Abstract
Polydorid species (Annelida, Spionidae), which inhabit the shells of the commercially important oyster Crassostrea gigas, were investigated along the coast of Normandy, France. Eight species, including five new records for Normandy (Polydora onagawaensis, Polydora websteri, Boccardia pseudonatrix, Boccardia proboscidea, and Boccardiella hamata) and two first records in European waters (P. onagawaensis and B. pseudonatrix), were identified based on morphological, molecular biological, and ecological characteristics. Polydora onagawaensis, which belongs to the Polydora ciliata/websteri complex, was discovered in the shells of wild and suspended cultured oysters, as well as in limestone substrates. In the phylogenetic analysis of mitochondrial COI gene sequences, specimens of P. onagawaensis collected from Normandy were grouped together with specimens from the USA into a single clade and were distinguished from the other three lineages that comprised Japanese and USA specimens. Polydora websteri inhabited shells of suspended cultured oysters. Polydora hoplura, Dipolydora giardi, and Dipolydora sp. were observed in shells from the sandy oyster culture grounds. Boccardiella hamata has been found in wild oyster shells from muddy oyster culture grounds. Boccardia pseudonatrix was observed in the shells of both the wild and cultured oysters. Adult and juvenile Boccardia proboscidea were observed in coralline algae, as well as in suspended cultured oysters. Mud tubes were observed to protrude from the outer surface of the shells, and abnormal black and calcareous deposits were secreted on the inner surface of the shells against polydorid penetration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family Spionidae Grube, 1850, is found in a wide variety of marine environments worldwide. It is one of the most abundant polychaete groups in Annelida in terms of the number of species and biomass. Within the family Spionidae, the so-called polydorid species comprises nine genera: Polydora Bosc, 1802; Dipolydora Verrill, 1881; Pseudopolydora Czerniavsky, 1881; Boccardia Carazzi, 1893; Polydorella Augener, 1914; Tripolydora Woodwick, 1964; Boccardiella Blake & Kudenov, 1978; Carazziella Blake & Kudenov, 1978; and Amphipolydora Blake, 1983; each of them has the common morphological characteristics of specially modified major spines in the fifth chaetigerous segment (Blake 1996). They are widely distributed in coastal benthic environment. A variety of habitat types are observed depending on the species, that is, some species are known to create mud tubes in the bottom sediment, utilize sand or mud deposits in crevices of various substrates (e.g., rocks and mollusk shells), to inhabit the surface of various organisms (e.g., sponges, brachiopods, and mollusk shells), inside of various organisms (e.g., calcareous algae and sponges), and some excavate burrows in calcareous substrates, such as limestone, mollusk shells, and corals (Blake and Evans 1973; Simon and Sato-Okoshi 2015; Abe et al. 2019a; Abe and Sato-Okoshi 2020). Many have been reported to have symbiotic associations with other marine benthic invertebrates (Martin and Britayev 1998; 2018), and new symbiotic relationships continue to be discovered (Abe and Sato-Okoshi 2020; Abe et al. 2022). Polydorids feed on suspended organic particles or deposited organic matter by exposing a pair of long palps from mud tubes (Dauer et al. 1981). In cases where symbiotic relationships have been observed, it is assumed that they compete with their hosts for food (Abe and Sato-Okoshi 2020).
Some genera in polydorids have long been well known and reported, especially in fisheries (Takahashi 1937; Evans 1969; Kent 1979; Okoshi and Sato-Okoshi 1996; Mortensen et al. 2000; Lleonart et al. 2003; Simon et al. 2006; Sato-Okoshi et al. 2008). For species inhabiting the shells of commercially important mollusk species, whether they excavate the shells (boring) or just inhabit the shells as interstitial or epifaunal (non-boring), a significant infestation can negatively affect the host shells. They often reduce the commercial value of mollusks by damaging their shells, decreasing the growth rate and meat yield, and causing heavy mortality in some cases (Okoshi and Sato-Okoshi 1996; Sato-Okoshi et al. 2008; 2012; 2013; Simon and Sato-Okoshi 2015). With the globalization of the aquaculture industry, commercially important mollusks are constantly transported between countries. Some mollusk-associated organisms, such as boring and non-boring polydorid species, are unintentionally introduced to newer, non-native regions. (Elton 1958; Carlton 1975; Bailey-Brock 2000; Sato-Okoshi et al. 2008; Simon and Sato-Okoshi 2015). Therefore, polydorids have established invasive populations in the waters where they have been introduced (Sato-Okoshi et al. 2012). For species that cause damage to cultured shells, it is necessary to determine the species accurately and suggest preventive measures or control such infestations (Diggles et al. 2002; Simon et al. 2010), but this problem often remains unresolved. Moreover, these unintentionally introduced species are not only a source of economic concern but may also pose a threat ecologically as they can infest indigenous mollusk species in their invasive ranges if they disperse from aquaculture facilities (Elton 1958; Carlton 1975; Cohen and Carlton 1998; Bailey-Brock 2000). Ecological disturbances caused by invasive organisms associated with cultured mollusks, such as oysters, are currently a major cause of diversity losses worldwide (Mack et al. 2000; Miura 2007).
The similarity of morphological characteristics in polydorids has led to confusion in species identification (Sato-Okoshi 1999; 2000; Sato-Okoshi and Takatsuka 2001; Radashevsky and Pankova 2006; 2013; Read 2010; Sato-Okoshi and Abe 2012; 2013). Several species complexes within the polydorid group are morphologically indistinguishable, whereas others show high intraspecific morphological variations. Some species previously thought to be the same include more than one species (Abe et al. 2016; Kondoh et al. 2017; Simon et al. 2019), and cases have been reported in which two species thought to be different were the same (Sato-Okoshi et al. 2017; Malan et al. 2020). Thus, the identification of these species, based on morphology, is often difficult and complex. Accurate species identification is necessary to elucidate the means by which they are transported and to assess the severity of their impact as exotic invasive species.
The Japanese or Pacific oyster Crassostrea gigas (Thunberg, 1793) was introduced in European countries owing to the decline in native populations of the European flat oyster Ostrea edulis Linnaeus, 1758 (Robert et al. 1991) and the Portuguese oyster Crassostrea angulata (Lamarck, 1819) (Grizel and Héral 1991). In France, oyster production is thriving, and oysters have been cultured for many years. Crassostrea gigas oysters have been exported from Japan since 1880. After the disease of C. angulata (1969–1971), large numbers of juvenile (spat) and adult C. gigas were introduced into France at the beginning of 1970, coming directly from Japan and also from British Colombia and Canada (Grizel and Héral 1991). Many polydorid species probably accompanied such voluntary introduction and have since been reported in cultured shells (Ruellet 2004; Royer et al. 2006). Polydorids are not the only annelids to be transported with cultured mollusks. Recently, Marphysa victori (Eunicidae) was reported to have accompanied oysters to France (Abe et al. 2019b; Lavesque et al. 2020).
Successive inventories have reported polydorids in the English Channel (Fauvel 1927; Dauvin et al. 2003; Ruellet 2004; Gully and Cochu 2020; Le Mao et al. 2020). In recent years, it has become necessary to distinguish the species of morphologically similar polydorids, especially the so-called Polydora ciliata/websteri complex (Blake 1996); however, this has not yet been sufficiently re-examined in France. Therefore, species identification using molecular biological methods as well as morphology is required in this region. In this study, we attempted to identify the polydorid species inhabiting commercially important oyster shells along the coast of Normandy using both morphology and molecular biology. Shell infestation by polydorids was investigated not only in oyster shells but also in other calcareous substrates from the Normandy intertidal zone in March 2018. Here, we report the species and their habitat conditions of polydorids 50 years after the mass introduction of Pacific oysters into France, which began in the 1970s.
Materials and methods
Sampling of calcareous substrates and polydorid species
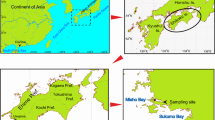
Sampling was conducted along the coast of Normandy in the English Channel from March 19 to 21, 2018 (Fig. 1). Crassostrea gigas oysters were collected from four oyster culture sites: Blainville-sur-Mer (sandy oyster culture ground dotted with rocks together) from the western coast of Cotentin, Saint-Vaast-La-Hougue (muddy oyster culture ground), Quineville (suspended culture) from the eastern coast of Cotentin, and Asnelles (suspended culture) along the Calvados coast. In Normandy, which is currently the most important center of oyster production in France, there has been a rapid increase in the number of oyster farms just after the introduction of the Japanese oyster C. gigas, in two main places of the Cotentin Peninsula: Blainville-sur-Mer on the western coast and Saint-Vaast-La-Hougue on the eastern coast. Blainville-sur-Mer has an extended intertidal zone of up to 5 km at low tide during spring tide. Here, the oyster farms were located between 1 and 3 km from the seashore, and the tables were placed on heterogeneous sediment from sand to gravel. Saint-Vaast-La-Hougue is another important hot spot for oyster production in Normandy, with a moderate intertidal zone of up to 2 km, on muddy and sandy sediments, and the tables occupy the entire intertidal zone. Twenty cultured oysters that fell out of the net bags and 20 wild oysters that were attached to rocks in Blainville-sur-Mer were randomly collected during the March spring tide. Twenty wild oysters from muddy oyster beds in Saint-Vaast-La-Hougue were randomly collected at sites during the March spring tide. Twenty cultured oysters each, from Quineville and Asnelles, were obtained randomly from harvested ones cultured in cages suspended in the intertidal zone. Additional samples were 20 harvested scallops Pecten maximus collected by dredging near Asnelles. Calcareous algae and clumps of limestone and concrete blocks covered with barnacles and mussels were also sampled from intertidal zones in Barfleur Cape, northeast Cotentin, and Luc-sur-Mer (Calvados), respectively, to identify polydorid species that inhabit wild calcareous substrates.
