Abstract
Since salmon lice have developed resistance to pesticides, non-medicinal delousing treatments have become increasingly used to remove the parasite from fish on salmon farms in Norwegian waters. These novel techniques are an important means of controlling epidemics of lice at farms for maintaining the health of farmed fish and for preventing the spread of lice to wild salmonid populations. However, some treatments are associated with higher mortality rates and negative welfare impacts on the host fish. Furthermore, it is unclear how effective each treatment is in removing lice compared to alternatives. Here, in a controlled laboratory environment, we tested the efficacy of mechanical, warm water (28 °C and 34 °C), and freshwater treatments alone and in combination, and examined their welfare impact on host fish. Regardless of treatment group or control, the handling of fish through the experiment led to a reduction in lice load and decline in fish welfare. Among the treatments examined, the freshwater bath alone and in combination with other treatments had the greatest delousing efficacy. The 34 °C warm water baths also significantly reduced lice loads but led to worse welfare outcomes with fish having a higher prevalence of injuries and reduced growth and condition factor. Delousing treatments were however not associated with long-term effects on neuroendocrine parameters or stress coping ability, suggesting that immediate welfare impacts from these procedures are generally reversible. It was also found that sedating fish prior to treatment was shown to mitigate the welfare impact. These findings are useful for the needed optimization of delousing strategies for greater delousing efficacy and reduced welfare impact on fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The salmon louse (Lepeophtheirus salmonis Krøyer, 1837) is both a major disease challenge for salmonid aquaculture and an environmental threat to wild Atlantic salmon (Salmo salar Linnaeus, 1758) infected by lice emanating from farms (Costello 2009; Vollset et al. 2018). The louse begins its lifecycle as a planktonic larva, after developing through two instars the resulting copepodid must find and infect a host fish before its lipid stores are exhausted (Samsing et al. 2016; Thompson et al. 2019). Once attached, the louse develops through two sessile chalimus stages, followed by two mobile preadult stages, and then the final adult stage (Hamre et al. 2013). The latter mobile stages of the salmon louse graze on the mucus, skin and blood of the host leading to sores, secondary infections, immune suppression, osmotic disruption, reduced growth, and physiological and behavioral stress responses (Bowers et al. 2000; Finstad et al. 2000; Fast et al. 2006; Overli et al. 2014; Fjelldal et al. 2020, 2022). Thus, the control of salmon lice epidemics has been a necessary component of salmonid aquaculture following the adoption of open-net pens with chemical delousing applied topically or in feed since the early 1980s (Costello 2006; Myhre Jensen et al. 2020).
Norway is the global leader of salmonid aquaculture, producing 1.6 million tons in 2021, with approximately 650 actively farmed sites holding 450 million fish (FAO-FIGIS 2021; Norwegian Ministry of Trade, Industry and Fisheries 2022). Farmed salmonids now host 99% of the adult female salmon lice in Norwegian waters, and infectious copepodids produced by them have the potential to infect wild salmonids, elevating their risk of mortality (Thorstad et al. 2012; Taranger et al. 2015; Bøhn et al. 2020; Dempster et al. 2021). In 1998, the Norwegian management authorities acknowledged the risk of salmon lice to both farmed and wild populations of salmonids and implemented rules to control their spread (Anon 1998). Subsequent updates to these louse control rules have progressively tightened, including the limit on the allowable lice load for farmed fish (Anon 2009; 2012). But by 2008, the decades-long use of a few chemical treatments in Norwegian waters had already led to the recognized development of drug resistance in the salmon louse populations (Aaen et al. 2015; Myhre Jensen et al. 2020). The declining efficacy of those treatments and the continued need to control lice epidemics on farms has motivated the development and widespread adoption of several non-medicinal treatments to remove lice (Overton et al. 2019).
In comparison to chemotherapeutics, non-medicinal treatments were a minor component of delousing operations in 2012 comprising 8% of the total, but by 2017, they were the majority and their popularity continued to increase with more than 74% of all reported operations in 2019 being non-medicinal (Myhre Jensen et al. 2020). The non-medicinal delousing treatments in common use and examined here fall under three categories: mechanical, thermal, and freshwater. While there are many variations on the application of those non-medicinal treatments the general procedure involves the concentration and confinement of fish at farms and multiple transfers through live fish pumps to delousing devices or treatment baths (Overton et al. 2019). Mechanical delousing systems can use physical brushes, high pressure sprays, or water turbulence to dislodge attached lice (Gismervik et al. 2017, 2022; Overton et al. 2019). Thermal delousing relies on the principle that the copepod parasite with its orders of magnitude smaller body size will be affected by the high temperature faster than the host fish and fall off with Roth et al. (2016) claiming that a greater difference between the ambient seawater temperature and treatment temperature yields a higher efficacy. In the commercial application of thermal treatments, the fish are pumped into a warm water bath between 28 and 34 °C and held there for 20–30 s (Grøntvedt et al. 2015; Roth 2016). Likewise, the salmon lice have a sensitivity to low salinity and a 3- to 5-h long bath in freshwater is used to kill or remove the lice (Powell et al. 2015). At farm sites, the fish are either transferred into a well-boat or into a temporary pond created out of a tarpaulin on site and filled with fresh or desalinated water (Mc Dermott et al. 2021).
Since the paradigm shift in delousing strategy from chemotherapeutics to non-medicinal treatments, the number of delousing operations per ton of fish farmed has increased along with poorer welfare outcomes for fish and higher mortality following non-medicinal operations (Overton et al. 2019). Although farmers and regulators know the delousing operations have negative welfare impacts on fish, frequent treatments are nonetheless required to remain below the inflexible lice limit (Stien et al. 2020). All handling and treatment of fish increases welfare and mortality risk, but there are indications that non-medicinal treatments, particularly thermal and mechanical treatments, lead to a greater increase in mortality with water temperature and fish weight being influencing factors among others (Overton et al. 2019; Bang Jensen et al. 2020; Oliveira et al. 2021; Sviland Walde et al. 2021; Persson et al. 2022). Between 2012 and 2014, 14.1% of the salmon put out to sea in net-pens died before they could be harvested, the percentage lost during the grow-out phase increased to 16.3% for the years 2019 to 2021 (Norwegian Ministry of Trade, Industry and Fisheries 2022). Meanwhile, the average weight at slaughter has been declining with Barrett et al. (2022) suggesting that the early harvests are a response by the farmers to the risky lice treatments, choosing to slaughter the fish, rather than performing another delousing. The increase in fish mortality is a complex phenomenon without a single cause, but the increase in non-medicinal treatments applied alone and in combination is acknowledged as a contributing factor (Grefsrud et al. 2022). In 2021, thermal treatments were the most reported treatment at 50%, followed by mechanical treatments at 28%, freshwater at 10%, and combination treatments and less commonly employed methods comprising the remainder (Gismervik et al. 2022; Grefsrud et al. 2022).
The unintended death of a farmed fish is both a production loss for the farm and an ethical failure for those who are responsible for its care. The welfare of fish was of little consideration until recently, but it is now broadly accepted that aquaculturalists and management institutions have an ethical obligation to prevent unnecessary death and suffering for these animals in our care (Bovenkerk & Meijboom 2013, 2020; Kristiansen & Bracke 2020). Beyond the increased mortality risk posed, the non-medicinal delousing operations can lead to unwanted welfare outcomes and directly cause suffering. Freshwater treatments have been found to stress fish and disrupt osmotic balance (Powell et al. 2015), while the application of mechanical and warm water treatments increases the prevalence of wounds and injuries (Gismervik et al. 2017, 2019; Moltumyr et al. 2021, 2022) and risk of mortality (Bui et al. 2022). Thermal treatments further instigate a strong behavioral response in fish which has been attributed to nociception (Nilsson et al. 2019). Citing the increase in mortality and the welfare impacts associated with thermal treatments, the council for animal ethics in Norway has called for a ban on the use of thermal treatments (Bøe & Ellingsen-Dalskau 2022). In a statement issued by the Food Safety Authority, the problems were acknowledged, but the practices would be allowed to continue while various research projects investigated the welfare impacts and delousing efficacy (Moore 2021).
Considering the ethical, environmental, and regulatory problems posed by the non-medicinal treatments this study seeks to investigate and compare the efficacy and welfare impact of freshwater, mechanical, and thermal treatments singly and in combination. Under controlled experimental conditions, efficacy and welfare outcomes were evaluated. Lice loads were enumerated before and after treatments, and welfare outcomes were evaluated through individual welfare indicators and stress response testing.
Methods
The efficacy and welfare impact of non-medicinal salmon louse treatments were evaluated through experiments designed to investigate the effect of individual treatments, combined treatments, and influencing factors. The study was conducted through two separate rounds of experiments taking place at the Matre Research Station (Matredal, Norway). At the facility, Atlantic salmon (AquaGen® Atlantic QTL-innOva® PRIME strain, AquaGen, Inc., Trondheim, Norway), were reared under controlled environmental conditions in state-of-the-art facilities and transferred from their stock tank (diameter = 7 m, ~ 69 m3, 8 °C) to the experimental tanks as needed. They were then infected with salmon lice, deloused with the various treatments, and observed for welfare impacts and lice load as described below.
