Abstract
The objective of this study was to explore the effects of black seed (Nigella sativa) and AQUA-IMMUNOPROTECT® on growth, feed utilization, serum constituents, and disease resistance against vibriosis in gilthead sea bream juveniles. The research involved three groups: TC (control) received a basal diet, TSI (received a basal diet with 2% AQUA-IMMUNOPROTECT®), and TNS (received a basal diet with 2% N. sativa). The experiment period extended for 2 months. Results revealed a significant rise in erythrogram (RBCs, HB, and PCV %), leucogram (total differential leucocytic count), serum total protein, and globulin in gilthead sea bream treated with 2% Nigella sativa supplemented diets after an 8-week trial. No substantial variations were found in liver enzymes, urea, uric acid, and creatinine between the groups. Respiratory burst activity was notably greater in the N. sativa group after 2 months of feeding compared to the other groups. In the experimental challenge, the control group experienced higher mortality rates than the other groups following an IP injection of V. harveyi at a dose of 106 CFU per 0.5 ml/fish (106 fish/challenge). Overall, the findings of this research indicate that N. sativa can enhance non-specific immunity and minimize susceptibility and pathogenicity to V. harveyi in gilthead sea bream.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture is a crucial global industry that provides essential food for the world’s population and a valuable source of animal protein. In recent times, Egypt has made significant progress in this sector and has experienced the strongest growth in fisheries-related activities in the country. As such, aquaculture is seen as the only practical solution to reducing the current gap between fish production and consumption in Egypt (Soliman and Yacout, 2016). Egypt leads African countries in aquaculture production, with Nigeria employing similar production systems. The presence of water bodies, institutional commitment, and high demand for fish make this industry very promising in these two countries (Kaleem and Sabi, 2020).
In 2018, the total value of aquaculture production and consumption, including aquatic plants, was estimated at USD 263 billion, with a total output of 114.5 million tons worldwide. This production included farmed aquatic animals, aquatic plants, and non-food products. Marine fish were the largest group of species produced that year, accounting for 52% of the total, followed by freshwater fish (32%), molluscs (13%), and crustaceans (9%), according to the Fishery and Aquaculture Statistics report in 2020.
Sparus aurata, commonly known as gilthead sea bream, is a highly sought-after food fish species. It is estimated that the total production of breams worldwide is 191,500 tons, with gilthead sea bream accounting for nearly 18% of the total production (FAO, 2018). Gilthead sea bream (Sparus aurata) is a sought-after porgy species prized for its culinary appeal and significant economic value. Predominantly, this species is sourced from intensive cultivation systems. Egypt has elevated its status as a major gilthead sea bream producer through national mariculture mega projects (Mehanna, 2007 & FAO 2018). While intensification offers substantial economic benefits, they also raise the likelihood of disease outbreaks (Barton & Fløysand 2010). The aquaculture sector is grappling with considerable global financial losses, reaching billions of US dollars annually, attributed to infectious diseases (Priya & Kappalli 2022). Disease outbreaks are a significant hurdle in aquaculture, resulting in economic losses and inadequate supply of fish and fish products. Bacterial infections, which are often introduced through water sources and diets, cause high mortality and morbidity rates, particularly during the summer season and in hatcheries (Enany et al., 2019; Jamabo et al., 2019). According to Mancuso et al. (2015), Vibrionaceae are a well-known threat to wild and farmed marine fish worldwide, inflicting significant financial losses in the aquaculture business. In accordance with Nor-Amalina et al. (2017), the most prevalent species that infect farmed aquatic animals are V. parahaemolyticus, V. alginolyticus, V. harveyi, V. owensii, and V. campbellii.
Antibiotics were once widely used to treat bacterial infections in farmed fish. However, antibiotics are becoming less applicable in aquaculture due to bacterial resistance, antibiotic residues in edible fish species, and potential environmental risks. As a result, attention has been focused on producing low-cost feed additives as an alternative to existing techniques (Burridge et al., 2010; Wamala et al., 2018; Antonelli et al., 2019).
Medicinal plant-derived immunostimulants have been proven to improve immune responses and disease resistance against Vibrio infections in many aquatic species (Awad and Awaad, 2017; Stratev et al., 2018 & Munaeni et al., 2020), promoting a sustainable and eco-friendly approach to control aquaculture diseases outbreaks. Furthermore, they are cost-effective simple strategy, and when applied for a long period of time, they do not contaminate the surrounding ecosystem (Citarasu, 2010).
Thus, this study aimed to analyze the impact of different natural and synthetic immunostimulants on the survival, growth, immune response, and resistance of cultured sea bream against Vibrio species infections. The research had the following objectives: first, to examine the inhibitory effects of certain natural and synthetic immunostimulants on selected pathogenic Vibrio species through in vitro experiments and second, to assess the effects of chosen natural and synthetic immunostimulants on the cultured sea bream’s including survival, growth, immune response, and resistance to pathogenic Vibrio species infections through in vivo experiments.