Polydorid species that bore into or inhabit the shells, limestone, and coralline algae were extracted by fracturing calcareous substrates with cutting pliers. Five to thirty individuals of each species of extracted worms were examined to determine their morphological characteristics, state of sexual maturity, and presence and conditions of oocytes and egg capsules under a stereomicroscope (SZX16, Olympus, Tokyo, Japan) and a biological microscope (BX53F, Olympus, Tokyo, Japan), in live individuals and specimens fixed in 10% formalin in seawater. For molecular analysis, one–eight worms per species were directly preserved in 99.5% ethanol.
Shell infestation was investigated by observing outer and inner shell surfaces and taking photographs using a digital camera (Tough TG-5, Olympus, Tokyo, Japan). Abnormal shell formation, along with the presence of black organic or calcareous deposits, indicates worm infestation. Worms, if present, were extracted from such shells and their species-level identification was determined. The number of shells with the abnormal formation due to polydorid invasion was examined for each species and valued as the occurrence rate of abnormal shell formation.
Specimens were deposited at the French National Natural History Museum, Paris, France, and the National Museum of Nature and Science, Tsukuba, Japan.
SEM observation
Six specimens of Polydora onagawaensis Teramoto et al., 2013 were used for scanning electron microscopy (SEM) (Hitachi SU8000, Hitachi, Japan). Each fixed specimen in 99.5% ethanol was freeze-dried in t-butyl alcohol, coated with platinum palladium, and viewed using an SEM equipped with a digital camera.
Molecular analysis
Two individuals each of Boccardia proboscidea Hartman, 1940, Boccardia pseudonatrix Day, 1961, Boccardiella hamata (Webster, 1879), Dipolydora giardi (Mesnil, 1893), Polydora hoplura Claparède, 1868, and Polydora websteri Hartman in Loosanoff & Engle, 1943, one individual of Dipolydora sp., and eight individuals of P. onagawaensis collected in the present study were used for DNA analysis. Additional samples of P. onagawaensis collected from Japan and China and Polydora sp. 3 sensu Abe and Sato-Okoshi (2021) collected from Japan were used for DNA analysis for sequence comparison (Table 1).
Genomic DNA was extracted from palps or a small piece of tissue from ethanol-preserved specimens by grinding and heating at 95 °C for 20 min in 50 μL of TE buffer (pH 8.0) with 10% Chelex 100 (Bio‐Rad; Richlen and Barber 2005). Undiluted or tenfold diluted extracted DNA in TE buffer was used as a template for the polymerase chain reaction (PCR). Partial sequences of nuclear 18S and 28S rRNA, mitochondrial 16S rRNA, and COI genes were amplified by PCR using the primer pairs 18S‐1F1/18S‐1R632, 18S‐2F576/18S‐2R1209, and 18S‐3F1129/18S‐R1772 for 18S, D1R/D2C for 28S, 16Sar /16Sbr for 16S, and Dorid COI.3F/Dorid COI.1R for COI (Table 2). We performed the PCR in a 20 μL reaction mixture containing 1.0–2.0 μL of template DNA, 8.8 μL of sterilized water, 10 μL of 2 × KOD One PCR Master Mix (TOYOBO, Osaka, Japan), 0.1 μL of 50 μM forward and reverse primers. The PCR cycling conditions were 36 cycles at 98 °C for 10 s, 60 °C (18S and 28S), 50–56 °C (16S), or 45 °C (COI) for 5 s, and 68 °C for 1 s. PCR products were purified using ExoSAP-IT (Affymetrix, Cleveland, OH) and sequenced by Eurofins Genomics (Tokyo, Japan). Forward and reverse complementary sequences and contigs were assembled using GeneStudio ver. 2.2.0.0 (GeneStudio, Inc. Suwanee, GA, USA). All sequences generated in the present study were deposited in the DDBJ/ENA/GenBank nucleotide sequence database under accession numbers LC682679–LC682767 (Table 1).
Gene sequences obtained in the present study were aligned using the MAFFT online service ver. 7 using the L‐INS‐i algorithm (Katoh et al. 2017) to reconstruct the molecular phylogeny with the sequences of other Polydora, Dipolydora, Boccardiella, and Boccardia species available in the DDBJ/ENA/GenBank databases (Table 1). For phylogenetic analyses of the 18S, 28S, and 16S rRNA gene regions, the gene sequences of Pseudopolydora paucibranchiata (Okuda, 1937), obtained from DDBJ/ENA/GenBank, were used as outgroup taxa, according to Abe and Sato-Okoshi (2021). Phylogenetic analysis of the COI gene was performed to investigate the geographical genetic variation in P. onagawaensis, and the P. websteri sequence obtained in the present study was used as the outgroup. Ambiguously aligned regions were eliminated using Gblocks ver. 0.91b (Castresana 2000) with less stringent settings. The final lengths of the alignments were 1769 (18S), 766 (28S), 471 (16S), and 656 (COI) bp for the multiple sequence alignment. Two phylogenetic trees were constructed based on the concatenated sequences of 18S, 28S, and 16S, and the sequences of the COI gene region by maximum likelihood (ML) analyses performed using IQ-TREE (Nguyen et al. 2015) implemented in PhyloSuite v.1.2.2 (Zhang et al. 2020) under an edge-linked partition model. The TIM2e + I + G4, TNe + R2, GTR + F + I + G4, and TPM + F + G4 models were selected for the 18S, 28S, 16S rRNA, and COI gene regions, respectively, as the best substitution model by ModelFinder (Kalyaanamoorthy et al. 2017) as implemented in IQ-TREE under the Bayesian information criterion. The robustness of the ML trees was evaluated using the Shimodaira–Hasegawa–like approximate likelihood-ratio test (SH-aLRT) with 5000 replicates (Guindon et al. 2010), approximate Bayes (aBayes) test (Anisimova et al. 2011), and ultrafast bootstraps (UFBoot) with 5000 replicates (Hoang et al. 2018). SH-aLRT ≥ 80%, aBayes ≥ 0.95, and UFBoot ≥ 95% were defined as robust statistical supports.
Results
Identification of polydorid species
Based on morphology and gene sequence results, a total of eight species were identified: three Polydora, P. onagawaensis, P. hoplura, and P. websteri; two Dipolydora, D. giardi, and Dipolydora sp.; two Boccardia, B. proboscidea, and B. pseudonatrix Day, 1961; and one Boccardiella, B. hamata. The sampling site, host species or associated substrate, culture or wild condition, habitat type, and condition of shell infestation and other calcareous substrates by each species are summarized in Table 3.
Molecular phylogenetic analysis of concatenated sequences of nuclear 18S and 28S and mitochondrial 16S rRNA gene sequences of polydorid species obtained in the present study and from the DDBJ/EMBL/GenBank database supported the morphological identification results. The species identified in the present study were genetically close to the same species previously reported from other localities (Fig. 2).
Maximum likelihood tree inferred from concatenated sequences of nuclear 18S, 28S, and mitochondrial 16S rRNA gene sequences of polydorid species obtained in the present study and from the DDBJ/EMBL/GenBank database (Table 1). The gene sequences obtained in this study are highlighted in bold. SH-aLRT/approximate Bayes support/ultrafast bootstrap support values of ≥ 80%/ ≥ 0.95/ ≥ 95% are shown beside the respective nodes in red. Nodes with red circles indicate triple high support values of SH-aLRT ≥ 80, approximate Bayes support ≥ 0.95, and ultrafast bootstrap support ≥ 95. Scale bar represents the number of substitutions per site. The sequence of Pseudopolydora paucibranchiata was used for outgroup rooting
Molecular phylogenetic analyses based on the mitochondrial COI gene sequences obtained in the present and previous studies showed that the individuals identified as P. onagawaensis were divided into four lineages (Fig. 3). One lineage consisted of individuals from Japan and China, two lineages consisted of individuals from the USA and Japan, and the remaining lineage consisted of individuals from the USA and France. The Japanese and USA specimens each contained three lineages, while the French specimens were of a single lineage.
