Abstract
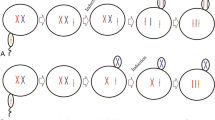
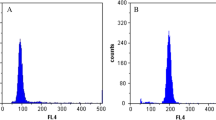
The sterility advantages of triploids make them paramount for oyster aquaculture. Tetraploids are always used as male parents for triploid industrial breeding in current hatcheries, thus they are the core asset for triploid oyster culture. The oyster Crassostrea angulata is the most productive oyster species in China. However, the optimal conditions for tetraploid induction have never been examined. In this study, we investigated the effectiveness of three induction methods (Method I, 2n ♀ × 2n ♂ + inducer abbreviated as DD + ind; Method II, 3n ♀ × 2n ♂ + inducer abbreviated as TrD + ind; Method III, 2n ♀ × 4n ♂ + inducer abbreviated as DT + ind) and three inducers (salinity; cytochalasin B, CB; and 6-dimethylaminopurine, 6-DMAP) for tetraploid induction in C. angulata. The optimal induction conditions for the three induction methods were a CB concentration of 0.5 mg/L treated for 20 min, 0.75 mg/L CB for a constant induction treatment of 20 min and 6-DMAP at a concentration of 100 mg/L for 20 min, respectively. When the optimal induction conditions were applied to a single pair of oysters, the D-larval tetraploid rate for the three methods were 18.93 ± 1.46%, 58.32 ± 1.03% and 51.61 ± 1.37%, respectively. After 30 days, the survival rates of the experimental groups were only 10.36 ± 2.51%, 28.07 ± 2.03% and 21.99 ± 1.27%, respectively, at day 30. The tetraploid rates in DD + ind and DT + ind were both decreased significantly over time, but they still reached 11.81% and 21.99% at 30 days, and 8% and 16% at day 180, respectively. Tetraploid rates in TrD + ind were 58.32 − 71.28% at the larval stage and 80% at day 180. In summary, despite very different induction rates, surviving tetraploids of C. angulata could be obtained in all three methods. Method II (TrD + inducer) was regarded as the optimal method to induce C. angulata tetraploids, and the optimal induction condition was a CB concentration of 0.75 mg/L and treated for 20 min under this method.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Aileen Tan SH, Teh CP, Chang GO, Yasin Z (2017) Tetraploid induction in tropical oysters, Crassostrea belcheri (Sowerby) and Crassostrea iredalei (Faustino). Aquac Res 48:1406–1412. https://doi.org/10.1111/are.12976
Allen SK (1983) Flow cytometry: assaying experimental polyploidy in fish shellfish. Aquaculture 33:317–328
Allen SK Jr, Bushek D (1992) Large scale production of triploid Crassostrea virginica (Gmelin) using “stripped” gametes. Aquaculture 103:241–251
Allen SK, Downing SL (1986) Performance of triploid Pacific oysters, Crassostrea gigas (Thunberg). I. Survival, growth, glycogen content, and sexual maturation in yearlings. J Exp Mar Biol Ecol 102:197–208. https://doi.org/10.1016/0022-0981(86)90176-0
Allen SK, Downing SL, Chew KK (1989) Hatchery manual for producing triploid oysters. Washington Sea Grant Program
Allen SK Jr, Shpigel M, Utting S, Spencer B (1994) Incidental production of tetraploid Manila clams, Tapes philippinarum (Adams and Reeve). Aquaculture 128:13–19
Barreto-Hernández A, Velasco LA, Winkler FM (2018) Effect of three triploidy induction methods on the growth and survival of larvae and post-larvae of the caribbean scallop Argopecten nucleus Aquac Res 49:1578–1587. https://doi.org/10.1111/are.13612
Benabdelmouna A, Ledu C (2007) Obtention de mollusques bivalves tétraploides á partir de géniteurs diploides. FR patent #2913982-A1
Benabdelmouna A, Ledu C (2015) Autotetraploid Pacific oysters (Crassostrea gigas) obtained using normal diploid eggs: induction and impact on cytogenetic stability. Genome 58:333–348. https://doi.org/10.1139/gen-2015-0014
Benabdelmouna A, Ledu C, Gérard A (2007) Obtention de mollusques bivalves tétraploides á partir du croisement de femelles diploädes et de males tétraploides. FR patent #2913983-A1