Materials and methods
In vitro antimicrobial sensitivity of the used immunostimulants
The antibacterial activity of natural and synthetic immunostimulants, Nigella sativa and AQUA-IMMUNOPROTECT® powders, against a pathogenic Vibrio harveyi strain was assessed using the agar well diffusion method (Valgas et al., 2007) as follows: 100 μl from a young broth culture of pathogenic Vibrio harveyi (with a cell density of 106 CFU/ml based on the McFarland turbidity standard) was spread over Muller Hinton agar plates using a sterile cotton swab. Three wells were made carefully on each plate, and 100 μl of 2% of each immunostimulant suspension was pipetted to each individual well separately. The plates were incubated overnight at 37 °C then examined for clearance around the wells. The examined immunostimulants, water and ethanolic suspensions, were done in triplicate, and DMSO and distilled water were used as control negatives for ethanolic and water extracts, respectively (Gonelimali et al., 2018). A clear zone around the disc (the inhibitory zone) confirms the antibacterial activity of the studied agents against the pathogenic Vibrio strain.
Experimental design
Juvenile gilthead sea bream (Sparus auratus), comprising a cohort of 60 fish with an average weight of approximately 60 ± 13 g, were sourced from a nearby fish farm. Fish were then transported to the Fish Farming and Technology Institute at Suez Canal University where subjected to acclimatization within aquaria with a capacity of 180 L, containing natural seawater. During 2 weeks of acclimation, fish fed basal diet and water temperature, fish behavior, appetite, as well as disease signs or mortality were monitored daily.
Afterward, the fish were randomly allocated into three distinct experimental groups (Table 1). Within each group, they were further divided into four equivalent replicates, with each replicate comprising 5 fish (20 fish/group in 4 replicates). The first group (TC) received a natural diet devoid of any additives. In the second group (TSI), the diet was supplemented with synthetic immunostimulants (AQUA-IMMUNOPROTECT®, Canal Aqua cure, Egypt), integrated at a proportion of 2% of the diet. Meanwhile, the third group (TNS) was administered a basal diet containing Nigella sativa, incorporated at a ratio of 2% of the diet. The fish were fed a commercial diet (3% of body weight and was served twice a day) supplied by Aller Aqua, Egypt®, with 60% protein and kept under constant 12-h light and 12-h dark conditions throughout the experiment period. The seawater temperature was maintained at 25–28 °C and within 36–40 ppt salinity.
Diet preparation
Synthetic pellets for feeding sea bream, provided by Aller Aqua Company®, Egypt, were processed by crushing. These crushed pellets were then mixed with supplements including either AQUA-IMMUNOPROTECT® or Nigella sativa powders both at a concentration of 2% per kilogram following the methodology of Latif et al. (2020). The resulting blend underwent a transformation into 3-mm pellets in Clinical Feeding and Food Department, Faculty of Veterinary Medicine, Suez Canal University (Table 2). Subsequently, the pellets were left to air-dry for a span of 24 h and subsequently stored in a refrigerator at 4 °C for daily dispensation, in accordance with Aly et al. (2019).
Measurement of growth parameters
At the beginning of the feeding experiment, all fish groups were initially weighted (initial weight, IW) then reweighted again each 2 weeks to adjust the required feed according to the gained weight as well as at the end of the experiment (final weight, FW). All growth parameters values were recorded along the experimental period (2 months) to calculate the growth performance parameters by the following equations:
(where Ln = the natural log, IW = initial fish weight, FW = the final fish weight in grams, and t = period in days).
Survival% = 100 × (initial fish number dead fish number)∕(initial fish number).
Blood and serum samples collection
Three fish from each replicate were collected and anesthetized using clove oil (12.5 mg/L) (Awad et al. 2022). Blood was drawn from the caudal vein using sterile syringe (23 Gag needles). The first portion of collected blood was immediately transferred to a test tube that contained 20 μL EDTA solution to act as an anticoagulant. The tube was shaken gently to avoid hemolysis of blood cells.
The other portion was for serum assays that were collected without anticoagulant. The blood was allowed to clot for 2 h and then centrifuged at 5000 x rpm for 5 min. After that, the serum was collected with a micropipette. These procedures were carried out according to the method described by Blaxhall and Daisley (1973).
Hematological and immunological parameters evaluation
Total erythrocytes and leukocytes count
Whole blood samples were diluted with the Natt and Herrick diluting reagent to be used for counting total erythrocytes (106/μL−1) and total leukocytes (103/μL−1) according to the method described by Natt and Herrick (1952). Additionally, the relative percent of different leukocytes was determined using the scheme from Schaperclaus (1992), by staining blood film with Gimsa stain. The percentage of different types of leukocytes was calculated after counting 100 white blood cells.
Nitroblue tetrazolium reduction test (NBT)
The respiratory burst activity of neutrophils and monocytes was measured by Nitro Blue Tetrazolium (NBT, Sigma-Aldrich Chemical, St. Louis, MO, USA) (Flückiger et al. 1988) following these steps; 0.1 ml of 0.2% NBT solution was added to an equal volume of whole blood in a microtiter plate. After that, the mixture was incubated at room temperature for 30 min. Following incubation, 0.05 ml of the NBT blood cell suspension was transferred to a glass tube containing 1 ml of N-dimethylformamide and centrifuged for 5 min at 3000 rpm. The supernatant optical density was measured using a spectrophotometer at 620 nm in 1-ml cuvettes (Baehner and Nathan, 1968).