Maximum likelihood tree inferred from mitochondrial COI gene sequences of Polydora onagawaensis obtained in the present study and from the DDBJ/EMBL/GenBank database (Table 1). The gene sequences obtained in this study are highlighted in bold. SH-aLRT/approximate Bayes support/ultrafast bootstrap support values of ≥ 80%/ ≥ 0.95/ ≥ 95% are shown beside the respective nodes in red. Nodes with red circles indicate triple high support values of SH-aLRT ≥ 80, approximate Bayes support ≥ 0.95, and ultrafast bootstrap support ≥ 95. Scale bar represents the number of substitutions per site. Sequences of Polydora websteri were used for outgroup rooting
Morphological characteristics of the specimens collected from Normandy
Polydora onagawaensis Teramoto et al., 2013 (Fig. 4)
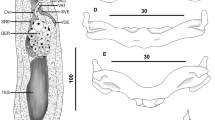
Polydora onagawaensis. Photographs showing morphological characteristics focused on pigmentation after fixation (A–F), SEM (G–H), and juveniles in live (I–J). A Black pigmentation on caruncle and dorsal side of anterior chaetigers. B Black pigmentation near the base of palp from lateral view. C Black pigmentation near the base of both palps from dorsal view. D Black pigmentation on prostomium, around the base of palp, and on palp from lateral view. E Black pigmentation on posterior chaetigers and along the edge of pygidium from lateral view. F Black pigmentation on lateral side of pygidium. G Anterior dorsal view. H Major falcate spines with lateral flange or sheath of modified chaetiger 5. I Juvenile with black pigmentation on its dorsal side. J Juvenile in mud tube
Adult morphology. Thirty individuals of Polydora onagawaensis were examined. After fixation, the maximum body length (95 chaetigers) was 15 mm, and the maximum width was 680 µm (at the 5th chaetiger). The body coloration of the live specimen was light tan. Body pigmentation in live and preserved specimens was weak; weak black pigmentation was present on and along the caruncle. Occasionally, intense black pigmentation was present on peristomium around the base of both palps (Fig. 4A–D). Palp pigmentation was either entirely absent or a faint black (Fig. 4D). Black pigment was occasionally present on the dorsal and lateral sides of the posterior chaetigers (Fig. 4E). The pygidium had no pigmentation or black pigmentation along the edge or on the side of the disc-like pygidium (Fig. 4E, F). The peristomium is wide and short. The prostomium was weakly incised on the anterior margin. The eyes were absent or up to four black eyes and were arranged in a trapezoid, with the anterior pair more widely spaced than the posterior pair (Fig. 4C). The caruncle extended posteriorly to the end of chaetigers 1 to 4, unrelated to body size, without a median antenna (Fig. 4G).
Chaetiger 1 had noto- and neuropodia; notochaetae were absent, and short capillary neurochaetae were present. Parapodial lobes and postchaetal lamellae were well-developed on anterior chaetigers, except chaetiger 5, gradually reducing on posterior chaetigers. Neuro- and notochaetae on chaetiger 2–4 and 6 were winged capillaries. The number of capillaries per fascicle and wings in the notopodia of succeeding chaetigers diminished gradually. Hooded hooks on neuropodia began from chaetiger 7 and were not accompanied by capillaries. Hooks were bidentate, with the main fang at a right angle to the shaft and an acute angle to the apical tooth. The shaft was slightly curved, with a constriction in the upper part. The size and number of hooks gradually diminished from anterior to posterior. Approximately six hooks were present in a series.
The modified chaetiger 5 was longer than adjacent chaetigers 4 and 6 (Fig. 4G) and possessed curved horizontal rows of up to six major spines alternating with pennoned companion chaetae. Both dorsal and ventral tufts of several short and winged capillaries were present. The major spines were falcate with lateral flanges or sheaths, and unworn spines were tooth-like (Fig. 4H).
Branchiae began from chaetiger 7, continuing to 1/2 to a maximum of 3/5 of the body. Nototrochs began from chaetiger 7 onwards.
Pygidium was white and disc-shaped, with a dorsal gap (Fig. 4E, F). Gizzard-like structures were absent in the digestive tract. Glandular pouches began with chaetiger 7.
Juvenile morphology. Twenty chaetigers juveniles were examined (Fig. 4I, J). The prostomium was rounded anteriorly. Conspicuous black pigmentation occurred on peristomium around the base of the palps. Dorsal pigmentation consisted of two rows of melanophores from chaetiger 2 with those of the first six band-shaped pairs, which were then replaced by ramified melanophores in posterior chaetigers. Lateral pigments were found in chaetigers 1–3. Dorsolateral pigments at the base of the parapodia started from chaetiger 7. A pair of black pigments appeared on the pygidium. The ventral pigment was absent. Modified chaetae developed in chaetiger 5.
Reproduction. Only a few large individuals had oocytes in their coeloms. Egg capsules were absent; however, numerous juveniles with approximately 20 chaetigers, representing characteristic larval pigmentation on the dorsal side, were observed in early March (Fig. 4I).
Remarks. Polydora onagawaensis resembles P. websteri and P. hoplura in that all three possess similar chaetal morphology, arrangement, and body pigmentation variation. However, P. onagawaensis can be distinguished from P. websteri by its small maximum size, few consecutive branchial chaetigers, wide and short peristomium, pygidium disc-like morphology rather than flared, and more pigment variations rather than only black pigment along the edge of the palps. Moreover, although body pigmentation resembles each other, P. onagawaensis can be distinguished from P. hoplura by its smaller body size, both in length and width, smaller pygidium, absence of a median antenna, and absence of posterior hooks in notopodia. The morphology of specimens from Normandy can be slightly distinguished from those from Japan (Teramoto et al. 2013), where the species was originally described by occasional intense black pigmentation around the base of both palps.
Polydora websteri Hartman in Loosanoff & Engle, 1943 (Fig. 5A, B)
Photographs showing morphological characteristics focused on pigmentation of newly recorded species in the English Channel; Polydora websteri (A, B), Boccardia pseudonatrix (C, D), and Boccardiella hamata (E, F) after fixation. A Black pigmentation along the edge of palp from anterior lateral view. B Flared pygidium without pigmentation. C Black pigmentation on prostomium from anterior dorsal view. D Black pigmentation on prostomium and on the dorsal side of anterior chaetigers. Mid-dorsal ridge can be observed from chaetiger 5 to 8. E No particular pigmentation on the whole body. F Special hooks on posterior chaetigers and pygidium from dorsal view
Twenty individuals of P. websteri were examined in this study. The maximum recorded body length after fixation was 18 mm. Polydora websteri from Normandy showed similar morphological characteristics to previous descriptions worldwide (Read 2010; Sato-Okoshi and Abe 2013; Rodewald et al. 2021). Palps had weak-to-clear black pigmentation along the margin (Fig. 5A). Branchiae began from chaetiger 7, and were fairly long throughout, continuing almost till the posterior end. The pygidium was flared outward (Fig. 5B).
Polydora hoplura Claparède, 1868
Twenty individuals of P. hoplura were examined in this study. The maximum recorded body length after fixation was 35 mm Morphological characteristics of P. hoplura from Normandy were similar to those previously described worldwide (Sato-Okoshi and Abe 2013; Walker 2013; Radashevsky et al. 2017; Sato-Okoshi et al. 2017). Black pigmentation on the palps, prostomium, and peristomium was feebly visible or entirely absent. A median antenna was present. Special recurved hooks were present on the posterior notopodia.
Dipolydora giardi (Mesnil, 1893)
We examined 20 individuals of Dipolydora giardi in this study. The maximum recorded body length after fixation was 5 mm. The morphological characteristics of D. giardi from Normandy were similar to those previously described in Asia (Sato-Okoshi 1999; Sato-Okoshi et al. 2012) and Australia (Sato-Okoshi et al. 2008; Walker 2013). Pigmentation was entirely absent on the body. Notochaetae were present on chaetiger 1, branchiae began from chaetiger 9, and capillaries were accompanied by hooded hooks.
Dipolydora sp.
The following description of Dipolydora sp. is based on an incomplete specimen. Branchiae began at chaetiger 9. While detailed morphological characteristics were unavailable, genetic data suggested that this species was different from D. giardi.
Boccardia pseudonatrix Day, 1961 (Fig. 5C, D)
We examined five individuals of Boccardia pseudonatrix in this study. The maximum recorded body length after fixation was 18 mm. The morphological characteristics of B. pseudonatrix from Normandy matched its previous descriptions from South Africa (Simon et al. 2010) and Australia (Sato-Okoshi et al. 2008, as B. knoxi: see Sato-Okoshi et al. 2015; Walker 2013). Palps were transparent with irregular colorless spots crossing transversely, giving the appearance of being crossed by white bars in the lateral view. Conspicuous black pigmentation was observed in the prostomium (Fig. 5C, D). A mid-dorsal ridge appeared from chaetiger 5 to the middle of chaetiger 8 (Fig. 5D).