Experimental design
Round 1
The first round of experiments began in August 2021, with 450 fish having a mean weight of 1.1 kg divided evenly between three experimental tanks (⌀ = 3 m, ~ 5.3 m3, 8 °C). The number of fish used in the experiments was determined by balancing the needed statistical power (informed by prior work) and the principle of reduction for animal welfare in experiments. Tanks were setup in a common garden design whereby all treatment groups were represented by 10 replicate fish in each tank. Four non-medicinal delousing treatments, combinations of those treatments, their procedural controls (see the “Delousing treatments” section), and a negative control were examined in this first round of experiments (Table 1). The treatments included a warm water bath of 28 °C or 34 °C, a mechanical delousing, and a 20-h freshwater bath.
Round 2
In March 2022, an additional experimental round investigated the influence of fish size and sedation in combination treatments which included a freshwater bath. The second round used a 4-h freshwater bath duration based on new data showing efficacy after this time. As in the first round, the treatments included warm water baths (28 °C or 34 °C) and a mechanical delousing. In half of the 6 combination treatment groups, the salmon were also sedated prior to treatment with warm water or mechanical delousing (‘sedation treatment’). This second round of experiments, like the first, used a common garden design with 140 new fish divided into two experimental tanks (⌀ = 3 m, ~ 5.3 m3, 8 °C), one tank holding ‘large’ fish and the other holding smaller fish. A malfunction of the tag reader during the March 2022 experiments prevented the recording of pre-treatment data for one of the tanks (2B, Table 1).
Experimental steps
The experimental timeline centers on the treatment day, with posttreatment sampling occurring 24 h or 3 weeks after treatments, and several experimental steps occurring prior to treatment day (Table 2). The fish were first transferred to their experimental tanks and allowed to recover and acclimated for 2 weeks; they were then sedated (tricaine methanesulfonate, 0.1 g L−1) and marked with 12 mm glass 2 PIT-tags (Biomark™, ID, USA) inserted into their stomach cavity. The salmon in the round 1 experiments were also measured for fork length (cm) and body weight (g) during the tagging. After a recovery period of approximately 10 days, the fish were infected with salmon lice. In round 1, the infection occurred over three separate instances timed to foster adult, pre-adult, and chalimus stages on the fish at the time of treatment application. An issue with the incubation system resulted in only one infection point (to foster adults) in round 2. The ambient temperature in the experimental tanks was 12 °C to speed up the development of the salmon lice and then decreased by 2 °C per day to reach the desired experimental temperature of 8 °C by the time of pre-treatment sampling. Three days prior to delousing treatments, 30 salmon from each of the experimental tanks were randomly sub-sampled and sedated (tricaine methanesulfonate, 0.1 g L−1) to assess lice load and welfare. The fish was held in a shallow tray with enough seawater to cover it while salmon lice were visually identified to stage and enumerated; meanwhile, welfare was assessed using elements from both the FISHWELL and LAKSVEL scoring system (Noble et al. 2018, see the “Welfare assessment and analysis” section; Nilsson et al. 2022). After the assessment was completed, the subsampled fish were placed in a recovery tank and transferred back to their original experimental tanks once consciousness was regained.
The delousing treatments began with the collection and transfer of fish for the freshwater bath from all single and combined treatment groups, and after the freshwater bath, the remaining delousing treatments were applied. During the treatments, the fish were temporarily moved to a different but identical tank, and after treatments were completed, they were transferred back to their original experimental tank. The negative control fish therein were crowded and netted to scan their PIT tags but not treated. After 24 h or 3 weeks, the fish were sampled to evaluate the delousing efficacy and welfare impact of the treatments (Table 2). As in the pre-sample, the lice load on the fish was enumerated, and their welfare was evaluated. Since that was the experimental endpoint, the fish were first euthanized by an overdose of tricaine methanesulfonate (1 g L−1), and final fork length and body weight were measured. During the 3-week post-treatment sampling of the round 1 experiments, a subsample of fish were further subjected to a stress test after which additional neurobiological samples were collected along with the other measures (see the “Stress test” section).
Infection with salmon lice
Following Hamre et al. (2009), egg strings were detached from adult female lice (Lepeophtheirus salmonis) and placed in incubation chambers with flowing seawater at 33 ppt and 10 °C where they were monitored daily for hatching and development into infectious stage copepodids. When the copepodids were 6–9 days old, they were added to the experimental fish tanks for infection. The intention was to reach a lice load of approximately 6–8 lice per fish (0.5 lice per stage per sex) and based on previous experience the number of copepods added was calculated to be 30 per fish in the tank to achieve that number. During the infection, the flowing seawater was reduced to 20% of standard rate, the salmon louse copepodids were added, and after 40 min, the water flow was turned back up which flushed any remaining unattached copepodids out of the tank. The developmental rate of lice to adults was determined using temperature dependent development functions (Hamre et al. 2019). In both rounds of experiments, wild adult female lice with egg strings were sourced from farmed salmon in Matre, Solheim, and Austevoll Norway, but in the second round of experiments, approximately 20% of the lice were sourced from laboratory strains at the Institute of Marine Research hatchery facilities in Bergen Norway.
Delousing treatments
All transfers from the holding tank were carried out by reducing the water level in the tank to crowd the fish and gently netting individuals with a knotless dip-net (5 × 5 mm mesh size, ⌀ = 38 cm, depth = 42 cm, Kayoba). While in the dip-net the individual PIT tags were scanned to record which fish were undergoing the designated treatments. In a randomized block design, fish were randomly selected from each tank in blocks of 10 and assigned to a treatment group. Transfers between tanks were aided by the use of runs with flowing seawater to reduce potential welfare or delousing impacts of extra netting.
Fish undergoing the freshwater treatment were transferred to a separate tank (round 1: groups of 10 fish to 1 × 1 m tanks, ~ 0.7 m3; round 2, all treatment fish from one tank, ⌀ = 3 m, ~ 5.3 m3). After the transfer the water quality in the tank was switched from sea- to freshwater via an automated system (round 1: 20-h freshwater bath; round 2: 4 h bath).
In the first round of experiments, the mechanical delousing used a custom device to spray a curtain of water from three directions across a box (60 × 60 cm) that fish passed through, and in the second round a larger, custom-designed device was used that sprayed water in a perpendicular line, at three points along a 2 m run. Both mechanical delousers were connected to a water pump providing 110-L per minute and delousing streams of water were valve adjusted to achieve 2 bars of pressure at the nozzles. Although the spray pressure used may be comparable to commercial delousers, replicating the commercial delousing treatment was not possible. Treatments vary between suppliers and the scale of the procedure and equipment is not compatible with a tank facility. Instead, the devices and procedure used here were designed to model mechanical disturbance and its welfare impacts and delousing efficacy at an experiment scale.
The warm water bath was applied in a ~ 1000 L vessel (140 × 100 × 74 cm), with the heated seawater monitored for salinity and temperature using a ProSolo Digital Water Quality probe, and total gas pressure monitored with a Handy Polary TGP (OxyGuard®, Farum, Denmark). Deviations of ± 0.4 °C from the target temperature were corrected by the addition of warmer/colder water, and oxygen stones were added as needed to ensure the oxygen saturation did not drop below 60%. Fish undergoing the thermal treatment were transferred to a custom-made basket (78 × 45 × 28 cm) that was placed within the warm water bath and covered with a lid. The basket had solid sides and the bottom was made of PVC pipes that created a grate without any edges, whereby the fish were confined in space yet could still move and exhibit responsive behaviors (albeit restricted). After the 30 s warm water bath, the basket was removed with the fish, and the fish was then transferred to a holding tank.
The mechanical delousing, freshwater bath, and warm water bath procedural controls were done following the same handling steps but without the delousing activity, i.e., the freshwater controls were transferred to another seawater holding tank, the mechanical controls passed through the device without the water jets turned on, and the warm water controls were held in the bath vessel under ambient temperature for 30 s. All combination treatments were applied in the following order (excluding a step if the treatment was not applied): freshwater bath, sedation (second round only), warm water bath, and then mechanical treatment. During the sedation treatment step, the fish would be netted into a smaller vessel with the sedative (tricaine methanesulfonate, 0.1 g L−1) and held there until it took effect.
Treatment efficacy and analysis
The effect of delousing strategy on the total number of lice remaining after treatment was analyzed by generalized linear modeling (GLM) following a negative binomial distribution (glm.nb, R Core Team 2022). Since mobile lice can transfer between fish (see Bui et al. 2018), the dataset examined included only the 24-h post treatment samples. The explanatory variables examined included experimental tank, fish weight, sedation, mechanical, thermal, and freshwater treatments. Fish weight was the only continuous variable included in the models. The factor levels for the treatment variables could be none, a procedural control, or the applied delousing treatment which in the case of thermal delousing was a 28 °C or 34 °C warm water bath, and for the freshwater treatment was a 4- or 20-h bath. Thus, for a negative control fish the factor levels were set to ‘none’ for sedation, mechanical, thermal, and freshwater while the maximal combination treatment group in the round 2 experiments would have levels set to ‘sedated’, ‘mechanically treated’, ‘34 °C warm water bath’, and’4-h freshwater bath’. Selection of the best performing model was determined through Akaike information criterion (AIC) with the lowest AIC model being selected, but an alternative model within 2 AIC may be chosen if it has fewer variables, which simplifies the model and often improves interpretation. After fitting the dataset to the selected model, the diagnostic functions in the R package DHARMa (Hartig 2019) were used to evaluate dispersion, dependency, and normality of model residuals.