Serum lysozyme activity and specific antibodies titer
Serum lysozyme activity of experimented gilthead sea bream groups was analyzed by the method described by Möck and Peters (1990). A 50 μL of serum sample was added to a 2 mL bacterial suspension, and the reduction in absorbance at 540 nm was measured after incubating the mixture at 22 °C for 0.5 and 4.5 min. The standard was chicken egg lysozyme from the Sigma brand, and the substrate used was 0.2 mg/mL of lyophilized Micrococcus lysodeikticus in 0.04 M sodium phosphate buffer with a pH of 5.75. Furthermore, the specific antibody titers were evaluated (Baba et al. 1988).
Serum biochemical assay
The serum levels of total proteins, aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum creatinine, and uric acid were measured using a commercially available diagnostic kit (Bioanalytic Diagnostic Industry, Co.).
Experimental infection
Bacterial culture preparation
A young culture of pathogenic V. harveyi was obtained by overnight incubation on tryptic soy agar at 28 °C as per Nur and Munaeni (2020), then suspended in sterile PBS, and washed several times, and then bacterial pellets resuspended and adjusted spectrophotometrically to reach a final concentration of 106 CFU ml−1 as described by Thompson et al. (2003).
Challenge test
At the end of the eighth week of the experiment, 10 fish from each experimented group were anesthetized with clove oil solution and then intraperitoneally infected by injecting 0.5 ml (106 CFU per ml/fish) of V. harveyi bacterial suspension that had been previously prepared. The challenged fish were monitored daily for 2 weeks to observe any disease signs, and mortality was also recorded to calculate the relative level of protection (RLP) as follows (Ruangroupan et al. 1986): RLP % = 1−(% stimulated mortality ÷ percent mortality in the control group) × 100.
Statistical analysis
The study conducted analysis of variance (ANOVA) and used Duncan’s Multiple Range Test (Duncan and Duncan, 1955) to identify differences between treatments (with means tested at a significance level of P < 0.05). Ordinary errors were also evaluated. The statistical analysis was performed using the SAS package (Spiegelman and Hertzmark, 2005).
Scientific Research Ethics Committee on animal researches
All procedures used in the present study were approved by the Scientific Research Ethics Committee on Animal Research, Faculty of Veterinary Medicine, Suez Canal University, Egypt, code: 2023015.
Results
In vitro antimicrobial activity assay of both natural and synthetic immunostimulants
The antimicrobial sensitivity of natural and synthetic immunostimulants, Nigella sativa and AQUA-IMMUNOPROTECT®, against V. harveyi strain was evaluated in vitro, and a clear zone of inhibitions was clearly noticed around both immunostimulants. However, a large measured zone was observed around N. sativa (14 mm), indicating that it had a greater antimicrobial effect than the synthetic immunostimulants products (12 mm).
Experimental studies
Growth performance and Survival rate
Body weight gain
Following the first month of the feeding experiment, the TNS group which was given natural immunostimulants (Nigella sativa) experienced a considerable increase in body weight gain compared to the TSI group which showed a minor increase in compare with the TC control group. Moreover, after, the second months of the experiment, the TNS group had a noticeable increase in body weight gain in comparison with the other groups, as evidenced by the data presented in Table 3.
Survival rate
Table 3 also shows that during the first and second month of the experiment, there was a significant increase in the survival rate of the experimental fish in the two groups (TNS and TSI) that were fed with synthetic and natural immunostimulants, respectively, compared to the TC group.
Feed conversion rate (FCR) and specific growth rate (SGR)
In the first month of the feeding trial, the TNS group treated with N. sativa had a notable increase in FCR, while the TSI group had a minor increase compared to the control group. After 2 months, both treated groups with N. sativa and AQUA-IMMUNOPROTECT® had a significant increase in FCR in comparison to the control group. Additionally, the specific growth rate was significantly higher in all treated groups compared to the control group in both the first and second months of the experiment, as presented in Table 4.
Erythrogram and leucogram
A noteworthy increase in RBCs, Hb, and PCV % was noticed among TNS and TSI groups while there were no significant differences observed in the control group. Total leucocytic and lymphocytic counts demonstrated a significant increase in (TNS and TSI), whereas the control group showed a minor increase, as indicated in Table 5.
Immunological parameter
Nitroblue tetrazolium reduction assay (NBT)
Table 6 presents the results of NBT values, where after 1 month of the experiment, there was a significant increase in groups TSI and TNS that received AQUA-IMMUNOPROTECT® and N. sativa, respectively, compared to the control group. However, the TSI group that received AQUA-IMMUNOPROTECT® showed a less significant increase than the control. After 2 months, the NBT values were significantly higher in the TNS group that received Nigella sativa compared to the other groups.
Serum lysozyme activity and specific antibodies titer
One month of the experiment, both treated groups experienced a noteworthy increase in lysozyme activity in comparison to the control group. Two months later, the TNS group 3 that received N. sativa had a significant increase in lysozyme activity compared to the control group. The remaining group (TSI group) had relatively lower values of increased lysozyme activity in comparison to the TNS group, as illustrated in Table 6.
Specific immunoglobulin titer among examined gilthead sea bream along the experimental period revealed significant upregulation in antibodies titer within the TNS group followed by the TSI group when compared with the control group as presented in Table 6.