Boccardia proboscidea Hartman, 1940
Twenty individuals of Boccardia proboscidea were examined in this study. The maximum recorded body length after fixation was 18 mm. The morphological characteristics of B. proboscidea from Normandy matched well with previous descriptions from Japan (Sato-Okoshi 2000), Australia (Blake and Kudenov 1978; Sato-Okoshi et al. 2008; Walker 2013), South Africa (Simon et al. 2006), USA (Bailey-Brock 2000), and Canada (Sato-Okoshi and Okoshi 1997). Black pigmentation was present on both sides of the caruncle. Their body color in life was greenish. The pygidium was divided into lobes.
Boccardiella hamata (Webster, 1879) (Fig. 5E, F)
We examined 20 individuals of Boccardiella hamata. The maximum recorded body length after fixation was 38 mm. The morphological characteristics of B. hamata from Normandy matched those of B. hamata from North America (Dean and Blake 1966) and Asia (Sato-Okoshi 2000; Sato-Okoshi et al. 2012; 2013). Pigmentation was entirely absent on the body (Fig. 5E). Special recurved hooks were present in posterior notopodia (Fig. 5F). Pygidium had two broad ventral lappets, each with a short process (Fig. 5F).
Shell infestation by polydorids
The conditions of shell infestation by each polydorid species at each sampling site are shown in Table 3. Shell infestation was determined by the occurrence rate of abnormal shell formation, and the degree of abnormal shell formation observed on the inner surface of the shell. The presence of abnormal black organic deposits or calcareous deposits is a direct response of the host mollusks to cover and defend the polydorid excavation and repair after their penetration. The degree “heavy infestation” was used only when multiple abnormal shell formations were observed per shell. We used the term “infestation” only when a host oyster formed an abnormal shell in response to an invasion, irrespective of the number of worms inhabiting the shell.
Sandy areas, including some rocks, were spread across Blainville-sur-Mer (Fig. 6A). Cultured Japanese oyster C. gigas that fell out of the net bags (Fig. 6B), along with wild Japanese oyster (Fig. 6C) and European flat oyster O. edulis (Fig. 6D) were collected and observed. They exhibited a large shell width (Fig. 6C–E) with calcareous layers (Fig. 6F). Shell chambers (French word: chambrage) are constructed with thin paper shells filled with jelly-like materials. Chalky deposits (Okoshi et al. 1987) or mud were observed in both shell valves. Small-sized D. giardi and large-sized P. hoplura were observed to inhabit the shells. Mud blisters, which were the responses against large-sized P. hoplura, were also confirmed. The number of individuals per shell fluctuated among the examined oysters, ranging from several to several tens of worms per shell. Although high densities of D. giardi (approximately 4 inds. /cm3 in aggregation) were detected in the shells of cultured oysters that fell from the net bags, the damage to the shells was less owing to their smaller body size. Large, aged O. edulis shells collected from the bottom showed traces of polydorid burrows, but no live individuals were found.
Wild and cultured oysters from Blainville-sur-Mer. A A large sandy oyster culture ground, including some rocky areas. B Cultured oysters in a mesh net. C Wild Crassostrea gigas attached to a rock. A limpet was attached to the right valve. The left valve is cup-shaped and thick. Scale bar: 2 cm. D Old and cup-shaped Ostrea edulis collected on the beach. Scale bar: 3 cm. E An oyster with a large shell width. F Fracture surface of right valve with multi- calcareous layers. Scale bar: 1 cm
Wild oyster shells from oyster beds in the muddy ground at Saint-Vaast-La-Hougue (Fig. 7A–C) were inhabited by P. onagawaensis, B. hamata, and B. pseudonatrix. Mud tubes were observed in, on, and within the shell crevices (Fig. 7D, E). The burrows created inside the shell are visible on the side of the inner surface of the shell (Fig. 7F, G). The number of individuals per shell fluctuated, ranging from several to several tens of worms per shell in P. onagawaensis.
Wild oysters from Saint-Vaast-La-Hougue. A Muddy culture ground with continuous oyster shelves. B Oyster bed. C Oysters with elongated shells collected from oyster bed. Scale bar: 2 cm. D Numerous mud tubes formed interstitially on the surface of the shell. E Mud tubes and burrows observed in a cross-section after peeling away the shells from each other. F, G Numerous burrows of different sizes visible from the inner surface of the shell. Scale bars: 1 cm
The number of worms extracted per shell (valve) was especially high (up to 135 individuals) in suspended cultured oysters from Quineville (Fig. 8A, B). Numerous mud tubes of P. onagawaensis and P. websteri protruded from the edge and near the umbo of the outer surface of the shell (Fig. 8C). The palps were observed waving from their tubes (Fig. 8D). The outer surface of the shell was peeled off, and a large number of burrows were found inside (Fig. 8E). Further, mud blisters were observed near the attachment site of the adductor muscle on the inner surface of the shell (Fig. 8F). Few cracks were observed along the burrows (Fig. 8G, H). Black or brown organic deposits and calcareous shell material were secreted against worm penetration on the inner surface of the shells (Fig. 8B, F–H).
Cultured oysters from Quineville. A Cultured oysters placed in mesh cages and suspended in culture pond. B Inner surface of the left and right valves, respectively, left valve with soft body. Brown deposits and calcareous shell materials were observed to secrete against polydorid infestation on the inner surface of the right valve. C Each arrow shows the mud tube protruding from along the edge of the valves in a triploid oyster. D Each arrow shows polydorid palp waving from its tube. E Outer surface of the shell revealing many polydorid burrows which were formed inside after the shell surface peeling off. F Mud blister near the attachment site of the adductor muscle. G Crack observed running across the polydorid burrow over the adductor muscle attachment and mud blister. H Enlarged view of a part of a crack in G
From the cultured oyster shells of Asnelles (Fig. 9A–D), three species, P. onagawaensis, P. websteri, and B. proboscidea, were documented. Mud blisters were also observed on the inner surface of the shells (Fig. 9D). Harvested scallops that were dredged near Asnelles had Cliona (Porifera) infestation; however, no polydorid infestations were observed on the shells.
Cultured oysters from Asnelles. A Cultured oysters displayed in the market. B Harvested cultured oysters. Scale bar: 2 cm. C Inner surface of the left and right valves, respectively, left valve with soft body. Each arrow shows the polydorid burrows. D Mud blister and polydorid burrows visible from the inner surface of the right valve shell. Scale bar: 1 cm
In the wild, numerous burrows of P. onagawaensis were observed on the surface of limestone (ca. 2 burrows/cm2), which covered the intertidal sea bottom in Luc-sur-Mer (Fig. 10A–D). Juveniles presenting larval pigmentation (Fig. 4I, J) were found from the shallow burrows on the surface of the concrete block covered with barnacles and mussel shells, which were installed during another study (Fig. 10E) (Dauvin et al. 2021).
High densities of approximately 1 ind./cm2 of B. proboscidea were observed to inhabit coralline algae that covered the entire surface of the intertidal rocks at Point de Barfleur (Fig. 11A–D). They created mud tubes in the crevices of coralline algae. Adults with egg capsules in their tubes were obtained (Fig. 11E, F).
Discussion
Diversity of environment along the coast of Normandy and calcareous substrates as a habitat
Eight species of polydorids were discovered during the present survey of the western half of the Normandy coast along approximately 450 km of the English Channel. In this study, we investigated two large oyster culture grounds and two suspended culture sites in different environments and the surrounding natural calcareous substrates.
The existence of varied habitats, such as the different environments at oyster farms, the diverse natural environments of mud, sand, and rocky areas, as well as the existence of artificial environments such as oyster farming facilities and structures, could be the reason for the coexistence of many of the eight species found in Normandy area. We also observed different morphological characteristics of shells between wild and cultured oysters. For example, all oysters cultivated in mesh bags were “single oysters” and were not stuck to each other. In contrast, the wild oysters that live on the muddy bottom in Saint-Vaast-La-Hougue were attached to each other (Fig. 7B). Crevices between individual oysters and mud accumulates created a conducive habitat for polydorids. Moreover, at Saint-Vaast-La-Hougue, oyster shells that live on the muddy bottom grow longitudinally upward and are thin to avoid burial under the mud (Fig. 7B, C). Since oysters with thin shells are less suitable as long-term habitats for large numbers of polydorid individuals, the abundance of mud provides a suitable alternate for species that build mud tubes to live in (Fig. 7D, E).
Oyster shells cultured in mesh bags on gravel and sandy ground farms and a wild single oyster grown on rocks or coarse sand tended to be thicker in Blainville-sur-Mer (Fig. 6B–E) compared to wild oyster shells in Saint-Vaast-La-Hougue. The cross-section of the shell was thicker (Fig. 6F), and a large number of shell chambers (Okoshi et al. 1987) were also observed. Owing to the thickness of the shell and the tendency for mud to collect in the shell chamber, it is inhabitable by more polydorid individuals. Triploid-cultured oysters were characterized by a cup-shaped valve on the left (Fig. 8C). In this case, the surface area of the shell increases, becoming a substrate on which more individuals can live. Thus, it is speculated that these microhabitats can be created, and multiple species can coexist within them.