Welfare assessment and analysis
The welfare assessment follows a simplified version of FISHWELL and LAKSVEL protocols with indicators in various categories scored for their welfare outcomes (Noble et al. 2018; Nilsson et al. 2022). For this study, the following categories were evaluated using their respective indicators of external damages: skin damage (wounds, scale loss, bleeding), snout damage, eye damage (injury, bleeding, opaqueness), and fin condition (caudal, dorsal, pectoral, pelvic, and anal fins). Under the FISHWELL and LAKSVEL protocols, indicators are scored on a scale of 0–3 with 0 signifying no damage and 3 a severe condition. Here, any score of 2 and greater was considered an unwanted welfare outcome, and if any indicator within a category signaled an unwanted welfare outcome the entire category was recorded as such. Thus, for each fish, there were 4 binary indicators of an unwanted welfare outcome, and each of these was analyzed separately through generalized linear modeling using a binomial distribution (GLM, R Core Team 2022). The statistical modeling and selection followed the same procedure used for evaluating delousing efficacy with separate models selected for each of the indicator categories. Like the delousing models the explanatory variables included experimental tank, fish weight, sedation, mechanical, thermal, and freshwater treatments. The analysis was separated by post-treatment sampling period to account for any healing after delousing or the later development of injuries. Since the fish in the round 2 experiments were all sampled 24-h after treatments, the models of welfare outcome 3 weeks after treatment only includes data from the first round of experiments in which there was no sedation treatment. In addition to the analysis of post-treatment welfare outcomes by GLM, the pre-treatment welfare status was analyzed for differences between tanks using the Fisher exact test (fisher.test, R Core Team 2022).
Growth and condition
Growth and condition factor were analyzed with respect to the impact of delousing strategy 3 weeks after treatment was applied in the round 1 experiments. Condition factor (K) was calculated using the formula \(K=\left({\mathrm{Weight}*\mathrm{Length}}^{-3}\right)*100\), and specific growth rate (SGR) was calculated following Brett and Groves (1979) with \(SGR=\left(\mathrm{ln}\left({W}_{2}\right)-\mathrm{ln}\left({W}_{1}\right)\right)*{T}^{-1}\), where W1 is start weight, W2 is end weight, and T is number of days between measurements. Both metrics were fit to linear models (LM, R Core Team 2022) with the explanatory variables experimental tank, mechanical, thermal, and freshwater treatments. Following previous statistical modeling analysis steps, the model selection was done through AIC, and model residuals were evaluated with DHARMa.
Stress test
In round 1 experiments, an additional physiological stress test was conducted during the 3-week post-treatment sampling on a subset of 38 fish collected from the following treatment groups: negative control; freshwater bath; 34 °C warm water bath; and the combination of mechanical delousing, freshwater and 34 °C warm water bath. The subsampled fish were handled and confined for 2 h in a smaller vessel (140 × 100 × 74 cm) to induce a stress response and assess possible long-term effects of delousing treatments on welfare in terms of stress coping ability (Vindas et al. 2016; c.f. Hoglund et al. 2021). For comparison, another subset of 37 fish from those same treatment groups was sampled directly from the experimental tank. Both subsets were anaesthetized with an overdose of sedation (tricaine methanesulfonate, 1 g L−1) then sampled for blood plasma cortisol, while brain stem serotonin (5-hydroxytryptamine, 5-HT) and its catabolite 5-hydroxyindoleacetic acid (5-HIAA) were analyzed by HPLC with electrochemical detection as described by Vindas et al. (2016). Statistical comparisons of response between stressed and unstressed fish were analyzed for both neuroendocrine indicators by applying t tests to each treatment group.
Results
Sample summary
Round 1 experiments were began in August of 2021, the mean weight of the salmon at the time of tagging and distribution into the experimental tanks was 1.13 kg, and they had a mean condition factor of 1.05. The fish in the 3-week post treatment tanks 1A and 1B had specific growth rates (SGR, Brett and Groves 1979) of 0.63 and 0.79 after 53 days, while the fish in the 24-h post treatment tank (1C) had a SGR of 0.60 after 34 days (Table 1). Ranging from 1.06 to 1.18, the mean condition factor of fish in the first-round experimental tanks was greater than at their initial measurements at tagging, indicating an improvement over the course of the experiment. During the second experimental round conducted in March 2022, two size classes of fish were examined but due to issues reported on in the methods limited pre-treatment data with lice counts is only available from the tank with the larger size class. The salmon in that tank (2A) had a mean weight of 4.18 kg, while the smaller class of fish (2B) had a mean weight of 2.08 kg (Table 1). Post-treatment, 10 fish died from the tanks sampled 3 weeks after delousing with a Fisher exact test finding no significance in prevalence of mortality due to treatment type (P value = 0.76). No fish died in tanks between the delousing treatments and sampling conducted 24 h later.
Delousing efficacy
Prior to delousing treatments, the three round 1 experimental tanks 1A, 1B, and 1C, had respective mean total lice loads and standard errors of 12.1 ± 0.8, 15.8 ± 1.1, and 12.6 ± 0.8. with 76%, 83%, and 86% of those being in the mobile stages (Table 1). In the round 2 experiments, the pre-treatment lice load in the recorded tank with larger fish (2A) was 12.3 ± 0.8, all of which were adults. The pre-treatment lice loads were higher than the target of 6–8 lice per fish which indicates that the infection success was greater than expected.
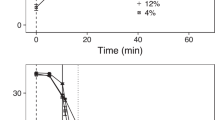
Tank 1C from the first round was sampled 24 h after treatment and was the only tank with attached stages of lice found on fish, with 6% of the total in the chalimus 2 stage and 1% in chalimus 1 stage. Due to the natural development rate of lice, attached stages had progressed to adult stages by the 3-week post-treatment sample date in the other round 1 tanks (1A, 1B). The louse loads decreased in all procedural control and treatment groups with mean total louse loads of experimental tanks found to be 4 to 40% of the pre-treatment totals if the negative control group was removed. If only considering the negative controls, the total number of lice was 63 to 71% of the pre-treatment totals (Fig. 1).
Post treatment salmon lice loads. Mean counts of adult, pre-adult, and attached stages are depicted for each treatment group, separated by experimental round and tank with sampling period indicated. Where available, the mean pre-treatment total lice loads are depicted with the dashed red line for each experimental tank
The decreases in louse loads across treatment groups were variable, but in those treatments with a freshwater bath, there were less lice found than in the alternatives. In the round 1 experiments, the freshwater delousing effect was particularly greater in the samples collected 24 h after application. The mean total lice load of those freshwater treatment groups was 8% of the pre-treatment level, lower than the other treatment groups and much lower than the freshwater control which was 75% of the pre-treatment level. With mean total lice at 10% of the pre-treatment lice load, the delousing treatments which included a 34 °C bath also had less lice than other non-freshwater groups (Fig. 1).
In the round 2 experiments, all treatment groups apart from the negative control included a freshwater bath treatment. While the treatment effects seen in the second round are comparable to the freshwater effects seen in the first round for the larger size class of fish (2A) the delousing efficacy for the smaller size class of fish is more variable (2B) (Fig. 1). Notably, the two combination treatments with the 34 °C bath had the lowest level of lice after treatment in this tank with the smaller size class of fish (2B). We also observed that across the two experimental tanks in the second round and not including the negative controls, the sedated fish have a mean louse load that is 10% higher than the non-sedated fish. However, while the sedated fish have more lice than non-sedated fish in the larger size class of fish (2A), the opposite is the case for the smaller size class (2B).
Model fitting total lice to delousing treatment and fish host characteristics
The observations of delousing efficacy were further scrutinized with statistical models, but only tanks sampled 24 h after treatment were included in the analysis. There were 64 possible models to select from to predict total lice load, with the null model having an AIC of 1318 on 291 observations. The selected model included 3 of the 6 explanatory variables and had the lowest AIC of all possible models with a ΔAIC of 205 from the null model (Eq. (1), Table 3). The selected model had an explained deviance of 53% (\(\mathrm{explained \: deviance}=100*(1-\mathrm{residual \: deviance}/\mathrm{null \: deviance}))\), and the dispersion test statistic p value of 0.616 lead us to reject the hypothesis that the model is over dispersed.
Under the model framework, the reference level of all treatment variables (Eq. 1, Table 2) was ‘none’ in which no treatment or procedural control was applied. When the reference level for the experimental tank variable is set to tank 2B the model predicts that both tank 2A and 1C have significantly less lice (p < 0.001). However, if the reference level is changed to 2A or 1C then the model indicates that there is no significant difference between tanks 2A and 1C (p = 0.321). Since the model does not account for pre-treatment lice loads that differ by tank, the tank variable captures the contribution of both tank effects and different pre-treatment lice loads to the predicted total lice. Furthermore, because the weight term is not included in the selected model and because fish weight varied with the experimental tank, it is also likely that the tank variable in the selected model captures the potential influence of weight on the total lice count. These influences together could contribute to the substantial variations between tanks, as indicated by their coefficients, which predict a difference of up to 2.7 lice between tank 2A and 2B (assuming all other variables are held constant and after converting from the log scale). Interestingly, despite the large differences in fish weight, there is no expected difference in total lice count between tanks 2A and 1C.
The chosen model excluded the mechanical and sedation terms, which indicates that these treatment variables were poor predictors of the observed total lice count and had no significant impact on the results. The thermal treatment variable was included, but among its categorical levels only the 34 °C warm water bath was significantly different from the reference level of ‘none’. Neither the 28 °C bath nor the procedural control was significantly different from the reference with respective p values of 0.393 and 0.927. For the included freshwater treatment term, both the 20-h and 4-h bath durations were significantly different than ‘none’ (p < 0.001). The coefficients of the two durations indicate that the 20-h bath had a greater delousing effect than the 4-h bath, but the difference is not significant (p value = 0.677). Meanwhile, the freshwater procedural control predicts a suggestive but not significant increase in total lice with a p value of 0.06. Overall, the 20-h freshwater bath had the greatest delousing effect, holding all other variables constant, it would result in 2.5 lice less than the 34 °C warm water bath.