Serum biochemical parameters
There was a significant increase in total protein in TSI and TNS groups while globulins levels increased significantly in the TNS group. On the other hand, no significant changes in liver (AST and ALT) and kidney (urea, uric acid, and creatinine) biomarkers among all experimental groups were recorded Table 7.
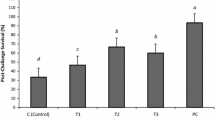
Mortality and relative level protection (RLP) of gilthead sea bream after bacterial challenge with V. harveyi
The mortality percentage of the challenged gilthead sea bream over a 2-week period of observation was 80% in the control group, while the two treated groups (TSI and TNS) with synthetic and natural immunostimulants had mortality rates of 40% and 20%, respectively. The RLP in the groups treated with Aqua-protect and N. Sativa was 50% and 75%, respectively, as shown in Table 8.
Discussion
Medicinal plants have been recognized as immunostimulants for thousands of years. The application of medicinal plants as natural and harmless compounds has potential in aquaculture as an alternative to antibiotics and immune prophylactics. The usage of these plants has increased world-wide because they are easy to prepare, cheap, and have few side effects on animals and the environment. A wide range of medicinal plants such as herbs, spices, seaweeds, herbal medicines, herbal extracted compounds, traditional Chinese medicines, and commercial plant-derived products has been studied in various aquatic animals. The whole plant or its parts, viz., roots, leaves, seeds, flowers or extract compounds, can be used. The extraction process is simple, with ethanol and methanol being commonly used. These herbs can be either single or in combination or even in a mixture with other immunostimulants, through water routine or feed additives and enrichment, where single administrations are as practical as combinations. There are variations in dosages and duration of time and the optimal levels have not been measured. Medicinal plants display their main assets as growth promoters, immune enhancers, where they act as antibacterial and antiviral agents to the host immune system. Inappropriately, the mechanisms are not fully understood (Van Hai, 2015).
Nowadays, natural immunostimulants are highly valued and have been confirmed to be effective for various plants, as stated by Tafalla et al. (2013). The Arab community has strong faith in the healing properties of Nigella sativa for treating multiple ailments and refers to it by different names such as Habbat Albarakah, Alhabahat Alsawda, and Alkamoun Alaswad (Aljawezjjah, 2001). It is also known as Shuniz and Khodhira and is commonly referred to as Black Cumin or Black Caraway in English (El-Tahir and Bakeet, 2006).
Nigella sativa has been traditionally used for various health problems related to respiratory, gastrointestinal, renal, hepatic, circulatory and for immune system functions, and overall well-being (Dey et al., 2020b). Several biological effects have been attributed to N. sativa or its active ingredients such as thymoquinone and nigellone, which include antiinflammatory, antinociceptive, anticancer, antibacterial, and fungicidal properties (Khan et al., 2003; Parvardeh and Fatehi, 2003; Diab et al., 2008; Randhawa et al., 2013; Dey et al., 2020b). Furthermore, N. sativa has been shown to have immunomodulatory effects and positive effects on the immune system of some fish species (Diab et al., 2008; Bektaş et al., 2019; Nur and Munaeni, 2020).
Vibriosis is a serious ailment that impacts numerous fish species, causing significant economic losses in the aquaculture industry (Austin, 2010). The disease typically spreads when fish are under stress or have a weakened immune system due to overcrowding, elevated temperatures, high salinity, and excessive organic matter in the water (Aldebasi et al., 2013). Vibriosis is commonly characterized by hemorrhagic septicemia, which results in extensive skin lesions and focal necrosis of vital organs such as the liver, spleen, and kidneys (Al-Taee et al., 2017).
Vibriosis outbreaks have caused significant declines in aquaculture production and resulted in additional costs to reach market size. Infections caused by Vibrio spp. can result in visible external lesions that reduce marketability. Affected fish often exhibit lethargic movement and various skin ulcerations. These symptoms make it challenging to maintain healthy populations of fish and can lead to economic losses (Aly, 2013a,b; Austin and Austin, 2016; Mohamad et al., 2019; Xie et al., 2020; de Souza Valente and Wan, 2021).
Our study employed the agar well diffusion method to determine the antimicrobial activity of the tested substances. The inhibition zone produced by the first substance (AQUA-IMMUNOPROTECT®) was 12 mm, while that of the second substance (N. sativa) was 14 mm against the pathogenic V. harveyi. Similar results were reported by Aly et al. (2019), who found that catfish vaccinated and supplemented with 3% N. sativa exhibited the highest relative level of protection (95%), followed by the vaccinated group (85%), then the supplemented group (55%). These findings suggest that N. sativa (3 gm/100 gm diet) can enhance the immune response and resistance of catfish to A. hydrophila, making it a potentially valuable supplement in aquaculture.
The diet used in the study was prepared by crushing a commercial fish feed and adding the tested substances, which had also been crushed, to make up 2% of the total ration. The mixture was then repelleted using a pelleting machine in the feeding department of Suez Canal University and stored in a refrigerator until feeding. This approach has been previously employed in other studies, including those conducted by Raval et al. (2010), .Aly et al. (2019), and Bektaş et al. (2019).