Polydorid species inhabiting the coast of Normandy and the English Channel
In the past, 13 polydorid species, Polydora ciliata (Johnston, 1838), P. hoplura, Dipolydora giardi, D. coeca (Ӧrsted, 1843), D. flava (de Claparède, 1870), D. quadrilobata (Jacobi, 1883), D. caulleryi (Mesnil, 1897), D. armata (Langerhans, 1880), Boccardia polybranchia (Haswell, 1885), B. semibranchiata Guérin, 1990, Boccardiella ligerica (Ferronnière, 1898), Pseudopolydora antennata (Claparède, 1868), and Ps. pulchra (Carazzi, 1893) has been reported in this vicinity along the coast of the English Channel in France (Fauvel 1927; Dauvin et al. 2003; Ruellet 2004; Le Mao et al. 2020). Of these, Fauvel (1927) reported three shell-boring polydorids, D. coeca (Polydora), D. flava (Polydora), and P. hoplura from Ostrea edulis Linnaeus, 1758, and Ruellet (2004) recently reported five polydorids, P. hoplura, P. ciliata, B. polybranchia, B. semibranchiata, and unidentified Boccardia from Crassostrea gigas east and west of the Cotentin Peninsula in Normandy. Among the eight Normandy species identified in this survey, only two, P. hoplura and D. giardi, have been previously reported. The remaining species that remained undocumented during this survey may have moved away from this area or could have been misidentified. Future work is needed to confirm each of the previously reported species individually. Polydora ciliata is known to be a non-borer. Species in the P. ciliata/websteri complex are morphologically very similar and may be misidentified as P. websteri (see discussion by Blake and Kudenov 1978) or other morphologically similar species. In contrast, B. semibranchiata was described from the Mediterranean coast of France in 1990 (Guérin 1990) and subsequently from the Atlantic coast of Spain (Martinez et al. 2006). This species has been reported in oyster shells in Veys Bay (Ruellet 2004). Boccardia semibranchiata is morphologically very similar to B. pseudonatrix, and further species elucidation is required. It has been suggested that B. polybranchia may also be morphologically confusing (Simon et al. 2010). Six species, P. onagawaensis, P. websteri, Dipolydora sp., B. proboscidea, B. pseudonatrix, and B. hamata, were added to Normandy in the present survey. Of these, three species, namely P. websteri, B. proboscidea, and B. hamata, have been recently reported in nearby waters (Kerckhof and Faasse 2014; Spilmont et al. 2018; Waser et al. 2020; Gully and Cochu 2020).
Interestingly, the species composition differed between the west and east across the Cotentin Peninsula. While from Blainville-sur-Mer, which is a gravel and sandy oyster culture ground located in the west, P. hoplura and D. giardi, both species were already known to be distributed in this locality, were observed to inhabit the oyster shells. In contrast, the two species were not reported from the east except Quineville. However, P. onagawaensis and P. websteri, both species previously unknown from this locality, abundantly inhabited muddy oyster beds in Saint-Vaast-La-Hougue and suspended culture oysters from Quineville and Asnelles, respectively, located in the east. None of these two species was observed on the west side of the peninsula during the present survey. Differences in species composition between the west and the east may be related to differences in habitat environment conditions, such as coastal current, sediment type, and sea water temperature, which was higher in the eastern part of the Cotentin (Dauvin 2019). At the same time, it is crucial to clarify the origin and distribution route of oysters brought to each farm since cultured oysters are transported and moved manually.
In this study, a newly recorded species, P. onagawaensis, was found to be distributed on the east side of the Cotentin Peninsula from wild and cultured oyster shells and limestone substrates. Notably, P. websteri was extracted only from suspended cultured oysters, and not from the wild. Further, our previous survey showed that P. websteri did not inhabit the shells of cultured oysters from Arcachon Bay, located along the Bay of Biscay, south of Normandy, in 2017 (Sato-Okoshi et al. in prep). Polydora websteri was recently reported to be abundant in the shells of cultured oysters and naturalized oyster reefs in the Wadden Sea, Netherlands, and Germany (Waser et al. 2020). These results suggest the possibility of new transportation of the species to the east through anthropogenic factors. Further studies on the distribution of P. onagawaensis and P. websteri along the western European coast, based on accurate species identification, are required.
Specimens of a newly recorded species, P. onagawaensis, reported for the first time in Normandy, were compared to the type locality in northeastern Japan and USA (Silverbrand et al. 2021). Polydora onagawaensis in France was grouped with a USA specimen in a single clade and was differentiated from three other lineages containing specimens from Japan and the USA (Fig. 3). Moreover, the morphological characteristics of French P. onagawaensis differed slightly from those of Japanese P. onagawaensis, as described in the remarks section. In the future, the P. onagawaensis complex should be studied in more detail by examining its genetic information, morphology, and ecology from additional habitat areas.
Boccardia proboscidea was originally described on the Pacific coast of North America (Hartman 1940), but the number of reports of this species from new locations globally continues to increase (Sato-Okoshi 2000; Sato-Okoshi et al. 2008; Simon and Sato-Okoshi 2015; Jaubet et al. 2018; Spilmont et al. 2018; Radashevsky et al. 2019). Boccardia proboscidea was found in coralline algae and the shells of cultured oysters during the present survey. Moreover, it was recently reported along the French coast of the English Channel, on the Opal Coast in northern France in 2014 (Spilmont et al. 2018), and in 2018 in North Brittany (Gully and Cochu 2020).
Boccardiella hamata was also reported for the first time in the English channel. This species was originally described from the Atlantic coast of North America (Webster 1879) and is now commonly known from the Pacific coast, for example, Vancouver Island, Canada (Sato-Okoshi and Okoshi 1997), Japan (Sato-Okoshi 2000), China (Zhou et al. 2010), Korea (Sato-Okoshi et al. 2012), and recently near Belgium and the North Sea (Kerckhof and Faasse 2014). The species has been reported from muddy environments, particularly sandstones (Sato-Okoshi and Okoshi 1997) and rigid man-made substrates such as coastal defense structures on sandy coasts (Kerckhof and Faasse 2014). Boccardiella hamata was also recently found to inhabit sponges (Abe et al. 2019a).
Boccardia pseudonatrix, which has never been reported in European waters, was found in the shells of wild and cultured oysters. Boccardia pseudonatrix was originally described in South Africa (Day 1967; Simon et al. 2010), from which it may have been artificially transferred to rest of the world by aquaculture or ship ballast water. This species has recently been found in Australia (Sato-Okoshi et al. 2008 (as B. knoxi), 2015; Walker 2013) and Japan (Sato-Okoshi et al. 2015; Abe et al. 2019a) and is likely to be found worldwide after accurate identification in the future.
It was unclear from this survey whether each of the eight species inhabiting this water area was introduced as non-indigenous or misidentified due to confusion in species identification. Since the life history of polydorids has been reported to range from less than a year to several years, it can be assumed that the species extracted from the shells of cultured and wild oysters from the oyster farms of Saint-Vaast-La-Hougue and Blainville-sur-Mer could include both the species that originally inhabited the water areas and those that were transported to the areas accompanying the host shells.
Notably, all species of polydorids were identified to the species level in the present study (except Dipolydora sp.), which are also found in Japan, especially along the Pacific coast of Tohoku District (Sato-Okoshi 1999; 2000; Abe and Sato-Okoshi 2021). Considering the historical background of cultured oysters in France and Japan, Japanese oysters were abundantly exported from the Pacific coast of Tohoku District of Japan to France (Koike 2015). Future discussions on the species of polydorids inhabiting the waters should consider the anthropogenic transfer of oysters, which could be their hosts and species genetic information.
Analyzing historical trends may be challenging; however, considering the future range expansion of these species, their larval developmental patterns should be studied. Of the eight species confirmed during the survey in March, only a few large individuals of P. onagawaensis were found to have oocytes. Further, only B. proboscidea produced egg capsules with developed larvae and many nurse eggs. Polydora onagawaensis, D. giardi, and B. hamata have shown simultaneous development and produce planktotrophic larvae with a relatively long planktonic larval phase before settling (Sato-Okoshi 1999; 2000; Teramoto et al. 2013). In contrast, P. websteri, P. hoplura, and B. proboscidea are known to produce adelphophagic larvae, that usually emerge soon before settlement or as a mixture of these and planktotrophic larvae (reviewed by Simon and Sato-Okoshi 2015); B. pseudonatrix is known to produce adelphophagic larvae (Sato-Okoshi et al. 2008, as B. knoxi; Simon et al. 2010). Further confirmation of the developmental pattern of larvae in this water may lead to the elucidation of distribution expansion.