Welfare outcomes
Across all experimental tanks, Fisher exact tests indicated no significant differences in the pre-treatment samples for the welfare categories of eye (p = 1.0), fin (p = 0.25), skin (p = 0.87), and snout damage (p = 0.75). In both rounds, the prevalence of an unwanted welfare status is higher in the post-treatment datasets than in the pre-treatment samples (Fig. 2). Overall, the post treatment fish in the round 1 experiments had a similar prevalence of unwanted scores to those in round 2 with 70% and 64% respectively. The proportion of fish with an unwanted welfare outcome was lowest for the eye category (Figs. 2 and 3); otherwise, unwanted welfare outcomes were widely prevalent in the experimental fish and assessing the impact of treatment strategies on prevalence was done through statistical modeling. Prevalence of an unwanted outcome was analyzed for each of the welfare categories and the datasets were further separated by 24-h and 3-week post treatment sampling periods to account for both immediate and longer-term impacts.
Prevalence of unwanted welfare outcomes for each category of external damage. Welfare data is presented per delousing treatment and experimental rounds-tank with sampling period indicated. The size of the bubble shows the % of the outcomes tallied as unwanted for each category. The color indicates the treatment group. Treatment abbreviation: FW = Fresh Water Bath; WW = Warm Water; Mech. = Mechanical; Ctrl. = Control
In the 24-h post-treatment dataset of injury prevalence, there were 6 possible explanatory variables to select from for each of the indicator categories producing 64 possible models to choose from. The reference level for experimental tank was 1C, experimental tank was the only variable included in the selected models of skin and fin damage (Table 4). The intercept and experimental tank coefficients of the skin model showed that the model predicts tank 1C to be less likely to have an unwanted outcome, but only the estimate of 2B is significantly different and overall the model explains just 1.8% of the deviance. The experimental tank groups in the model of fin damage are both significant and predict those tanks to have a lower prevalence of unwanted outcomes than the 1C reference tank with the overall model having an explained deviance of 7.6%. The model of snout damage predicts a significantly lower prevalence of unwanted outcomes in tank 2A compared to the reference level of 1C, but tank 2B was not significantly different from the reference. The snout model also predicts that if a 1C fish is sedated there is a 10% chance of an unwanted outcome compared to a 35% chance for a non-sedated fish, but this model also has a low percentage of explained deviance at 8.3%. The model of eye damage had the greatest explained deviance with 13.5%, and it had the lowest intercept of all models at − 2.77 which predicts a baseline 5.9% probability of an unwanted outcome. Overall, this follows the observed pattern of fewer unwanted outcomes in the eye category (Fig. 2). Sedated fish also have a significantly lower likelihood of having an unwanted injury to their eyes which follows the findings for the snout model. The pattern of lower prevalence of eye and snout damage in sedated fish is particularly apparent if only examining the data from the round 2 experiments (Fig. 3). The model of eye damage indicates that all freshwater treatments increase the likelihood of an unwanted outcome, but the procedural control level predicts the greatest increase and the 20-h bath treatment was not significant. According to the model, a fish that underwent the procedural control would have a 30% chance of having eye damage while a fish treated with the 4-h bath would have a 24% chance which would go down to 3% if it were also sedated. Although the mechanical variable was included in the selected model of eye damage, no factor level was significant.
Samples collected 3 weeks after treatment were only available from the round 1 experiments and thus do not include a treatment strategy of sedating the fish prior to delousing. The 5 explanatory variables produced 32 possible models to select from for each of the 4 external damage categories. Similar to the models selected for the 24-h dataset, those selected from the skin and fins category here did not include any treatment strategy variables, but they did include weight in addition to tank (Table 5). There were two experimental tanks with the reference level set to tank 1A which significantly predicted a lower probability of an unwanted outcome than tank 1B for all categories. Although weight was included in all 3-week post treatment models it was not found to be significant. Notably, weight was not included in the 24-h models despite fish in that dataset ranging in weight from approximately 1 to 5 kg, while weights of fish in the 3-week dataset had a lower range centered near 1.5 kg (Table 1). Also contrasting from the 24-h models, the freshwater bath variable was not selected in any of the models while the warm water bath variable was included in the models predicting snout and fin damage. In both of those models, the highest temperature treatment of 34 °C significantly predicted a higher likelihood of an unwanted outcome while the lower treatment of 28 °C and the procedural control predicted a lower likelihood, but not all those levels are significant. According to those models, a 1.5 kg fish from the 1A tank would have 6% and 22% respective chance of having an unwanted snout and fin outcome if treated with the warmwater procedural control, and a 53% and 52% chance if treated with the 34 °C bath. As in the 24-h model, the 3-week model of eye damage included the mechanical variable which predicted that the delousing treatment significantly lowered the likelihood of an unwanted outcome compared to the negative control. Here, a 1.5 kg fish from the 1B tank would have a 7% probability of developing unwanted eye damage if treated with mechanical delousing and an 18% probability otherwise. The percentage of explained deviance for the 3-week models was similar to the 24-h post treatment models with overall low percentages, the lowest being for the skin model and the highest for the eye category.
Growth and condition factor
In the round 1 experiments, two tanks of fish were measured for fork length and body weight at the time of tagging and again when they were sampled 3 weeks after treatment. The treatments were applied 33 days after the first measurement, and the second measurement was taken 3 weeks later which was 54 days since the first. Thus, there was time for the treatments to impact growth and condition factor which were analyzed using statistical models. For both growth and condition factor, the 5 explanatory variables produced 16 possible models to choose from including the null model. The null model for condition factor had an AIC of − 630.3 on 284 observation and the best performing model which was selected (Eq. 2, Table 6) had an AIC − 653.5 with an adjusted r square of 0.084. Evaluation of the residuals (with DHARMa; Hartig 2019) indicated no issues with the model of condition factor but the initially selected model of growth displayed heteroscedasticity and featured outliers. Further inspection of the outlier fish showed that their growth was negative; out of the 285 fish observed for growth 6 had negative growth and 5 of them were the outliers. The outliers were removed and a new model was selected in which the residual variance was acceptable. The null model of growth had an AIC of − 23.6 on 279 observations and the selected model, which matches the model for condition factor (Eq. 2, Table 6), had an AIC of − 70.5 with an adjusted r-square of 0.167.
Model fitting dependent variable \({{{Y}}}_{{{i}}}\) which is either condition factor or growth to delousing treatment and fish host characteristics
Fish sampled 3-week post-treatment had a mean weight gain over the 53 days of the experiment of 471 g and 587 g for 1A and 1B respectively. According to the statistical models, the condition factor and growth was greater in tank 1B compared to tank 1A (Table 6, Fig. 3). The selected models also both included thermal treatment as an explanatory variable with only the 34 °C warm water bath significantly predicting a decrease of condition factor by 0.029 and growth by 0.079. Nevertheless, like the welfare GLMs, the goodness of fit of these models was low as indicated by their adjusted r squares, and there were many outliers, especially in growth (Fig. 4).
Condition factor and growth boxplots with groupings by experimental tank and thermal treatment. According to linear models, experimental tanks for both metrics were significantly different (Table 6) and further significant differences between the reference level of none and the paired boxplots of treatments are indicated with P value significance codes: * < 0.05; ** < 0.01. The outliers shown in the growth boxplots do not include those removed prior to fitting the linear models
Stress test: cortisol and serotonergic activity
Fish from all treatment groups subjected to the additional confinement stress test exhibited elevated blood plasma cortisol and serotonin turnover in the brain (Fig. 5). A t-test was performed in each treatment group for both indicators, and in all cases, significant differences were found compared to unstressed fish directly sampled from the tank (P values < 0.001).
Boxplots of stress responsiveness as indicated by blood plasma cortisol and brain stem serotonergic activity measured by the ratio of 5-hydroxytryptamine (5-HT) and its catabolite 5-Hydroxyindoleacetic acid (5-HIAA). In all treatments, the differences between the confined fish and those sampled directly were significant (t test, P values < 0.001)
Discussion
Delousing efficacy
This study aimed to compare non-medicinal delousing strategies, particularly their efficacy in removing the salmon louse and their impact on the hosts. Several delousing treatments were examined singularly and in combination through multiple experiments with various conditions. Freshwater baths, on their own and in combination with other treatments, had the greatest delousing efficacy, and there was no statistical difference in efficacy due to bath duration. Thus, the most efficacious treatment in this study is currently the least used non-medicinal treatment at Norwegian salmon farms (see Gismervik et al. 2022). The most used are thermal treatments, and as shown here, they are an effective delousing strategy when the warm water bath is 34 °C but not at 28 °C. This lack of delousing effect at 28 °C compared to at 34 °C is also confirmed in a study by Nilsson et al. (2023). The mechanical treatment was shown to not have a delousing effect in this study, yet it is a widely used treatment with previously documented efficacy removing 81–100% of lice (Gismervik et al. 2017; Stien et al. 2023). The treatment experience at an experimental facility is undoubtedly different from a commercial setting. Nevertheless, in this study, the delousing effect of the mechanical treatment could not be separated from the handling effect or the handling itself was solely responsible for the delousing. Although sedated fish from one of the experimental tanks had higher lice loads than non-sedated fish, the treatment was not found to have an effect on the delousing efficacy.