Bodenstein S, Callam BR, Walton WC et al (2023) Survival and growth of triploid eastern oysters, Crassostrea virginica, produced from wild diploids collected from low-salinity areas. Aquaculture 564:739032. https://doi.org/10.1016/j.aquaculture.2022.739032
Brake J, Evans F, Langdon C (2004) Evidence for genetic control of pigmentation of shell and mantle edge in selected families of Pacific oysters, Crassostrea gigas Aquaculture 229:89–98. https://doi.org/10.1016/S0044-8486(03)00325-9
Buestel D, Ropert M, Prou J, Goulletquer P (2009) History, status, and future of oyster culture in France. J Shellfish Res 28:813–820
Callam BR, Allen SK, Frank-Lawale A (2016) Genetic and environmental influence on triploid Crassostrea virginica grown in Chesapeake Bay: growth. Aquaculture 452:97–106. https://doi.org/10.1016/j.aquaculture.2015.10.027
Cheng G, Jiang G, Xu C et al (2023) A comparative study in inducing tetraploid Crassostrea gigas “Haida No.2” by three pathways. unpublished data
Comai L (2005) The advantages and disadvantages of being polyploid. Nat Rev Genet 6:836–846. https://doi.org/10.1038/nrg1711
Desrosiers RR, Gérard A, Peignon J-M et al (1993) A novel method to produce triploids in bivalve molluscs by the use of 6-dimethylaminopurine. J Exp Mar Biol Ecol 170:29–43. https://doi.org/10.1016/0022-0981(93)90127-A
Dufresne L, Néant I, St-Pierre J, Dubé F, Guerrier P (1991) Effects of 6- dimethylaminopurine on microtubules and putative intermediate filaments in sea urchin embryos. J Cell Sci 99:721–730
Eudeline B, Allen SK, Guo X (2000a) Optimization of tetraploid induction in Pacific oysters, Crassostrea gigas, using first polar body as a natural indicator. Aquaculture 187:73–84. https://doi.org/10.1016/S0044-8486(00)00302-1
Eudeline B, Allen SK, Guo X (2000b) Delayed meiosis and polar body release in eggs of triploid Pacific oysters, Crassostrea gigas, in relation to tetraploid production. J Exp Mar Biol Ecol 248:151–161. https://doi.org/10.1016/S0022-0981(00)00158-1
Gérard A, Naciri Y, Peignon JM, Ledu C, Phelipot P (1994) Optimization of triploid induction by the use of 6-DMAP for the oyster Crassostrea gigas (Thuneberg). Aquacult Fish Manage 25:709–719
Gérard A, Ledu C, Phélipot P, Naciri-Graven Y (1999) The induction of MI and MII triploids in the Pacific oyster Crassostrea gigas with 6-DMAP or CB. Aquaculture 174:229–242. https://doi.org/10.1016/S0044-8486(99)00032-0
Guo X (1991) Studies on tetraploid induction in the Pacific oyster, Crassostrea gigas (Thuneberg). Ph.D. dissertation, University of Washington, Seattle, Wash
Guo XM, Allen SK (1994a) Viable tetraploids in the Pacific oyster (Crassostrea gigas Thunberg) produced by inhibiting polar body I in eggs from triploids. Mol Mar Biol Biotechnol 3:42–50
Guo XM, Allen SK (1994b) Sex determination and polyploid gigantism in the dwarf surfclam (Mulinia lateralis Say). Genetics 138:1199–1206
Guo X, Cooper K, Hershberger WK, Chew KK (1992a) Genetic consequences of blocking polar body I with cytochalasin B in fertilized eggs of the Pacific Oyster, Crassostrea gigas: I. Ploidy of Resultant embryos. Biol Bull 183:381–386. https://doi.org/10.2307/1542013
Guo XM, Hershberger WK, Cooper K, Chew KK (1992b) Genetic consequences of blocking polar body I with cytochalasin B in fertilized eggs of the Pacifc oyster, Crassostrea gigas: II. Segregation of chromosomes. Bio Bullet 183:387–393
Guo X, DeBrosse GA, Allen SK (1996) All-triploid Pacific oysters (Crassostrea gigas Thunberg) produced by mating tetraploids and diploids. Aquaculture 142:149–161. https://doi.org/10.1016/0044-8486(95)01243-5
Guo XM, Wang J, Landau BJ, Li L, DeBrosse GA, Buono KD (2002) The successful production of tetraploid eastern oyster, Crassostrea virginica Gmelin. J Shellfish Res 21:380–381 (abstract)