The study conducted found a noteworthy increase in the body weight of fish treated with N. sativa supplemented diet in comparison to other treated groups and control group after 1 and 2 months. Additionally, the FCR and SGR of all treated groups were higher than that of the control group in the first and second months. These results were consistent with the findings of Abdelkhalek et al. (2008) and Awad et al. (2013), who observed that the body weight gain and survival rate of sea bream significantly increased after a feeding experiment of 30 to 60 days with N. Sativa, either synthetic immunostimulants.
In this study, the effect of N. sativa powder supplementation on body weight was investigated over a period of 30 and 60 days. Although there was a difference in body weight after adding 20 g/kg, it was not statistically significant until the 60th day of feeding. These findings differ from previous studies that used methanolic extracts of N. sativa at rates of 0.1 and 0.5 g/kg, where significant improvements in growth performance and immunity of fish and a decrease in FCR were reported (Altunoglu et al., 2017; Niroomand et al., 2019). Furthermore, the use of black cumin seeds has been shown to enhance growth and immunity in fish and reduce FCR in other studies (John et al., 2007; Diab et al., 2008; Dorucu et al., 2009; Al-Ngada et al., 2017;).
The present study showed a significant increase in the overall erythrogram and predictable blood indices, including RBCs, Hb, HCT%, MCV, MCH, and MCHC, in the group treated with N. sativa powder compared to the control group. There was a slight increase in the group treated with synthetic immunostimulants. Similarly, Bektaş et al. (2019) reported significant differences in blood parameters among rainbow trout groups fed with N. sativa powder at various doses for different feeding periods. These parameters included WBC, LYM, MID, GRAN, RBC, HGB, HCT, MCV, MCH, MCHC, RDW-SD, RDW-CV, PLT, MPV, and PDW. The group fed with 20.0 g/kg doses of N. sativa showed a higher increase in these parameters compared to other groups.
Additionally, the current study measured various immunological parameters including NBT, lysozyme activity, leucogram, and serum immunoglobulins (IgG & IgM). After 1 month of the experiment, both the groups that received N. sativa and AQUA-IMMUNOPROTECT® showed a significant increase in NBT and lysozyme activity compared to the control group. After 2 months, the group that received N. sativa showed a significant increase in lysozyme activity compared to the control group. These findings suggest that N. sativa as a herbal immunostimulant can help increase the immunity of the fish being studied. This is supported by previous research (Dorucu et al. 2009) which found that using herbal additives, particularly Nigella sativa, significantly increases immunological parameters in sea bream including the NBT assay, neutrophil adherence, and lysozyme activity as well as serum bactericidal activity.
In vertebrates, serum protein and immunoglobulin play a crucial role in the humoral immune system (Salem, 2005). The rise in serum total protein and globulin levels observed in catfish that received Nigella sativa supplementation was consistent with the findings in previous catfish studies (Aly et al., 2005). The results showed a noteworthy increase in total protein levels in TSI and TNS groups, while the globulins levels rose significantly in TNS compared to TSI. In contrast, no significant changes were found in liver enzymes, urea, uric acid, and creatinine levels among all experimental groups. This study did not find any noteworthy alterations in liver enzymes and kidney function, which is consistent with the findings of previous studies (Aly et al., 2019) and Latif et al., 2020).
Following 2 months, the experimental fish were injected with 0.5 ml of pathogenic strain of V. harveyi. The mortality rate of the control group was 80%, while the two groups treated with synthetic and natural immunostimulants (TSI and TNS) had mortality rates of 40% and 20%, respectively. The RLP was 50% and 75% in the groups treated with AQUA-IMMUNOPROTECT® and N. sativa, respectively. These findings are consistent with those of Harikrishnan et al. (2011), who observed mortality rates of 40%, 20%, and 30% in fish fed with diets supplemented with 2.0%, 4.0%, and 6.0% for 30 days against P. fluorescens, with the 4.0% dose showing the highest relative percentage survivability (85.7%). The highest mortality rate of 70% was observed in fish fed with the control diet (0% supplementation), and the survival rate was highest in the 4% dose.
According to the findings of this research, Nigella sativa can improve certain non-specific immune parameters in sea bream. However, additional studies are necessary to determine the best dose, potential effects when used with other immunostimulants, and the duration of treatment. Overall, this study implies that herbal remedies may be utilized to enhance the immune system of sea bream. A study by Fadeifard et al. (2018) found that Nigella sativa, Zingiber officinale, and Echinacea angustifolia essential oils can enhance some non-specific immune parameters in rainbow trout. Nevertheless, further research is needed to determine the optimum dose, effects when combined with other immunostimulants, and treatment duration. Overall, this research suggests that herbal essential oils may be utilized to improve the immune system of rainbow trout.
The use of natural compounds as immunostimulants in aquaculture has become an area of increasing interest. Three studies explored the effects of black cumin seed crude extract (BCSO), nettle extract, Quercetin, and N. sativa oil as immunostimulants in fish feed. Nur and Munaeni (2020) found that adding 7500 ppm of BCSO to shrimp feed increased the survival of vannamei shrimp and inhibited bacteria growth, but further studies are needed to determine optimal extraction methods and long-term administration at lower concentrations. Dey et al. (2020a) demonstrated that 2% BCSO in fish feed improved the survival of Nile tilapia by enhancing their innate immunity, while Awad et al. (2013) showed that using nettle extract, Quercetin, and N. sativa oil in rainbow trout feed enhanced their innate and adaptive immune responses. These studies suggest that natural compounds can act as promising immunomodulatory components in fish feed to help fish resist disease.