Effects of polydorids on cultured oysters in Normandy
A high occurrence rate of abnormal shell formation was observed in wild and cultured oysters, with particularly high rates in wild oysters caused by P. hoplura and in suspended cultured oysters caused by P. websteri and P. onagawaensis. Host oysters secrete excess organic and calcareous shell materials to cover and defend against polydorid excavation and repair part of the shell after its penetration. Although we did not confirm oyster death or apparent soft body shrinkage due to the infestation of the oyster shells by polydorids, based on the levels of shell infestation observed, negative impacts on oysters were a serious concern. The decrease in the commercial value of the host oysters, unpredictable environmental conditions, and other unfavorable factors could easily lead to growth inhibition and mortality. For these high-risk polydorid species groups, biological and ecological control measures, such as avoiding the period when the planktonic larvae settle on the host shells and avoiding water areas where larvae aggregate, to prevent invasion, are required to minimize damage to the culture system. At the same time, considering the high density and wide distribution of these species reported today, we need to recognize that invasive alien species have already established their populations in non-indigenous waters and continue to expand their distribution. It is necessary to discuss and evaluate global impacts on ecosystems.
Data availability
Results from the experiments performed by the authors are available upon reasonable request.
Code availability
Not applicable.
References
Abe H, Sato-Okoshi W (2020) Novel symbiotic relationship between a spionid polychaete and Lingula (Brachiopoda: Lingulata: Lingulidae), with description of Polydora lingulicola sp. nov. (Annelida: Spionidae). Zoosymposia 19:103–120
Abe H, Sato-Okoshi W (2021) Molecular identification and larval morphology of spionid polychaetes (Annelida, Spionidae) from northeastern Japan. ZooKeys 1015:1–86
Abe H, Kondoh T, Sato-Okoshi W (2016) First report of the morphology and rDNA sequences of two Pseudopolydora species (Annelida: Spionidae) from Japan. Zool Sci 33:650–658
Abe H, Takeuchi T, Taru M, Sato-Okoshi W, Okoshi K (2019a) Habitat availability determines distribution patterns of spionid polychaetes (Annelida: Spionidae) around Tokyo Bay. Mar Biodivers Rec 12:7
Abe H, Tanaka M, Taru M, Abe S, Nishigaki A (2019b) Molecular evidence for the existence of five cryptic species within the Japanese species of Marphysa (Annelida: Eunicidae) known as “Iwa-mushi.” Plankton Benthos Res 14:303–314
Abe H, Hoshino O, Yamada K, Ogino T, Kawaida S, Sato-Okoshi W (2022) A novel symbiotic relationship between ascidians and a new tunic-boring polychaete (Annelida: Spionidae: Polydora). Zootaxa 5159:1–22
Andrews EA (1891) A commensal annelid. Am Nat 25:25–35
Anisimova M, Gil M, Dufayard JF, Dessimoz C, Gascuel O (2011) Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Syst Biol 60:685–699
Augener H (1914) Polychaeta II: Sedentaria, pp 1–72. In: Michaelsen W, Hartmeyer R (eds) Die Fauna Südwest-Australiens. Ergebnisse der Hamburger südwest-australischen Forschungsreise 1905. Volume 5 Gustav Fischer Jena
Bailey-Brock JH (2000) A new record of the polychaete Boccardia proboscidea (family Spionidae), imported to Hawaii with oysters. Pac Sci 54:27–30
Berkeley E (1927) Polychaetous annelids from the Nanaimo district. Part 3. Leodicidae to Spionidae. Contributions to Canadian Biol Fisheries 3:407–422
Berkeley E, Berkeley C (1950) Notes on Polychaeta from the coast of Western Canada. IV Polychaeta Sedentaria. Annals Magazine Natural History 3:50–69
Blake JA (1983) Polychaetes of the family Spionidae from South America, Antarctica and adjacent seas and islands. In: Biology of the Antarctic Seas XIV, vol 39. Antarctic Research Series, pp 205–287
Blake JA (1996) Family Spionidae Grube, 1850. In: Blake J, Hilbig B, Scott PH (eds) Taxonomic atlas of the benthic fauna of the Santa Maria Basin and Western Santa Barbara Channel, vol 6. The Annelida, Part 3. Santa Barbara Museum of Natural History, California, pp 81–224
Blake JA, Evans JD (1973) Polydora and related genera (Polychaeta: Spionidae) as borers in mollusk shells and other calcareous substrates. Veliger 15:235–249
Blake JA, Kudenov JD (1978) The Spionidae (Polychaeta) from Southeastern Australia and adjacent areas with a revision of the genera. Mem Mus Vic 39:171–280
Bosc LAG (1802) Histoire Naturelle des Vers : contenant leur description et leurs moeurs, avec figures dessinées d'après nature. Guilleminet, Paris chez Deterville 3 vols 324 pp
Carazzi D (1893) Revisione del genere Polydora Bosc e cenni su due specie che vivono sulle ostriche. Mittheilungen aus der Zoologischen Station zu Neapel 11:4–45
Carlton JT (1975) Introduced intertidal invertebrates. In: Smith RI, Carlton JT (eds) Light’s manual: Intertidal invertebrates of the Central California Coast. University of California Press, California, pp 17–25
Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17:540–552
Chlebovitsch VV (1959) Species of Polychaeta worms from the Kurile Islands, which are new or recorded for the first time in the USSR fauna. Zoologicheskii zhurnal 38:167–181
Claparède É (1868) Les annelides chétopodes du Golfe de Naples. p 499
Claparède É (1870) Les Annélides Chétopodes du Golfe de Naples. Supplément. Mémoires de la Société de physique et d'histoire naturelle de Genève 20:365–542
Cohen A, Carlton JT (1998) Accelerating invasion rate in a highly invaded estuary. Science 279:555–558
Czerniavsky V (1881) Materialia ad zoographiam Ponticam comparatam. Fasc. III Vermes [Second part]. In: Bulletin de la Société Impériale des Naturalistes de Moscou (= Byulletin' Moskovskogo obshchestva ispytatelei prirody), vol 56, pp 338–420
Dauer DM, Maybury CA, Ewing RM (1981) Feeding behavior and general ecology of several spionid polychaetes from the Chesapeake Bay. J Exp Mar Biol Ecol 54:21–38
Dauvin J-C, Gentil F, Dewarumez J-M (2003) Liste actualisées des espèces d’Annèlides Polychètes presents en Manche. Cah Biol Mar 44:67–95
Dauvin J-C, Deloor M, Pezy JP, Raoux A, Claquin P, Foveau A (2021) Four years-temporal survey of an intertidal artificial reef in the English Channel. J Mar Sci Eng 9:1174
Dauvin J-C (2019) English Channel: La Manche. In: Sheppard C (ed), World of the seas: an environmental evaluation, 2nd Edition, vol. I: Europe, the Americas and West Africa. Academic Press, pp 153–188.
Day JH (1955) The Polychaeta of South Africa. Part 3. Sedentary species from Cape shores and estuaries. J Linnean Society London, Zoology 42:407–452
Day JH (1961) The Polychaet [sic] Fauna of South Africa. Part 6. Sedentary species dredged off Cape coasts with a few new records from the shore. J Linnean Society London 44:463–560
Day JH (1967) A monograph on the Polychaeta of Southern Africa Part 2, Sedentaria. Trustees of the British Museum (Natural History), London.
Dean D, Blake JA (1966) Life history of Boccardia hamata (Webster) on the east and west coasts of North America. Biol Bull 130:316–330
Diggles BK, Hine PM, Handley S, Boustead NC (2002) A handbook of diseases of importance to aquaculture in New Zealand. NIWA Sci Tech Ser 49:1–200
Elton CS (1958) The ecology of invasions by animals and plants. University of Chikago Press.