Through the two rounds of experiments, it was shown that lice load decreases with time and that handling of the salmon had an additional delousing effect. This incidental delousing effect due to handling and crowding has been observed on commercial farms and documented in studies where control groups have experienced reductions in lice along with the treatment groups (Reynolds 2014; Powell et al. 2015; Coates et al. 2021). Specifically, Powell et al. (2015) reported a 40% decrease due to handling in an experimental setting, and in a commercial scale investigation by Reynolds (2014) there was a 51% reduction in lice in a saltwater bath while the freshwater treatment lice were reduced by 95%. Coates et al. (2021) suggests that synergy between mechanical treatment and either freshwater or warm water baths increases the delousing efficacy particularly on farms. While this study examined the efficacy of combined treatments, it did not establish if synergy occurs when two or more treatments are used together. Rather, the delousing models calculated the relative contribution of each treatment in combination to the observed lice load with the assumption that the effects are additive. Neither freshwater nor thermal treatments are necessarily fatal to the mobile stages of lice (Andrews and Horsberg 2020; 2021) but can cause them to detach from their hosts with the aid of incidental mechanical delousing (Groner et al. 2019; Coates et al. 2021). Detached mobile lice can potentially recover and re-infect their hosts and thus it is important that at farms the lice are filtered from treatment water and removed (Groner et al. 2019).
While this study and others (Reynolds 2014; Powell et al. 2015; Mc Dermott et al. 2021; Thompson et al. 2023) found freshwater bath treatments to have high efficacy still others have documented a limited effect (Stone et al. 2002; Connors et al. 2008; Wright et al. 2016; Thompson et al. 2023). This observed inconsistency of freshwater bath efficacy may be partly attributed to the duration of the bath, chemistry and salinity of the water used, phenotypic variability of louse populations, and developmental stage of the lice. Those studies which featured a bath of 3 h or less showed limited efficacy (Stone et al. 2002; Wright et al. 2016), but after a 4-h bath, high efficacy has been demonstrated (Reynolds 2014; Powell et al. 2015, this study). Furthermore, because attached stages of lice are more sensitive to low salinity, a shorter bath at a higher salinity could remove attached stages; on the other hand, mobile stages require low salinity and a longer bath, or a combined treatment to effectively remove them (Connors et al. 2008; Powell et al. 2015; Wright et al. 2016; Andrews & Horsberg 2020, this study). Other aspects of the water chemistry may also be influential but are not well understood (Powell et al. 2015; Thompson et al. 2023). Hyposaline water created through membrane filtration of seawater has a higher salinity (~ 5 ppt) but lower calcium and magnesium than freshwater, and a 4.5 h treatment removes nearly all mobile stages (Mc Dermott et al. 2021). When placed in fresh or brackish water, the mobile stages have the capacity to osmoregulate, and depending on the size of the host may take refuge in its mucus and cutaneous layer (Hahnenkamp & Fyhn 1985; Connors et al. 2008; Borchel et al. 2021). A selection for and enhancement of those responses is one path through which the louse may develop a resistance to the freshwater treatments (Coates et al. 2021). Although the freshwater treatment was shown to be generally and highly effective at removing lice in this study, there was some variability between tanks and delousing never reached 100% as seen elsewhere. As discussed, some of those differences may be due to different tolerances of the lice across the experimental rounds, the lesser incidental mechanical delousing here in comparison to commercial scale applications, or due to some combination of fish size and tank effect.
The efficacy of thermal treatments or freshwater baths freshwater is influenced by the specific parameters of the treatment and the different tolerances of the lice. Andrews et al. (2021) demonstrated that geographically isolated populations of salmon lice have different sensitivities to thermal treatment temperatures, and Coates et al. (2021) suggests that further adaptation could be selected for through repeated warm water treatments. Nevertheless, it is possible that there is an upper thermal limit for salmon lice which has been demonstrated with other arthropods (Gilchrist & Huey 1999; Coates et al. 2021), but because of welfare concerns for the host that upper limit is unattainable (Gismervik et al. 2019; Nilsson et al. 2019; Bui et al. 2022). Still, as seen in this study the efficacy of the thermal treatment is dependent on the exposure temperature with the lower temperature tried here (28 °C) having no effect. A study by Nilsson et al. (2023) further examined delousing over a range of exposure temperatures from 28 to 36 °C and for durations of up to 2 min found that higher temperatures led to greater detachment of lice with little additional detachment occurring past 30 s. Nevertheless, no more than 80% of mobile stages detached within the first 30 s (Nilsson et al. 2023), but at commercial scale where there is a greater potential for incidental mechanical delousing higher rates of 75–100% can be achieved (Grøntvedt et al. 2015). Apart from the handling effect, thermal treatments have no reported delousing effect on the attached stages of lice (Grøntvedt et al. 2015; Roth 2016; Nilsson et al. 2023). The finding by Andrews and Horsberg (2021) of infectious copepodids regaining normal behavior after a 34 °C exposure further suggests that the attached stages are largely unaffected by the treatment.
As seen in this study and as discussed above, both warm water and freshwater baths can be effective delousing treatments under certain conditions, and where one has a lesser impact on certain stages the other has a demonstrated strength. Thus, a treatment strategy which applies them in combination could be more reliable and effective than either alone, especially when targeting both mobile and attached stages. It was also found here that a freshwater bath alone and combination treatments with other treatments besides the 34 °C exposure were highly effective. Although the mechanical treatment was not found to predict significant delousing, combination treatments did involve greater handling and likely more incidental delousing than would otherwise be found. At commercial scale, there is also a greater degree of crowding and handling which implies greater incidental delousing. However, when considering delousing strategies, the marginal increases of combined treatments in delousing efficacy should be balanced against the possibility of additional welfare impact.
Delousing impact on welfare
This investigation was carried out on a laboratory scale and conditions at commercial farms will be different, with greater application and welfare challenges. The fish used here were in good health condition relative to what is often found on farms. Diseases, including pancreatic disease, cardiomyopathy syndrome, and amoebic gill disease are prevalent on farms and such comorbidities are believed to lead to a greater cumulative mortality when they interact with treatments but the topic remains understudied (Oliveira et al. 2021; Sviland Walde et al. 2021; Stien et al. 2023). The overall welfare of the fish declined throughout the duration of the experiment regardless of treatment group. Although poor welfare outcomes were found in all fish, the neuroendocrine results showed that fish in all treatment groups examined were able to respond to a new stressor. In this aspect, our results suggest that although acutely stressful, none of the treatments induced any long-term effects on stress coping ability. Had the treatment or experimental conditions induced a severe chronic activation of the stress response in the fish their ability to respond to an additional stressor would likely have been reduced (Wendelaar Bonga 1997).
Statistical analysis of the welfare assessment was challenged by the high prevalence and large variability of unwanted welfare outcomes in all post-treatment groups including negative controls, and by the numerous variables examined. Thus, the statistical models generally had poor fits, but nonetheless show a differential impact of treatments with the 34 °C warm water bath leading to worse welfare outcomes. Three weeks following the treatment application fish treated with a 34 °C warm water bath, but not the lower temperature bath or any other treatment, were predicted to have an increased prevalence of severe injuries to their snouts and fins. The 34 °C thermally treated fish were the only group with lower condition factor and growth which supports the hypothesis that 34 °C thermal treatment affects welfare more than the other treatments. The initial reports documenting the welfare impact of commercially applied thermally delousing concluded that the welfare impact was no worse than what is typically observed after handling and crowding though there was an increase in snout and fin injuries as seen here (Grøntvedt et al. 2015). Expanding the scope of investigation, later studies found that thermal exposure induced an extreme behavioral response indicative of pain (Nilsson et al. 2019). In an effort to distinguish between handling and thermal damage, Moltumyr et al. (2021) sedated fish and held them for 30 s at 34 °C, finding no difference from the control except for minor fin damage attributed to a strong behavioral response in the treatment chamber despite sedation. The results of this investigation conform to these earlier findings with much of the unwanted welfare outcomes likely due to handling and crowding, but a significant if minor increase caused by the 34 °C thermal treatment. Furthermore, the sedation treatment here led to a decrease in the prevalence of unwanted outcomes when combined with other treatments including warm water baths. This is in line with Folkedal et al. (2021) who found that anesthesia prior to delousing led to improved welfare during thermal treatment for salmon lice.
This investigation also showed that the application of a freshwater bath led to no additional welfare impact beyond those caused by the handling and crowding of fish. Models indicated that freshwater treatment increased eye injuries in the short term, but the procedural control predicted the greatest increase in the likelihood of unwanted outcomes. Thus, it is probable that the extra handling steps taken to transfer fish undergoing freshwater treatment led to the increase in eye injuries. Freshwater baths were first used at Australian salmon farms in the 1980s as a treatment against amoebic gill disease (AGD), but the typically duration of the bath was 2 h for that application (Powell et al. 2001; 2015). Previous studies have found that treatment of AGD and salmon lice using freshwater baths caused no acute osmoregulatory disturbance, no prolonged behavioral abnormalities, and minor physiological disruption consistent with a normal stress response (Powell et al. 2001, 2015; Reynolds 2014; Thompson et al. 2023). Thus, the literature and this study demonstrate that the freshwater bath itself is not necessarily harmful. As suggested by Barrett et al. (2022), freshwater baths are often chosen as a delousing method when the farmed fish are in a weakened state because of the belief that it is the gentlest of the non-medicinal treatment options. However, a recognized challenge of applying freshwater treatments at farms is the sourcing of water and maintaining of its quality throughout the duration of the day long operation (Powell et al. 2015). One solution to the sourcing problem is to use semi-permeable membrane desalinization to create hyposaline water which has ~ 5 ppt salinity but nonetheless has been shown to remove nearly all mobile stages (Mc Dermott et al. 2021; Thompson et al. 2023). However, there is a need for greater investigation since the physiological and welfare impacts of hyposaline water are understudied, and because little is known about the potential for salmon lice to evolve resistance to these treatments (Coates et al. 2021; Thompson et al. 2023).