This study emphasizes the negative effects of antibiotics on fish immunity and the development of immunity from the Vibrio microbe against the antibiotic. To address this issue, the use of natural herbs as an alternative to boosting immunity is proposed. Nigella sativa is a highly immune-boosting herb that is antibacterial, antiviral, and antioxidant and improves the efficiency of blood and immune cells in the body. In addition, AQUA-IMMUNOPROTECT®, an industrial product of natural compounds and herbs, has been shown to have similar activity to the Nigella sativa seed and can increase the ability of fish to withstand pressure.
To control infections and minimize antimicrobial use in fish farms, strict veterinary hygienic regulations should be implemented. Appropriate biosecurity and health management practices, including strong vaccination programs, are necessary to support the aquaculture industry. The use of immunostimulants should be prioritized over antibiotics to avoid their harmful effects and the development of microbial resistance. In conclusion, prevention is better than cure, and the combination of Nigella sativa can be used as an immune booster to give fish the strength to overcome stress and be more resistant to diseases.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Abdelkhalek, N. K., Zaki, V. H. & Yousef, M. (2008) Effect of some immunostimulants on health status and disease resistance of Nile tilapia (Oreochromis niloticus). 8th International Symposium on Tilapia in Aquaculture, 2008. 1037-1088.
Aldebasi YH, Aly SM, Ahmad MI, Khan AA (2013) Incidence and risk factors of bacteria causing infectious keratitis. Saudi Med J 34:1156–1160
Aljawezjjah A (2001) Study and comment: Ammar Zaki Albaroodi, Eygypt, pp 291–293 Alttawfeeqiah library
Al-Ngada R, Abdelwahab A, El-Bahr S (2017) Effect of dietary supplementation of green tea (Camellia sinensis) on growth, body composition and serum biochemistry of the Asian seabass, Lates calcarifer fingerlings. J Aquacult Res Develop 8:518–522
Al-Taee A, Khamees N, Al-Shammari N (2017) Vibrio species isolated from farmed fish in Basra city in Iraq. Aquacult Res Develop 8:1–4
Altunoglu YC, Bilen S, Ulu F, Biswas G (2017) Immune responses to methanolic extract of black cumin (Nigella sativa) in rainbow trout (Oncorhynchus mykiss). Fish shellfish immunol 67:103–109
Aly S, Abeer S, Awad M (2005) A new systematic approach for water network design. Clean Technol Environ Policy 7:154–161
Aly, S. M. (2013a) A review of fish diseases in the Egyptian aquaculture sector: Working report
Aly SM (2013b) Risk of antimicrobial misuse. Int J Health Sci 7(1):V–VI Qassim University
Aly, S M., M Ismail, M., Fathi, M. & A Al Zohairy, M. (2019) The Role of Nigella sativa in improving the immune response of the African Catfish (Clarias gariepinus) to Aeromonas hydrophila Vaccine. Egyptian J Aquatic Biol Fisher, 23, 373-384.
Antonelli P, Belluco S, Mancin M, Losasso C, Ricci A (2019) Genes conferring resistance to critically important antimicrobials in Salmonella enterica isolated from animals and food: a systematic review of the literature, 2013–2017. Res veterin sci 126:59–67
Austin B (2010) Vibrios as causal agents of zoonoses. Veterin microbiol 140:310–317
Austin B, Austin DA (2016) Bacterial fish pathogens: disease of farmed and wild fish. Springer
Awad E, Austin D, Lyndon AR (2013) Effect of black cumin seed oil (Nigella sativa) and nettle extract (Quercetin) on enhancement of immunity in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquacult 388:193–197
Awad E, Awaad A (2017) Role of medicinal plants on growth performance and immune status in fish. Fish shellfish immunol 67:40–54
Awad LZ, El-Mahallawy HS, Abdelnaeim NS, Mahmoud MM, Dessouki AA, ElBanna NI (2022) Role of dietary Spirulina platensis and betaine supplementation on growth, hematological, serum biochemical parameters, antioxidant status, immune responses, and disease resistance in Nile tilapia. Fish Shellfish Immunol 126:122–130
Baba T, Masumoto K, Nishida S, Kajikawa T, Mitsui M (1988) Harderian gland dependency of immunoglobulin A production in the lacrimal fluid of chicken. Immunology 65(1):67
Baehner RL, Nathan DG (1968) Quantitative Nitroblue tetrazolium test in chronic granulomatous disease. New England J Med 278:971–976
Barton JR, Fløysand A (2010) The political ecology of Chilean salmon aquaculture, 1982–2010: a trajectory from economic development to global sustainability. Global Environ Change 20(4):739–752
Bektaş ZH, Savaşer S, Akcimen U, Ceylan M, Yener O, Bulut C (2019) Using of black cumin seed powder (Nigella sativa) as immunostimulant and growth promoter in rainbow trout, Oncorhynchus mykiss (Walbaum). Turkish J Fisher Aquatic Sci 19:987–999
Blaxhall P, Daisley K (1973) Routine haematological methods for use with fish blood. J fish biol 5:771–781