Evans JW (1969) Borers in the shell of the sea scallop, Placopecten magellonicus. Am Zool 9:775–7782
Fauvel P (1927) Polychètes Sédentaires. Faune De France 16:1–412
Ferronnière G (1898) 1er contribution à l'étude de la faune de la Loire-Inférieure (Polygordiens, Spionidiens, Némertien). Bulletin de la Société des Sciences Naturelles de l'Ouest de la France 8:101–115
Grizel H, Héral M (1991) Introduction into France of the Japanese oyster (Crassostrea gigas). ICES J Mar Sci 47:399–403
Guérin J-P (1990) Description d’une nouvelle espèce de spionidé (Annélides, Polychètes) Boccardia semibranchiata. Annales De L’institut Océanographique 66(1–2):37–45
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321
Gully F, Cochu M (2020) Première mention en Bretagne de l’annélide Boccardia proboscidea Hartman, 1940. An Aod-Les Cahiers Naturaliste De L’observatoire Marin 8:1–9
Hartman O (1940) Boccardia proboscidea, a new species of spionid worm from California. Washington Acad Sci 30:382–387
Haswell WA (1885) Proceedings of the Linnean Society of New South Wales. 10:273–279
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol 35:518–522
Jacobi R (1883) Anatomisch-histologische Untersuchung der Polydoren der Kieler Bucht. 1–37 Kiel
Jaubet ML, Bottero MAS, Hines E, Elias R, Garaffo GV (2018) Boccardia proboscidea (Polychaete: Spionidae) in the SW Atlantic: how far has the invasion spread? Aquat Invasions 13:351–363
Johnston G (1838) Miscellanea Zoologica Ariciadae. Magazine of Zoology and Botany, Edinburgh 2:63–73
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589
Katoh K, Rozewicki J, Yamada KD (2017) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20:1160–1166
Kent RML (1979) The influence of heavy infestations of Polydora ciliata on the flesh content of Mytilus edulis. J Mar Biol Ass UK 59:289–297
Kerckhof F, Faasse MA (2014) Boccardia proboscidea and Boccardiella hamata (Polychaeta: Spionidae: Polydorinae) introduced mud worms new for the North Sea and Europe, respectively. Mar Biodivers Rec 7:1–9
Koike Y (2015) An example of friendship and cooperation between France and Japan: oyster farming in Sanriku area (Tohoku region, northern Japan) before and after tsunami – restoration and technical adaptation of culture systems. In: Ceccaldi HJ, Hénocque Y, Koike Y, Komatsu T, Stora G, Tusseau-Vuillemin M-H (eds) Marine productivity: perturbations and resilience of socio-ecosystems. Springer, Switzerland, pp 3–14
Kondoh T, Abe H, Sato-Okoshi W (2017) Reproduction and larval development of two sympatric Pseudopolydora species (Annelida: Spionidae) in Japan. Invertebr Reprod Dev 61:172–181
Lamarck J-BM de (1819) Histoire naturelle des animaux sans vertèbres. Tome 6(1): vi + 343 pp. Paris: published by the author. Available online at http://www.biodiversitylibrary.org/item/47441
Langerhans P (1880) Die Wurmfauna von Madeira. III. Zeitschrift für wissenschaftliche Zoologie 34:87–143
Lavesque N, Hutchings P, Abe H, Daffe G, Gunton LM, Glasby CJ (2020) Confirmation of the exotic status of Marphysa victori Lavesque, Daffe, Bonifácio & Hutchings, 2017 (Annelida) in French waters and synonymy of Marphysa bulla Liu, Hutchings & Kupriyanova, 2018. Aquat Invasions 15:355–366
Le Mao P, Godet L, Fournier J, Desroy N, Gentil F, Thiébaut E, Pourinet L, Cabioch L, Retière C, Chambers P (2020) Atlas de la faune marine invertébrée du golfe Normano-Breton - Volume 2/7 - Annélides. Éditions de la Station biologique de Roscoff, France. 9782951802940.
Lleonart M, Handlinger J, Powell M (2003) Spionid mudworm infestation of farmed abalone (Haliotis spp.). Aquaculture 221:85–96
Loosanoff VL, Engle JB (1943) Polydora in oysters suspended in the water. Biol Bull 85:69–78
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 10:689–710
Malan A, Williams JD, Abe H, Sato-Okoshi W, Matthee CA, Simon CA (2020) Clarifying the cryptogenic species Polydora neocaeca (Annelida: Spionidae): a shell-boring invasive pest of molluscs from locations worldwide. Mar Biodivers 50:51
Martin D, Britayev TA (1998) Symbiotic polychaetes: review of known species. In: Ansell AD et al (eds) Oceanography and marine biology: an annual review, vol 36. University College London Press, London, pp 217–340
Martin D, Britayev TA (2018) Symbiotic polychaetes revisited: an update of the known species and relationships (1998–2017). Oceanogr Mar Biol 56:371–448
Martínez J, Adarraga I, López E (2006) Neuvos datos del género Boccardia Carazzi, 1893 (Polychaeta: Spionida) para la península Ibérica y el océano Atlantico. Boletín Instituto Español De Oceanografía 22:53–64
Mesnil F (1893) Sur le genre Polydora Bosc (Leucodore Johnston) Comptes Rendus Hebdomadaires des seances de l'Academie des scieces. 117:643–645
Miura O (2007) Molecular genetic approaches to elucidate the ecological and evolutionary issues associated with biological invasions. Ecol Res 22:876–883
Mortensen S, van der Meeren T, Fosshagen A, Hernar I, Harkestad L, Torkildsen L, Bergh O (2000) Mortality of scallop spat in cultivation, infested with tube dwelling bristle worms Polydora sp. Aquac Int 8:267–271
Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol and Evol 32:268–274
Nishitani G, Nagai S, Hayakawa S, Kosaka Y, Sakurada K, Kamiyama T, Gojobori T (2012) Multiple plastids collected by the dinoflagellate Dinophysis mitra through kleptoplastidy. Appl Environ Microbiol 78:813–821
Okoshi K, Sato-Okoshi W (1996) Biomineralization in molluscan aquaculture – growth and disease. Bull L’inst Océanogr Monaco 14:151–169
Okoshi K, Mori K, Nomura T (1987) Characteristics of shell chamber formation between the two local races in the Japanese oyster, Crassostrea gigas. Aquaculture 67:313–320
Okuda S (1937) Spioniform polychaetes from Japan (With 27 Textfigures). J Fac Sci Hokkaido Imp Univ Ser 6 Zool 5:217–254
Örsted AS (1843) Annulatorum danicorum conspectus. Auctore AS Örsted. Fasc. I. Maricolæ. (Quæstio ab universitate Hafniensi ad solvendum proposita et proemio ornata). Available online at http://www.biodiversitylibrary.org/bibliography/11849
Palumbi S, Martin A, Romano S, McMillan WO, Stice L, Grabowski G (1991) The simple fool’s guide to PCR. Version 2.0. Department of Zoology and Kewalo Marine Laboratory, University of Hawaii, Honolulu, p 45
Radashevsky VI (1993) Revision of the genus Polydora and related genera from the north west Pacific (Polychaeta: Spionidae). Pub Seto Mar Biol Lab 36:1–60
Radashevsky VI, Hsieh HL (2000) Polydora (Polychaeta: Spionidae) species from Taiwan. Zool Studies 39:203–217
Radashevsky VI, Pankova VV (2006) The morphology of two sibling sympatric Polydora species (Pollychaeta: Spionidae) from the Sea of Japan. J Mar Biol Ass UK 86:245–252
Radashevsky VI, Pankova VV (2013) Shell-boring versus tube-dwelling: is the mode of life fixed or flexible? Two cases in spionid polychaetes (Annelida, Spionidae). Mar Biol 160:1619–1624
Radashevsky VI, Choi J-W, Gambi MC (2017) Morphology and biology of Polydora hoplura Claparède, 1868 (Annelida: Spionidae). Zootaxa 4282:543–555
Radashevsky VI, Pankova VV, Malyar VV, Neretina TV, Wilson RS, Worsfold TM, Diez ME, Harris LH, Hourdez S, Labrune C, Houbin C, Kind B, Kuhkenkamp R, Nygren A, Bonifácio P, Bachelet G (2019) Molecular analysis and new records of the invasive polychaete Boccardia proboscidea (Annelida: Spionidae). Mediterr Mar Sci 20:393–408
Read GB (2010) Comparison and history of Polydora websteri and P. haswelli (Polychaeta: Spionidae) as mud-blister worms in New Zealand shellfish. N Z J Mar Freshwater Res 44:1–18
Richlen ML, Barber PH (2005) A technique for the rapid extraction of microalgal DNA from single live and preserved cells. Mol Ecol 5:688–691
Robert R, Borel M, Pichot Y, Trut G (1991) Growth and mortality of the European oyster Ostrea edulis in the Bay of Arcachon (France). Aquat Living Resour 4:265–274
Rodewald N, Snyman R, Simon CA (2021) Worming its way in—Polydora websteri (Annelida: Spionidae) increases the number of non-indigenous shell-boring polydorin pests of cultured molluscs in South Africa. Zootaxa 4969:255–279. https://doi.org/10.11646/zootaxa.4969.2.2
Royer J, Ropert M, Mathieu M, Costil K (2006) Presence of spionid worms and other epibionts in Pacific oysters (Crassostrea gigas) cultured in Normandy, France. Aquaculture 253:461–474
Ruellet T (2004) Infestation des coquilles d’huîtres Crassostrea gigas par les polydores en Basse-Normandie: recommandations et mise au point d’un traitement pour éliminer cette nuisance. PhD University of Caen Basse-Normandie, France, p 538