Achieving good welfare outcomes for farmed Atlantic salmon requires the consideration of the animals’ ‘quality of life as perceived by the animals themselves’ which can be partly achieved by maintaining optimal welfare conditions (Stien et al. 2013). Throughout the experiments efforts were made to provide an optimal environment for the welfare of the experimental animals which is assumed to be better than the conditions typically found on a farm. However, as with experimental fish the husbandry of farmed fish requires their occasional crowding and handling which can lead to injury, stress, and reduced welfare outcomes (AHAW 2008). In this investigation, efforts were made beyond what is typical at a commercial farm to reduce the impact of the handling, crowding, and treatments on the fish, but the results still indicate generally poor welfare outcomes. Seemingly, the negative welfare impact is unavoidable when handling and crowding fish though the outcome from additional treatment applications differs. Still, there were no indications that delousing treatments were associated with long-term effects on neuroendocrine parameters or stress coping ability. The experimental scale mechanical treatment used here, like the freshwater treatment, did not lead to worse outcomes than procedural controls, but it is essentially little more than an additional handling step. At a commercial scale, it is a more intense procedure found to increase gill bleeding and scale loss, but also found to remove 82–100% of lice (Gismervik et al. 2017). The mechanical treatment here did not result in any additional decline in lice which suggests that the delousing device, and the precautions taken to reduce welfare impact also diminished the physical mechanism responsible for delousing.
Conclusion
The decision to apply a non-medicinal delousing treatment at a farm represents a tradeoff between the welfare of the fish and the environmental impact of the salmon lice on wild salmonid populations (Macaulay et al. 2022). Thus, under the current paradigm with open net pens (Dempster et al. 2021) and regulatory lice limits (Anon 2012), delousing treatments and their welfare impacts are unavoidable. However, the delousing strategies can be optimized to reduce the welfare impact and maximize the delousing efficacy. Here, we showed that all treatments and handling have negative welfare impacts on fish but the use of 34 °C warm water baths leads to relatively worse welfare outcomes while freshwater baths do not. Furthermore, we found that freshwater bath treatments alone and in combination had the best efficacy, while 34 °C warm water baths also had good efficacy. Although the freshwater results are promising and suggest that it should be the preferred delousing treatment, the reports of its efficacy in previous studies are mixed. Therefore, further efforts should be made to optimize the freshwater bath delousing method by investigating efficacy over time, the sources of variance, evolved resistance of lice, and the suitability of hyposaline water.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information. Three spreadsheets are included in the supplementary files with data that were used for the analyses presented in this paper.
References
Aaen SM, Helgesen KO, Bakke MJ, Kaur K, Horsberg TE (2015) Drug resistance in sea lice: a threat to salmonid aquaculture. Trends Parasitol 31:72–81. https://doi.org/10.1016/j.pt.2014.12.006
AHAW (2008) Scientific Opinion of the Panel on Animal Health and Welfare on a request from the European Commission on Animal welfare aspects of husbandry systems for farmed Atlantic salmon. EFSA J 736:1–31. https://doi.org/10.2903/j.efsa.2008.736
Andrews M, Horsberg TE (2020) Sensitivity towards low salinity determined by bioassay in the salmon louse, Lepeophtheirus salmonis (Copepoda: Caligidae). Aquac 514:734511. https://doi.org/10.1016/j.aquaculture.2019.734511
Andrews M, Horsberg TE (2021) In vitro bioassay methods to test the efficacy of thermal treatment on the salmon louse. Lepeophtheirus salmonis. Aquac. 532:736013. https://doi.org/10.1013/j.aquaculture.2020.736013
Anon (1998) Forskrift om tiltak mot lakselus, Hordaland og Sogn og Fjordane (Regulations on measures against salmon lice, Hordaland and Sogn og Fjordane). In Lovedata, Ministry of Justice and the Faculty of Law in Oslo. Accessed May 19, 2023. https://lovdata.no/dokument/LFO/forskrift/1998-06-01-540
Anon (2009) Strategi for en miljømessig bærekraftig havbruksnæring (Strategy for an environmentally sustainable aquaculture industry). Fiskeri- og kystdepartementet Rapport (Minister of Fisheries and Coastal Affairs Report). https://www.regjeringen.no/no/dokumenter/strategi-for-en-miljomessig-barekraftig-/id571066/. Accessed 23 May 2023
Anon (2012) Forskrift om bekjempelse av lakselus i akvakulturanlegg (Regulations on the control of salmon lice in aquaculture facilities). In Lovedata, Ministry of Justice and the Faculty of Law in Oslo. Accessed May 19, 2023. https://lovdata.no/dokument/SF/forskrift/2012-12-05-1140
Bang Jensen B, Qviller L, Toft N (2020) Spatio-temporal variations in mortality during the seawater production phase of Atlantic salmon (Salmo salar) in Norway. J Fish Dis 43:445–457. https://doi.org/10.1111/jfd.13142
Barrett L, Oldham T, Kristiansen TS, Oppedal F, Stien LH (2022) Declining size-at-harvest in Norwegian salmon aquaculture: lice, disease, and the role of stunboats. Aquac 559:738440. https://doi.org/10.1016/j.aquaculture.2022.738440
Bøe K, Ellingsen-Dalskau K (2022) Er det mulig å benytte termisk behandling mot lakselus i norsk oppdrettsnæring på en velferdsmessig forsvarlig måte? (Is it possible to use thermal treatment against salmon lice in the Norwegian aquaculture industry in a welfare-responsible way?). Accessed May 19, 2023. https://www.radetfordyreetikk.no/wp-content/uploads/2022/06/Radet-for-dyreetikk-Uttalelse-om-termisk-avlusning.pdf
Bøhn T, Gjelland KØ, Serra-Llinares RM, Finstad B, Primicerio R, Nilsen R, Karlsen Ø, Sandvik AD, Skilbrei OT, Elvik KMS, Skaala Ø, Bjørn PA, Vamosi S (2020) Timing is everything: survival of Atlantic salmon Salmo salar postsmolts during events of high salmon lice densities. J Appl Ecol 57:1149–1160. https://doi.org/10.1111/1365-2664.13612
Borchel A, Heggland EI, Nilsen F (2021) The transcriptomic response of adult salmon lice (Lepeophtheirus salmonis) to reduced salinity. Comp Biochem Physiol Part D Genomics Proteomics 37:100778. https://doi.org/10.1016/j.cbd.2020.100778
Bovenkerk B, Meijboom F (2020) Ethics and the welfare of fish. In: Kristiansen TS, Fernö A, Pavlidis MA, van de Vis H (eds) The welfare of fish. Springer International Publishing
Bovenkerk B, Meijboom FLB (2013) Fish welfare in aquaculture: explicating the chain of interactions between science and ethics. J Agric Environ Ethics 26:41–61. https://doi.org/10.1007/s10806-012-9395-x
Bowers JM, Mustafa A, Speare DJ, Conboy GA, Brimacombe M, Sims DE, Burka JF (2000) The physiological response of Atlantic salmon, Salmo salar L., to a single experimental challenge with sea lice. Lepeophtheirus Salmonis J Fish Dis 23:165–172. https://doi.org/10.1046/j.1365-2761.2000.00225.x
Brett J, Groves T (1979) Physiological Energetics Fish Physiology 8:280–352
Bui S, Halttunen E, Mohn AM, Vagseth T, Oppedal F (2018) Salmon lice evasion, susceptibility, retention, and development differ amongst host salmonid species. ICES J Mar Sci 75:1071–1079. https://doi.org/10.1093/icesjms/fsx222
Bui S, Madaro A, Nilsson J, Fjelldal PG, Iversen MH, Brinchman MF, Venas B, Schroder MB, Stien LH (2022) Warm water treatment increased mortality risk in salmon. Vet Anim Sci 17:100265. https://doi.org/10.1016/j.vas.2022.100265
Coates A, Phillips BL, Bui S, Oppedal F, Robinson NA, Dempster T (2021) Evolution of salmon lice in response to management strategies: a review. Rev Aquac 13:1397–1422. https://doi.org/10.1111/raq.12528
Connors BM, Juarez-Colunga E, Dill LM (2008) Effects of varying salinities on Lepeophtheirus salmonis survival on juvenile pink and chum salmon. J Fish Biol 72:1825–1830. https://doi.org/10.1111/j.1095-8649.2008.01839.x
Costello MJ (2006) Ecology of sea lice parasitic on farmed and wild fish. Trends Parasitol 22:475–483. https://doi.org/10.1016/j.pt.2006.08.006
Costello MJ (2009) How sea lice from salmon farms may cause wild salmonid declines in Europe and North America and be a threat to fishes elsewhere. Proc Biol Sci 276:3385–3394. https://doi.org/10.1098/rspb.2009.0771