Burridge L, Weis JS, Cabello F, Pizarro J, Bostick K (2010) Chemical use in salmon aquaculture: a review of current practices and possible environmental effects. Aquacult 306:7–23
Citarasu T (2010) Herbal biomedicines: a new opportunity for aquaculture industry. Aquacult Int 18:403–414
De Souza Valente C, Wan AH (2021) Vibrio and major commercially important vibriosis diseases in decapod crustaceans. J Inverteb Pathol 181:107527
Dey BK, Hossain M, Mosharraf M, Alam M (2020a) Effect of black cumin seed oil on growth, innate immunity and resistance against Pseudomonas fluorescens infection in Nile tilapia Oreochromis niloticus. Aquacult Int 28:1485–1499
Dey BK, Hossain MMM, Alam ME (2020b) Effect of black cumin seed oil on growth, innate immunity and resistance against Pseudomonas fluorescens infection in Nile tilapia Oreochromis niloticus. Aquacult Int:1–15
Diab AS, Aly SM, John G, Abde-Hadi Y, Mohammed MF (2008) Effect of garlic, black seed and Biogen as immunostimulants on the growth and survival of Nile tilapia, Oreochromis niloticus (Teleostei: Cichlidae), and their response to artificial infection with Pseudomonas fluorescens. African J Aquatic Sci 33:63–68
Dorucu M, Ispir U, Colak S, Altinterim B, Celayir Y (2009) The effect of black cumin seeds, Nigella sativa, on the immune response of rainbow trout, Oncorhynchus mykiss. Mediterran Aquacult J 2:27–33
Duncan OD, Duncan B (1955) A methodological analysis of segregation indexes. Am Sociol Rev 20:210–217
El-Tahir KE-DH, Bakeet DM (2006) The black seed Nigella sativa Linnaeus-A mine for multi cures: a plea for urgent clinical evaluation of its volatile oil. J Taibah University Med Sci 1:1–19
Enany M, Eidaroos N, Eltamimy N (2019) Microbial causes of summer mortality in farmed fish in Egypt Suez Canal Veterinary Medical. J SCVMJ 24:45–56
Fadeifard F, Raissy M, Jafarian M, Boroujeni HR, Rahimi M, Faghani M (2018) Effects of black seed (Nigella sativa), ginger (Zingiber officinale) and cone flower (Echinacea angustifolia) on the immune system of rainbow trout, Oncorhynchus mykiss. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 70:199–204
FAO (2018) Globefish Highlights, a quarterly update on world seafood markets. Food Agricult Organ United Nations 2:1–70
Flückiger R, Woodtli T, Gallop PM (1988) The interaction of aminogroups with pyrroloquinoline quinone as detected by the reduction of nitroblue tetrazolium. Biochem Biophys Res Commun 153(1):353–358
Gonelimali FD, Lin J, Miao W, Xuan J, Charles F, Chen M, Hatab SR (2018) Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front microbiol 9:1639
Harikrishnan R, Balasundaram C, Heo M-S (2011) Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquacult 317:1–15
Jamabo N, Ukwe I, Amachree D (2019) Growth assessment and microbial flora presence in African catfish (Clarias gariepinus) larvae fed live and commercial feeds. Int J Sci 8:1–6
John, G., Mesalhy, S., Rezk, M., El-Naggar, G. & Fathi, M. (2007) Effect of some immunostimulants as feed additives on the survival and growth performance of Nile tilapia, Oreochromis niloticus and their response to artificial infection.
Kaleem O, Sabi A-FBS (2020) Overview of aquaculture systems in Egypt and Nigeria, prospects, potentials, and constraints. Aquacult Fisher
Khan M, Ashfaq M, Zuberi H, Mahmood M, Gilani A (2003) The in vivo antifungal activity of the aqueous extract from Nigella sativa seeds. Phytotherapy Res: an Int J Devoted to Pharmacol Toxicol Evaluat Natural Product Derivat 17:183–186
Latif M, Faheem M, Hoseinifar SH, Van Doan H (2020) Dietary black seed effects on growth performance, proximate composition, antioxidant and histo-biochemical parameters of a culturable fish, rohu (Labeo rohita). Animals 11:48
Mancuso M, Genovese M, Guerrera MC, Casella G, Genovese L, Piccolo G, Maricchiolo G (2015) First episode of vibriosis in wild specimens of Pagellus bogaraveo (Brünnich, 1768) in the Mediterranean Sea. Cah Biol Mar 56:355–361
Mehanna SF (2007) A preliminary assessment and management of gilthead bream Sparus aurata in the Port Said fishery, the Southeastern Mediterranean, Egypt. Turkish J Fisher Aquatic Sci 7(2)
Möck A, PETERS G (1990) Lysozyme activity in rainbow trout, Oncorhynchus mykiss (Walbaum), stressed by handling, transport and water pollution. J Fish Biol 37:873–885
Mohamad N, Amal MNA, Yasin ISM, Saad MZ, Nasruddin NS, Al-Saari N, Mino S, Sawabe T (2019) Vibriosis in cultured marine fishes: a review. Aquacult 512:734289
MUNAENI W, YUHANA M, SETIAWATI M, WAHYUDI AT (2020) Effect in white shrimp Litopenaeus vannamei of Eleutherine bulbosa (Mill.) Urb. Powder on immune genes expression and resistance against Vibrio parahaemolyticus infection. Fish shellfish immunol 102:218–227
