecule of fish innate immune system. Aquac Res 39(3):223–239
Schoech SJ, Rensel MA, Heiss RS (2011) Short-and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: a review. Curr Zool 57(4):514–530
Sergaliyev NH, Absatirov GG, Tumenov AN, Sariyev BT, Ginayatov NS (2017) Nosological description of fish pathologies in RAS. J Pharm Sci Res 9(9):1637
Shahsavani D, Mohri M, GholipourKanani H (2010) Determination of normal values of some blood serum enzymes in Acipenser stellatus Pallas. Fish Physiol Biochem 36:39–43
Sugita H, Nakamura H, Shimada T (2005) Microbial communities associated with filter materials in recirculating aquaculture systems of freshwater fish. Aquac 243(1–4):403–409
Zago AC, Franceschini L, Garcia F, Schalch SH, Gozi KS, Silva RJD (2014) Ectoparasites of Nile tilapia (Oreochromis niloticus) in cage farming in a hydroelectric reservoir in Brazil. Rev Bras Parasitol Vet 23:171–178
Zhi T, Huang C, Sun R, Zheng Y, Chen J, Xu Yang T (2020) Mucosal immune response of Nile tilapia Oreochromis niloticus during Gyrodactylus cichlidarum infection. Fish Shellfish Immunol 106:21–27 (Zhi et al. 2020)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors equally contributed to the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was run in accordance with the guidelines instated by the Institutional Animal Care and Use Committee, Faculty of Veterinary Medicine, Cairo University, Egypt.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Amany Abbass
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eissa, A., Attia, M.M., El Zlitne, R.A. et al. The puzzling etiologies of transient black discoloration in Nile Tilapia (Oreochromis niloticus) intensively cultured under RAS system. Aquacult Int 32, 581–592 (2024). https://doi.org/10.1007/s10499-023-01328-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01328-9