Dempster T, Overton K, Bui S, Stien LH, Oppedal F, Karlsen O, Coates A, Phillips BL, Barrett LT (2021) Farmed salmonids drive the abundance, ecology and evolution of parasitic salmon lice in Norway. Aquac Environ Interact 13:237–248. https://doi.org/10.3354/aei00402
FAO-FIGIS (2021) Fisheries Global Information System (FAO-FIGIS). Accessed August 23rd, 2021. http://www.fao.org/fishery
Fast MD, Muise DM, Easy RE, Ross NW, Johnson SC (2006) The effects of Lepeophtheirus salmonis infections on the stress response and immunological status of Atlantic salmon (Salmo salar). Fish Shellfish Immunol 21:228–241. https://doi.org/10.1016/j.fsi.2005.11.010
Finstad B, Bjorn PA, Grimnes A, Hvidsten NA (2000) Laboratory and field investigations of salmon lice [Lepeophtheirus salmonis (Kroyer)] infestation on Atlantic salmon (Salmo salar L.) post-smolts. Aquac Res 31:795–803. https://doi.org/10.1046/j.1365-2109.2000.00511.x
Fjelldal PG, Hansen TJ, Karlsen O (2020) Effects of laboratory salmon louse infection on osmoregulation, growth and survival in Atlantic salmon. Conserv Physiol 8:coaa023. https://doi.org/10.1093/conphys/coaa023
Fjelldal PG, Fraser TWK, Hansen TJ, Karlsen O, Bui S (2022) Effects of laboratory salmon louse infection on mortality, growth, and sexual maturation in Atlantic salmon. ICES J Mar Sci 79:1530–1538. https://doi.org/10.1093/icesjms/fsac078
Folkedal O, Utskot SO, Nilsson J (2021) Thermal delousing in anaesthetised small Atlantic salmon (Salmo salar) post-smolts: a case study showing the viability of anaesthesia prior to delousing for improved welfare during treatment for salmon lice. Anim Welfare 30:117–120. https://doi.org/10.7120/09627286.30.2.117
Gilchrist GW, Huey RB (1999) The direct response of Drosophila melanogaster to selection on knockdown temperature. Heredity (edinb) 83(Pt 1):15–29. https://doi.org/10.1038/sj.hdy.6885330
Gismervik K, Gasnes SK, Gu J, Stien LH, Madaro A, Nilsson J (2019) Thermal injuries in Atlantic salmon in a pilot laboratory trial. Vet Anim Sci 8:100081. https://doi.org/10.1016/j.vas.2019.100081
Gismervik K, Nielsen K, Lind M, Viljugrein H (2017) Mekanisk avlusing med FLS-avlusersystem-dokumentasjon av fiskevelferd og effekt mot lus (mechanical lice removal with FLS lice removal system documentation of fish welfare and effect against lice). Veterinærinstituttets Rapport (Norwegian Veterinary Institute Report). Accessed May 23, 2023. https://www.vetinst.no/rapporter-og-publikasjoner/rapporter/2017/mekanisk-avlusing-dokumentasjon-av-fiskevelferd-og-effekt-mot-lus
Gismervik K, Harasimczuk E, Nielsen K, Gåsnes S, Tørud B, Mejdell C (2022) Fish welfare. In: Sommerset I (Ed) Norwegian Fish Health Report 2021. Norwegian Veterinary Institute Report series #2a
Grefsrud ES, Bjørn PA, Grøsvik BE, Hansen PK, Husa V, Karlsen Ø, Kvamme BO, Samuelsen OB, Sandlund N, Solberg MF (2022) Risikorapport norsk fiskeoppdrett 2022-kunnskapsstatus-Effekter på miljø og dyrevelferd i norsk fiskeoppdrett (Norwegian fish farming risk report 2022- Knowledge status: effects on the environment and animal welfare in Norwegian fish farming). Rapport fra havforskningen (Report from the Institute of Marine Research). Accessed May 19, 2023. https://www.hi.no/hi/nettrapporter/rapport-fra-havforskningen-2022-13
Groner ML, Laurin E, Stormoen M, Sanchez J, Fast MD, Revie CW (2019) Evaluating the potential for sea lice to evolve freshwater tolerance as a consequence of freshwater treatments in salmon aquaculture. Aquac Environ Interact 11:507–519. https://doi.org/10.3354/aei00324
Grøntvedt RN, Nerbøvik I-KG, Viljugrein H, Lillehaug A, Nilsen H, Gjerve A (2015) Termisk avlusing av laksefisk–dokumentasjon av fiskevelferd og effekt (thermal deworming of salmon fish – documentation of fish welfare and effect). Veterinærinstituttets Rapport (Norwegian Veterinary Institute Report). Accessed May 23, 2023. https://www.vetinst.no/rapporter-og-publikasjoner/rapporter/2015/termisk-avlusning-av-laksefisk-dokumentasjon-av-fiskevelferd-og-effekt
Hahnenkamp L, Fyhn HJ (1985) The osmotic response of salmon Louse, Lepeophtheirus Salmonis (Copepoda, Caligidae), during the transition from sea water to fresh water. J Comparative Physiol B-Biochem Systems Environmental Physiol 155:357–365. https://doi.org/10.1007/Bf00687479
Hamre LA, Glover KA, Nilsen F (2009) Establishment and characterisation of salmon louse (Lepeophtheirus salmonis (Krøyer 1837)) laboratory strains. Parasitol Int 58:451–460. https://doi.org/10.1016/j.parint.2009.08.009
Hamre LA, Eichner C, Caipang CM, Dalvin ST, Bron JE, Nilsen F, Boxshall G, Skern-Mauritzen R (2013) The salmon louse Lepeophtheirus salmonis (Copepoda: Caligidae) life cycle has only two chalimus stages. PLoS One 8:e73539. https://doi.org/10.1371/journal.pone.0073539
Hamre LA, Bui S, Oppedal F, Skern-Mauritzen R, Dalvin S (2019) Development of the salmon louse Lepeophtheirus salmonis parasitic stages in temperatures ranging from 3 to 24 degrees C. Aquac Environ Interact 11:429–443. https://doi.org/10.3354/aei00320
Hartig F (2019) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R Package Version 02:4
Hoglund E, Hogberget R, Atland A, Haraldstad T, Overli O, Vindas MA (2021) Effects of repeated short episodes of environmental acidification on Atlantic salmon (Salmo salar) from a landlocked population. Sci Total Environ 753:141403. https://doi.org/10.1016/j.scitotenv.2020.141403
Kristiansen TS, Bracke MBM (2020) A brief look into the origins of fish welfare science. In: Kristiansen TS, Fernö A, Pavlidis MA, van de Vis H (eds) The Welfare of Fish. Springer International Publishing
Macaulay G, Barrett LT, Dempster T (2022) Recognising trade-offs between welfare and environmental outcomes in aquaculture will enable good decisions. Aquac Environ Interact 14:219–227. https://doi.org/10.3354/aei00439
Mc Dermott T, D’Arcy J, Kelly S, Downes JK, Griffin B, Kerr RF, O’Keeffe D, O’Ceallachain M, Lenighan L, Scholz F (2021) Novel use of nanofiltered hyposaline water to control sea lice (Lepeophtheirus salmonis and Caligus elongatus) and amoebic gill disease, on a commercial Atlantic salmon (Salmo salar) farm. Aquac Rep 20:100703
Moltumyr L, Gismervik K, Gu J, Gasnes SK, Kristiansen TS, Ronnestad I, Nilsson J, Stien LH (2021) Does the thermal component of warm water treatment inflict acute lesions on Atlantic salmon (Salmo salar)? Aquac 532:736048. https://doi.org/10.1016/j.aquaculture.2020.736048
Moltumyr L, Nilsson J, Madaro A, Seternes T, Winger FA, Rønnestad I, Stien LH (2022) Long-term welfare effects of repeated warm water treatments on Atlantic salmon (Salmo salar). Aquac 548:737670. https://doi.org/10.1016/j.aquaculture.2021.737670
Moore G (2021) Thermal delousing allowed to continue in Norway. Fish Farming Expert. Bergen Norway. Accessed May 23, 2023. https://www.fishfarmingexpert.com/norwegian-food-safety-authority-salmon-farming-sea-lice/thermal-delousing-allowed-to-continue-in-norway/1383096
Myhre Jensen E, Horsberg TE, Sevatdal S, Helgesen KO (2020) Trends in de-lousing of Norwegian farmed salmon from 2000–2019-consumption of medicines, salmon louse resistance and non-medicinal control methods. PLoS. One 15:e0240894. https://doi.org/10.1371/journal.pone.0240894
Nilsson J, Moltumyr L, Madaro A, Kristiansen TS, Gasnes SK, Mejdell CM, Gismervik K, Stien LH (2019) Sudden exposure to warm water causes instant behavioural responses indicative of nociception or pain in Atlantic salmon. Vet Anim Sci 8:100076. https://doi.org/10.1016/j.vas.2019.100076
Nilsson J, Barrett LT, Mangor-Jensen A, Nola V, Harboe T, Folkedal O (2023) Effect of water temperature and exposure duration on detachment rate of salmon lice (Lepeophtheirus salmonis); testing the relevant thermal spectrum used for delousing. Aquac 562:738879. https://doi.org/10.1016/j.aquaculture.2022.738879
Nilsson J, Gismervik K, Nielsen KV, Iversen MH, Noble C, Kolarevic J, Frotjold H, Nilsen K, Wilkinson E, Klakegg B (2022) Laksvel-Standardisert operasjonell velferdsovervåking for laks i matfiskanlegg (Salmon welfare - Standardized operational welfare monitoring for salmon in food fisheries). Rapport fra havforskningen (Report from the Institute of Marine Research). Accessed May 19, 2023. https://www.hi.no/hi/nettrapporter/rapport-fra-havforskningen-2022-14