Natt MP, Herrick CA (1952) A new blood diluent for counting the erythrocytes and leucocytes of the chicken. Poultry Sci 31:735–738
Niroomand M, Akbarzadeh A, Ebrahimi E, Sobhani SA, Sheikhahmadi A (2019) Effects of dietary black cumin seed on haemolymph biochemical parameters of Pacific white shrimp (Penaeus vannamei). J Animal Environ 11:279–286
Nor-Amalina Z, Dzarifah M, Mohd-Termizi Y, Amal M, Zamri-Saad M, Ina-Salwany M (2017) Phenotypic and genotypic characterization of Vibrio species isolates from Epinephelus species in Selangor, Malaysia. Proceed Int Conference Adv Fish Health:4–6
Nur I, Munaeni W (2020) Assessment of antibacterial and immunostimulating activity of black cumin (Nigella sativa) extract against vibriosis in white shrimp (Litopenaeus vannamei). Thai J Veterin Med 50:549–557
Parvardeh S, Fatehi M (2003) Effects of thymoquinone, the major constituent of Nigella sativa seeds, on the contractile responses of rat vas deferens. Pharmaceut biol 41:616–621
Priya TJ, Kappalli S (2022) Modern biotechnological strategies for vaccine development in aquaculture–prospects and challenges. Vaccine 40(41):5873–5881
Randhawa MA, Alghamdi MS, Maulik SK (2013) The effect of thymoquinone, an active component of Nigella sativa, on isoproterenol induced myocardial injury. Pak J Pharm Sci 26:1215–1219
Raval BP, Shah TG, Suthar MP, Ganure AL (2010) Screening of Nigella sativa seeds for antifungal activity. Ann Biol Res 1:164–171
Ruangroupan L, Kitao T, Yoshida T (1986) Protective efficacy of Aeromonas hydrophila vaccines in Nile tilapia. Vet Immunol Immunopathol 12:345–350
Salem ML (2005) Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol 5:1749–1770
Schaperclaus, W. (1992) Prophylaxis and therapy of fish diseases. Fish diseases.
Soliman NF, Yacout DM (2016) Aquaculture in Egypt: status, constraints and potentials. Aquacult Int 24:1201–1227
Spiegelman D, Hertzmark E (2005) Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 162:199–200
Stratev D, Zhelyazkov G, Noundou XS, Krause RW (2018) Beneficial effects of medicinal plants in fish diseases. Aquacult Int 26:289–308
Tafalla C, Bøgwald J, Dalmo RA (2013) Adjuvants and immunostimulants in fish vaccines: current knowledge and future perspectives. Fish Shellfish Immunol 35:1740–1750
Thompson F, Li Y, Gomez-Gil B, Thompson C, Hoste B, Vandemeulebroecke K, Rupp G, Pereira A, De Bem M, Sorgeloos P (2003) Vibrio neptunius sp. nov., Vibrio brasiliensis sp. nov. and Vibrio xuii sp. nov., isolated from the marine aquaculture environment (bivalves, fish, rotifers and shrimps). Int J Syst Evolution Microbiol 53:245–252
Valgas C, Souza SMD, Smânia EF, Smânia A Jr (2007) Screening methods to determine antibacterial activity of natural products. Brazil J Microbiol 38:369–380
Van Hai N (2015) The use of medicinal plants as immunostimulants in aquaculture: a review. Aquacult 446:88–96
Wamala SP, Mugimba KK, Mutoloki S, Evensen Ø, Mdegela R, Byarugaba DK, Sørum H (2018) Occurrence and antibiotic susceptibility of fish bacteria isolated from Oreochromis niloticus (Nile tilapia) and Clarias gariepinus (African catfish) in Uganda. Fisher Aquatic Sci 21:1–10
Xie J, Bu L, Jin S, Wang X, Zhao Q, Zhou S, Xu Y (2020) Outbreak of vibriosis caused by Vibrio harveyi and Vibrio alginolyticus in farmed seahorse Hippocampus kuda in China. Aquacult 523:735168
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Noha I. ElBanna, Mai Hegazy and Mohamed Fathi. The first draft of the manuscript was written by Salah M. Aly, Mohamed A. Elatta, all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The trial was performed following the Universal Directive on the protection of animals used for scientific purposes according to ethical guidelines approved by the ethics of scientific research committee, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt, code: 2023015.
Consent for publication
We understand that our data and potentially identifying information, such as our names or likeness, may be used in research publications. We have been provided with information about how our data will be used, who will have access to it, and any potential risks associated with its publication. We voluntarily agree to allow our data to be used and published in accordance with the study protocol.
Competing interests
The authors have no relevant financial or non-financial interests to disclose
Additional information
Handling editor: Amany Abbass
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aly, S.M., ElBanna, N.I., Elatta, M.A. et al. Effects of natural and synthetic immunostimulants on growth, feed utilization, immune status, and disease resistance against vibriosis in sea bream (Sparus aurata). Aquacult Int 32, 2739–2756 (2024). https://doi.org/10.1007/s10499-023-01294-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01294-2