Ibarra AM, Ascencio-Michel R, Ramirez JL et al (2017) Performance of diploid and triploid Crassostrea gigas (thunberg, 1793) grown in tropical versus temperate natural environmental conditions. J Shellfish Res 36:119–139. https://doi.org/10.2983/035.036.0113
Jeung H-D, Keshavmurthy S, Lim H-J et al (2016) Quantification of reproductive effort of the triploid Pacific oyster, Crassostrea gigas raised in intertidal rack and bag oyster culture system off the west coast of Korea during spawning season. Aquaculture 464:374–380. https://doi.org/10.1016/j.aquaculture.2016.07.010
Jouaux A, Heude-Berthelin C, Sourdaine P, Mathieu M, Kellner K (2010) Gametogenic stages in triploid oysters Crassostrea gigas: irregular locking of gonial proliferation and subsequent reproductive effort. J Exp Mar Biol Ecol 395:162–170. https://doi.org/10.1016/j.jembe.2010.08.030
Komaru A, Wada KT (1990) Gametogenesis of triploid japanese pearl oyster, Pinctada fucata martensii. In: Hoshi M, Yamashita O (eds) Advances in Invertebrate Reproduction. Elsevier, Amsterdam, pp 469–474
Kong J, Wang ZP, Yu RH, Liu J, Zhang YH (2011) Triploid induction in Pacific oyster (Crassostrea gigas) by hypotonic treatment and comparison with other induction methods. J Fish Sci China 18:581–587 (in Chinese)
Ledu C, Mccombie H (2003) Effects of cytochalasin B on fertilization and ploidy in the Pacific oyster Crassostrea gigas Invertebrate Reprod Dev 44:131–137. https://doi.org/10.1080/07924259.2003.9652563
Li Q, Wang Q, Liu S, Kong L (2011) Selection response and realized heritability for growth in three stocks of the Pacific oyster Crassostrea gigas. Fish Sci 77:643–648. https://doi.org/10.1007/s12562-011-0369-0
Li HK, Zhang Z, Yu RH, Wang YW, Li LW, Ma PZ (2021) A comparative study on the effectiveness of 6-DMAP and different salinities in inducing tetraploid Crassostrea gigas “Haida No.1”. J Ocean Univ China 51:136–145 (in Chinese)
Li H, Yu R, Li Q, Ma P (2022) Evaluation of advantages in the growth, survival and reproductive aspects of triploid hybrids derived from Crassostrea gigas tetraploids and C. ariakensis diploids in northern China. Aquaculture 548:737675. https://doi.org/10.1016/j.aquaculture.2021.737675
Maclean-Fletcher S, Pollard TD (1980) Mechanism of action of cytochalasin B on actin. Cell 20:329–341
Matt JL, Allen SK (2021) A classification system for gonad development in triploid Crassostrea virginica Aquaculture 532:735994. https://doi.org/10.1016/j.aquaculture.2020.735994
McCombie H, Ledu C, Phelipot P et al (2005) A complementary method for production of tetraploid Crassostrea gigas using crosses between diploids and tetraploids with cytochalasin B treatments. Mar Biotechnol 7:318–330. https://doi.org/10.1007/s10126-004-0440-2
McCombie H, Cornette F, Beaumont AR (2009) Short sharp shock produces viable tetraploids in crosses of diploid blue mussels Mytilus edulis Aquac Res 40:1680–1682. https://doi.org/10.1111/j.1365-2109.2009.02273.x
Melo EMC, Sühnel S, de Oliveira ACS et al (2020) Growth, mortality and reproductive traits of diploid and triploid Pacific oysters (Crassostrea gigas, THUNBERG, 1793) in Southern Brazil. Aquac Res 51:3631–3640. https://doi.org/10.1111/are.14713
Ministry of Agriculture and Rural Affairs of the People’s Republic of China, National Fisheries Technology Extension Center, China Society of Fisheries (2021) China fishery statistical yearbook. China Agriculture Press, pp 1–144
Nell JA (2002) Farming triploid oysters. Aquaculture 210:69–88. https://doi.org/10.1016/S0044-8486(01)00861-4
Nell JA, Hand RE, Goard LJ, McAdam SP, Maguire GB (1996) Studies on triploid oysters in Australia: evaluation of cytochalasin B and 6-dimethylaminopurine for triploidy induction in Sydney rock oysters Saccostrea commercialis (Iredale and Roughley). Aquaculture Res 27:689–698. https://doi.org/10.1111/j.1365-2109.1996.tb01304.x