Sato-Okoshi W (1998) Three new species of polydorids (Polychaeta, Spionidae) from Japan. Species Diversity 3:277–288
Sato-Okoshi W (1999) Polydorid species (Polychaeta, Spionidae) in Japan, with descriptions of morphology, ecology and burrow structure. 1 Boring species. J Mar Biol Ass UK 79:831–848
Sato-Okoshi W (2000) Polydorid species (Polychaeta, Spionidae) in Japan, with descriptions of morphology, ecology and burrow structure. 2 Non-boring species. J Mar Biol Ass UK 80:443–456
Sato-Okoshi W, Abe H (2012) Morphological and molecular sequence analysis of the harmful shell boring species of Polydora (Polychaeta: Spionidae) from Japan and Australia. Aquaculture 368–369:40–47
Sato-Okoshi W, Abe H (2013) Morphology and molecular analysis of the 18S rRNA gene of oyster shell borers, Polydora species (Polychaeta: Spionidae), from Japan and Australia. J Mar Biol Ass UK 93:1279–1286
Sato-Okoshi W, Okoshi K (1997) Survey of the genera Polydora, Boccardiella and Boccardia (Polychaeta, Spionidae) in Berkeley Sound (Vancouver Island, Canada), with special reference to boring activity. Bull Mar Sci 60:482–493
Sato-Okoshi W, Takatsuka M (2001) Polydora and related genera (Polychaeta, Spionidae) around Puerto Montt and Chiloe Island (Chile), with description of a new species of Dipolydora. Bull Mar Sci 68:485–503
Sato-Okoshi W, Okoshi K, Shaw J (2008) Polydorid species (Polychaeta, Spionidae) in southwestern Australian waters with special reference to Polydora uncinata and Boccardia knoxi. J Mar Biol Ass UK 88:491–501
Sato-Okoshi W, Okoshi K, Koh B-S, Kim Y-H, Hong J-S (2012) Polydorid species (Polychaeta, Spionidae) associated with commercially important mollusc shells in Korean waters. Aquaculture 350–353:82–90
Sato-Okoshi W, Okoshi K, Abe H, Li J-Y (2013) Polydorid species (Polychaeta, Spionidae) associated with commercially important mollusk shells from eastern China. Aquaculture 406–407:153–159
Sato-Okoshi W, Abe H, Okoshi K, Teramoto W, Shaw J, Koh B-S, Kim Y-H, Hong J-S, Li J-Y (2015) Harmful shell borers, Polydora species (Polychaeta: Spionidae), from commercially important mollusk shells in East Asia and Australia. In: Ceccaldi H-J et al (eds) Marine Productivity: Perturbations and Resilience of Socio-ecosystems. Springer, Switzerland, pp 31–41
Sato-Okoshi W, Abe H, Nishitani G, Simon CA (2017) And then there was one: Polydora uncinata and Polydora hoplura (Annelida: Spionidae), the problematic polydorid pest species represent a single species. J Mar Biol Ass UK 97:1675–1684
Schmarda LK (1861) Neue wirbellose Thiere beobachtet und gesammelt auf einer Reise un die Erdr 1853 bis 1857. Erster Band (zweite halfte) Turbellarian, Rotatorien un Anneliden:1–164
Scholin CA, Herzog M, Sogin M, Anderson DM (1994) Identification of group-and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene. J Phycol 30:999–1011
Silverbrand SJ, Lindsay SM, Rawson PD (2021) Detection of a novel species complex of shell-boring polychaetes in the northeastern United States. Invertebr Biol 140:e12343
Simon AC, Sato-Okoshi W (2015) Polydorid polychaetes on farmed molluscs: distribution, spread and factors contributing to their success. Aquac Environ Interact 7:147–166
Simon CA, Ludford A, Wynne S (2006) Spionid polychaetes infesting cultured abalone Haliotis midae in South Africa. Afr J Mar Sci 28:167–171
Simon CA, Worsfold TM, Lange L, Sterley J (2010) The genus Boccardia (Polychaeta: Spionidae) associated with mollusc shells on the south coast of South Africa. J Mar Biol Ass UK 90:585–598
Simon CA, Sato-Okoshi W, Abe H (2019) Hidden diversity within the cosmopolitan species Pseudopolydora antennata (Claparède, 1869) (Spionidae: Annelida). Mar Biodivers 49:25–42
Spilmont N, Hachet A, Faasse MA, Jourde J, Luczak C, Seuront L, Rolet C (2018) First record of Ptilohyale littoralis (Amphipoda: Hyalidae) and Boccardia proboscidea (Polychaeta: Spionidae) from the coast of the English Channel: habitat use and coexistence with other species. Mar Biodivers 48:1109–1119
Takahashi K (1937) Notes on the polychaetous annelid, Polydora pacifica n. sp., which bores holes in Pinctada margaritifera (LINNE). PalaoTropical Biol Stn Stud 2:155–167
Templeton R (1836) A catalogue of the species of annulose animals, and of rayed ones, found in Ireland, as selected from the papers of the late J. Templeton, Esq., of Cranmore, with localities, descriptions, and illustrations. The Magazine of Natural History and Journal of Zoology, Botany, Mineralogy, Geology and Metereology 9(233240):301–305 417–422, 466–475
Teramoto W, Sato-Okoshi W, Abe H, Nishitani G, Endo Y (2013) Morphology, 18S rRNA gene sequence, and life history of a new Polydora species (Polychaeta, Spionidae) from northeastern Japan. Aquat Biol 18:31–45
Thunberg CP (1793) Tekning och Beskrifning på en stor Ostronsort ifrån Japan. Kongliga Vetenskaps Academiens Nya Handlingar 14:140–142
Verrill AE (1881) New England Annelida. Part I. Historical sketch, with annotated lists of the species hitherto recorded. Transactions of the Connecticut Academy of Arts and Sciences 4:285–324
Walker LM (2013) A revision of the Polydora-complex (Annelida: Spionidae) fauna from Australia. PhD. Thesis. Australia: University of Queensland. 200 pp.
Waser AM, Lackschewitz D, Knol J, Resise K, Wegner KM, Thieltges DW (2020) Spread of the invasive shell-boring annelid Polydora websteri (Polychaeta, Spionidae) into naturalized oyster reefs in the European Wadden Sea. Mar Biodivers 50:63
Webster HE (1879) Annelida Chaetopoda of the Virginian coast. Trans Albany Inst 9:202–269
Williams JD, Radashevsky VI (1999) Morphology, ecology, and reproduction of a new Polydora species from the east coast of North America. Ophelia 51:115–127
Williams L-G, Karl S, Simon C (2017) Molecular identification of polydorid polychaetes (Annelida: Spionidae): Is there a quick way to identify pest and alien species? Afr Zool 52:105–117
Woodwick KH (1953) Polydora nuchalis, a new species of polychaetous annelid from California. J Washington Acad Sci 43:381–383
Woodwick KH (1964) Polydora and related genera (Annelida, Polychaeta) from Eniwetok, Majuro, and Bikini Atolls, Marshall Islands. Pacific Science 18:146–159
Ye L, Tang B, Wu K, Su Y, Wang R, Yu Z, Wang J (2015) Mudworm Polydora lingshuiensis sp. n is a new species that inhabits both shell burrows and mudtubes. Zootaxa 3986:88–100
Zachs I (1933) [Annelid worm fauna North-Japanese sea (Polychaeta)] K. faune kol´chatykh chervi Severo-Yaponskogo morya (Polychaeta). Gosudarstvennyi Gidrologicheskii Institut, Issledovaniia Morei SSSR, Leningrad 14:125–137
Zhang D, Gao F, Jakovlić I, Zou H, Zhang J, Li WX, Wang GT (2020) PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour 20:348–355
Zhou J, Ji W, Li X (2010) Records of Polydora complex spionids (Polychaeta: Spionidae) from China’s coastal waters, with emphasis on parasitic species and the description of a new species. Mar Fish 32:1–14
Acknowledgements
We sincerely thank Thierry Ruellet and oyster farmers from Quineville and Blainville-sur-Mer for their hospitality. We are grateful to Dr. Cheryl Ames for her critical English reading and comments on an earlier version of this manuscript.
Funding
This study was partially supported by a research grant from JSPS KAKENHI (Grant Numbers: JP15K07540, JP18K05777, and JP19K15899) and the Environment Research and Technology Development Fund of the Environmental Restoration and Conservation Agency of Japan (Grant Number: JPMEERF20204R01). This study was conducted in the framework of both the French and Japanese Associations of Oceanography.
Author information
Authors and Affiliations
Contributions
W. S.-O.: Research design, field sample collection, specimen examination, data analyses, and manuscript drafting. K. O.: Field sample collection, specimen examination, data analyses, review, and manuscript editing. H. A.: Specimen examination, molecular analyses, review, and editing of the manuscript, and J.-C. D. Research design, field sample collection, and review and editing of the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sato-Okoshi, W., Okoshi, K., Abe, H. et al. Polydorid species (Annelida: Spionidae) associated with commercially important oyster shells and their shell infestation along the coast of Normandy, in the English Channel, France. Aquacult Int 31, 195–230 (2023). https://doi.org/10.1007/s10499-022-00971-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-00971-y