Noble C, Gismervik K, Iversen MH, Kolarevic J, Nilsson J, Stien LH, Turnbull JF (2018) Welfare Indicators for farmed Atlantic salmon: tools for assessing fish welfare. Nofima report. Accessed May 19, 2023. https://nofima.com/publication/1636395/
Norwegian Ministry of Trade, Industry and Fisheries (2022) Aquaculture statistics: Atlantic salmon and rainbow trout statistics. Accessed October 20th, 2022. https://www.fiskeridir.no/English/Aquaculture/Statistics/Atlantic-salmon-and-rainbow-trout
Oliveira VHS, Dean KR, Qviller L, Kirkeby C, Bang Jensen B (2021) Factors associated with baseline mortality in Norwegian Atlantic salmon farming. Sci Rep 11:14702. https://doi.org/10.1038/s41598-021-93874-6
Overli O, Nordgreen J, Mejdell CM, Janczak AM, Kittilsen S, Johansen IB, Horsberg TE (2014) Ectoparasitic sea lice (Lepeophtheirus salmonis) affect behavior and brain serotonergic activity in Atlantic salmon (Salmo salar L.): Perspectives on animal welfare. Physiol Behav 132:44–50. https://doi.org/10.1016/j.physbeh.2014.04.031
Overton K, Dempster T, Oppedal F, Kristiansen TS, Gismervik K, Stien LH (2019) Salmon lice treatments and salmon mortality in Norwegian aquaculture: a review. Rev Aquac 11:1398–1417. https://doi.org/10.1111/raq.12299
Persson D, Nodtvedt A, Aunsmo A, Stormoen M (2022) Analysing mortality patterns in salmon farming using daily cage registrations. J Fish Dis 45:335–347. https://doi.org/10.1111/jfd.13560
Powell MD, Parsons HJ, Nowak BF (2001) Physiological effects of freshwater bathing of Atlantic salmon (Salmo salar) as a treatment for amoebic gill disease. Aquac 199:259–266. https://doi.org/10.1016/S0044-8486(01)00573-7
Powell MD, Reynolds P, Kristensen T (2015) Freshwater treatment of amoebic gill disease and sea-lice in seawater salmon production: Considerations of water chemistry and fish welfare in Norway. Aquac 448:18–28. https://doi.org/10.1016/j.aquaculture.2015.05.027
R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Reynolds P (2014) Ferskvannsavlusing i brønnbåt: Oppsummeringsrapport/analyser av tidligere forsøk (The use of freshwater to control infestations of the sea louse Lepeophtheirus salmonis K on Atlantic salmon Salmo salar). Gildeskal Research Station Technical Report. Accessed May 19, 2023. https://www.fhf.no/prosjekter/prosjektbasen/901006/
Roth B (2016) Avlusing av laksefisk med Optilice: Effekt på avlusing og fiskevelferd (Deworming of salmon with Optilice: Effect on deworming and fish welfare). Nofima Report. https://nofima.no/publikasjon/1408716/. Accessed 23 May 2023
Samsing F, Oppedal F, Dalvin S, Johnsen I, Vagseth T, Dempster T (2016) Salmon lice (Lepeophtheirus salmonis) development times, body size, and reproductive outputs follow universal models of temperature dependence. Can J Fish Aquat Sci 73:1841–1851. https://doi.org/10.1139/cjfas-2016-0050
Stien LH, Bracke MBM, Folkedal O, Nilsson J, Oppedal F, Torgersen T, Kittilsen S, Midtlyng PJ, Vindas MA, Overli O, Kristiansen TS (2013) Salmon Welfare Index Model (SWIM 1.0): a semantic model for overall welfare assessment of caged Atlantic salmon: review of the selected welfare indicators and model presentation. Rev Aquac 5:33–57. https://doi.org/10.1111/j.1753-5131.2012.01083.x
Stien LH, Torud B, Gismervik K, Lien ME, Medaas C, Osmundsen T, Kristiansen TS, Storkersen KV (2020) Governing the welfare of Norwegian farmed salmon: three conflict cases. Mar Policy 117:103969. https://doi.org/10.1016/j.marpol.2020.103969
Stien LH, Thompson CRS, Fjelldal PG, Oppedal F, Kristiansen T, Sæther PA, Bølgen PM, Martinsen L (2023) Production, fasting and delousing of triploid and diploid salmon in Northern Norway: Report for the 2020 Generation. Rapport fra Havforskningen (Report from the Institute of Marine Research). Accessed May 19, 2023. https://www.hi.no/hi/nettrapporter/rapport-fra-havforskningen-en-2023-20
Stone J, Boyd S, Sommerville C, Rae GH (2002) An evaluation of freshwater bath treatments for the control of sea lice, Lepeophtheirus salmonis (Kroyer), infections in Atlantic salmon, Salmo salar L. J Fish Dis 25:371–373. https://doi.org/10.1046/j.1365-2761.2002.00370.x
Sviland Walde C, Bang Jensen B, Pettersen JM, Stormoen M (2021) Estimating cage-level mortality distributions following different delousing treatments of Atlantic salmon (Salmo salar) in Norway. J Fish Dis 44:899–912. https://doi.org/10.1111/jfd.13348
Taranger GL, Karlsen O, Bannister RJ, Glover KA, Husa V, Karlsbakk E, Kvamme BO, Boxaspen KK, Bjorn PA, Finstad B, Madhun AS, Morton HC, Svasand T (2015) Risk assessment of the environmental impact of Norwegian Atlantic salmon farming. ICES J Mar Sci 72:997–1021. https://doi.org/10.1093/icesjms/fsu132
Thompson CRS, Fields DM, Bjelland RM, Chan VBS, Durif CMF, Mount A, Runge JA, Shema SD, Skiftesvik AB, Browman HI (2019) The planktonic stages of the salmon louse (Lepeophtheirus salmonis) are tolerant of end-of-century pCO(2) concentrations. PeerJ 7:e7810. https://doi.org/10.7717/peerj.7810
Thompson CRS, Madaro A, Oppedal F, Stien LH, Bui S (2023) Delousing efficacy and physiological impacts on Atlantic salmon of freshwater and hyposaline bath treatments. Rapport fra Havforskningen (Report from the Institute of Marine Research). Accessed May 19, 2023. https://www.hi.no/hi/nettrapporter/rapport-fra-havforskningen-en-2023-11
Thorstad EB, Whoriskey F, Uglem I, Moore A, Rikardsen AH, Finstad B (2012) A critical life stage of the Atlantic salmon Salmo salar: behaviour and survival during the smolt and initial post-smolt migration. J Fish Biol 81:500–542. https://doi.org/10.1111/j.1095-8649.2012.03370.x
Vindas MA, Johansen IB, Folkedal O, Hoglund E, Gorissen M, Flik G, Kristiansen TS, Overli O (2016) Brain serotonergic activation in growth-stunted farmed salmon: adaption versus pathology. R Soc Open Sci 3:160030. https://doi.org/10.1098/rsos.160030
Vollset KW, Dohoo I, Karlsen O, Halttunen E, Kvamme BO, Finstad B, Wennevik V, Diserud OH, Bateman A, Friedland KD, Mahlum S, Jorgensen C, Qviller L, Krkosek M, Atland A, Barlaup BT (2018) Disentangling the role of sea lice on the marine survival of Atlantic salmon. ICES J Mar Sci 75:50–60. https://doi.org/10.1093/icesjms/fsx104
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625. https://doi.org/10.1152/physrev.1997.77.3.591
Wright DW, Oppedal F, Dempster T (2016) Early-stage sea lice recruits on Atlantic salmon are freshwater sensitive. J Fish Dis 39:1179–1186. https://doi.org/10.1111/jfd.12452
Acknowledgements
The authors thank the technicians that contributed substantially to the execution of the experiments, namely Tone Vågseth, Simon Flavell, Linda Neset, Audun Pedersen, Florian Sambraus, and other staff at the Matre Research Station. This work was funded by the Norwegian Seafood Research Fund (project number 901687), and we thank the project's reference group for their constructive discussions.
Funding
This study was part of the OptiDeLouse project funded by the Norwegian Seafood Research Fund (project number 901687). Open access funding provided by the Norwegian Institute Of Marine Research.
Author information
Authors and Affiliations
Contributions
SB, FO & LHS were responsible for project conception and funding acquisition. SB, FO & CT supervised various aspects of the investigation while SB & FO handled administrative responsibilities. The experiments were designed and resourced by SB, ØØ, JN, LHS, & AM. Further execution of experiments and analysis of samples was conducted by SB, AM, ØØ & WK. Data management and statistical analysis was done by CT with SB. Manuscript preparation was likewise done by CT with SB. All authors were responsible for critical review with contribution to the editing and revision of the manuscript prior to submission.
Corresponding author
Ethics declarations
Ethics approval
This experiment was conducted at the Institute of Marine Research’s facilities in Matre, which is authorized for animal experimentation by the Norwegian Food Safety Authority (facility ID 110), and in accordance with regulations for the use of animals in experimentation (application ID: 27999).
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thompson, C.R.S., Madaro, A., Nilsson, J. et al. Comparison of non-medicinal delousing strategies for parasite (Lepeophtheirus salmonis) removal efficacy and welfare impact on Atlantic salmon (Salmo salar) hosts. Aquacult Int 32, 383–411 (2024). https://doi.org/10.1007/s10499-023-01167-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01167-8