Normand J, Le Pennec M, Boudry P (2008) Comparative histological study of gametogenesis in diploid and triploid Pacific oysters (Crassostrea gigas) reared in an estuarine farming site in France during the 2003 heatwave. Aquaculture 282:124–129. https://doi.org/10.1016/j.aquaculture.2008.06.026
Peachey BL, Allen SK (2016) Evaluation of cytochalasin B and 6-dimethylaminopurine for tetraploidy induction in the Eastern oyster, Crassostrea virginica Aquaculture 450:199–205. https://doi.org/10.1016/j.aquaculture.2015.07.034
Piferrer F, Beaumont A, Falguière J-C et al (2009) Polyploid fish and shellfish: production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture 293:125–156. https://doi.org/10.1016/j.aquaculture.2009.04.036
Qin Y, Zhang Y, Mo R et al (2019) Influence of ploidy and environment on grow-out traits of diploid and triploid Hong Kong oysters Crassostrea hongkongensis in southern China. Aquaculture 507:108–118. https://doi.org/10.1016/j.aquaculture.2019.04.017
Qin Y, Noor Z, Li X et al (2021) Tetraploid induction of Crassostrea hongkongensis and C. sikamea by inhibiting the polar body 1 release in diploid fertilized eggs. Mar Life Sci Technol 3:463–473. https://doi.org/10.1007/s42995-021-00107-w
Stanley JG, Allen SK, Hidu H (1981) Polyploidy induced in the american oyster, Crassostrea virginica, with cytochalasin B. Aquaculture 23:1–10. https://doi.org/10.1016/0044-8486(81)90002-8
Tan K, Deng L, Zheng H (2021) Effects of stocking density on the aquaculture performance of diploid and triploid, pacific oyster Crassostrea gigas and portuguese oyster C. angulata in warm water aquaculture. Aquac Res 52(12):6268–6279
Wadsworth P, Wilson AE, Walton WC (2019) A meta-analysis of growth rate in diploid and triploid oysters. Aquaculture 499:9–16. https://doi.org/10.1016/j.aquaculture.2018.09.018
Wang ZP, Zhao T, Yu RH, Zhang CC (2009) A new method for triploid induction by hypotonic treatment in scallop Patinopecten yessoensis J Ocean Univ China 39:193–196 (in Chinese)
Wang Y, Gan Y, Zhang J et al (2022) Performance of triploid Haliotis discus hannai cultured in a subtropical area using sea-based suspended systems. Aquaculture 548:737722. https://doi.org/10.1016/j.aquaculture.2021.737722
Yang H, Zhang F, Guo X (2000) Triploid and tetraploid Zhikong scallop, Chlamys farreri Joene et Preston, produced by inhibiting polar body I. Mar Biotechnol 2:466–475
Zhang Y, Qin Y, Yu Z (2022) Comparative study of tetraploid-based reciprocal triploid portuguese oysters, Crassostrea angulata, from seed to marketsize. Aquaculture 547:737523. https://doi.org/10.1016/j.aquaculture.2021.737523
Funding
This work was supported by grants from the National Key R&D Program of China (2022YFD2400305), the China Agriculture Research System Project (CARS-49), and Shandong Province (2021ZLGX03, 2021LZGC027).
Author information
Authors and Affiliations
Contributions
Yuanxin Liang: Conceptualization, supervision, resources, project admi. Geng Cheng: Resources. Xianchao Bai: Resources. Jianmin Zhou: Conceptualization, supervision. Haining Zhang: Supervision. Yong Chi: Resources. Gaowei Jiang: Conceptualization. Chengxun Xu: Resources. Qi Li: Conceptualization, supervision, resources, project admi.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The present study was performed according to the standard operation procedures (SOPs) of the Guide for the Use of Experimental Animals of the Ocean University of China. All animal care and use procedures were approved by the Institutional Animal Care and Use Committee of Ocean University of China.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, Y., Cheng, G., Bai, X. et al. Comparative study on tetraploid induction of the Fujian oyster Crassostrea angulata utilizing three typical methods. Aquacult Int 32, 593–612 (2024). https://doi.org/10.1007/s10499-023-01174-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01174-9