Abstract
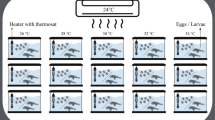
Temperature is among the critical determinants of the physiology of fishes and other heterotherms throughout the life history. Temperatures outside of the optimal range distress embryonic and larval development with untoward consequences later in life. To understand the effect of high temperature on embryogenesis and organogenesis, the commercially important rohu carp (Labeo rohita) was chosen. Rohu embryos and larvae were reared at four different temperatures (30, 32, 34, and 36 °C), and indices of their hatching, development, and mortality were observed throughout early development. Embryos exposed to 30 and 32 °C showed normal development with highest rates of hatching success. Embryos at 34 °C displayed evidence of damaged zygotes, cellular deformities, damaged yolk sac coupled with shortest incubation time, and the lowest rates of hatching success. No hatching was observed at 36 °C, and these embryos displayed coagulated organs, dark yolk sac, irregular segmentation, and pustules. Larvae of rohu exposed to 34 and 36 °C showed developmental deformities (fusion in the eye, axial curvature, yolk sac ulceration, blood coagulation, tail shortening, and ulceration) and minimal survival. Our results suggest that optimal temperature of rohu embryo and larvae is 30–32 °C or lower; higher temperatures disrupt embryonic metabolism and physiology, resulting in aberrant and unsuccessful early development.


Similar content being viewed by others
Data availability
The corresponding author (Md. Shahjahan) is entitled to provide the data that support the findings of this study.
References
Anguilleta MJ (2009) Thermal adaptation: a theoretical and empirical synthesis. Oxford Univ Press, Oxford
Ashaf-Ud-Doulah M, Shahjahan M, Islam SMM, Al-Emran M, Rahman MS, Hossain MAR (2019) Thermal stress causes nuclear and cellular abnormalities of peripheral erythrocytes in Indian major carp, rohu Labeo rohita. J Therm Biol 86:102450
Ashaf-Ud-Doulah M, Mamun AA, Rahman ML, Islam SMM, Jannat R, Hossain MAR, Shahjahan M (2020) High temperature acclimation alters upper thermal limits and growth performance of Indian major carp, rohu, Labeo rohita (Hamilton, 1822). J Therm Biol 93: 102738
Backiel T, Kokurewicz B, Ogorzalek A (1984) High incidence of skeletal anomalies in carp, Cyprinus carpio, reared in cages in flowing cages. Aquaculture 43:369–380
Bizuayehu TT, Johansen SD, Puvanendran V, Toften H, Babiak I (2015) Temperature during early development has long-term effects on microRNA expression in Atlantic cod. BMC Genomics 16(1):305
Boyd CE, Tucker CS (1998) Pond aquaculture water quality management. Kluwer Academic Publishers, Boston
Brahmane MP, Krishnani KK, Sarkar B, Sajjanar B, Kumar S, Nakhawa AD, Minhas PS (2014) Growth, thermal tolerance and oxygen consumption in rohu, Labeo rohita early fry acclimated to four temperatures. African J Agricultural Res 9(9):854–858
Bromage N, Porter M, Randall C (2001) The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture 197:63–98
Brown CL, Núñez JM (1998) Chapter 1, Disorders of development. Pages 1-17. In: Leatherland JF, Woo PTK (eds) Fish diseases and disorders Volume 2 Non-infectious disorders. CAB International Press, Wallingford, p 386
Brown CL, Power DM, Núñez JM (2010) Disorders of development in fish. Pages 166-182. In: Leatherland JK, Woo PTK (eds) Fish disease and disorders, volume 2. Non-infectious disorders, 2nd edn. CAB International Press, Wallingford, p 400. https://doi.org/10.1007/s10803-009-0846-9
Cahu C, Infante JZ, Takeuchi T (2003) Nutritional components affecting skeletal development in fish larvae. Aquaculture 227:245–258
Cheng SY, Chen CS, Chen JC (2013) Salinity and temperature tolerance of brown-marbled grouper Epinephelus fuscoguttatus. Fish Physiol Biochem 39:277–286
Coward K, Bromage NR, Hibbitt O, Parrington J (2002) Gametogenesis, fertilization and egg activation in teleost fish. Rev Fish Biol Fish 12:33–58
Dahanukar N (2010) Labeo rohita. The IUCN Red List of Threatened Species 2010:e. T166619A6248771
Das T, Pal AK, Chakraborty SK, Manush SM, Sahu NP, Mukherjee SC (2005) Thermal tolerance, growth and oxygen consumption of Labeo rohita (Hamilton 1822) acclimated to four temperatures. J Therm Biol 30: 378–383.
Das T, Pal AK, Chakraborty SK, Manush SM, Dalvi RS, Sarma K (2006) Thermal dependence of embryonic development and hatching rate in Labeo rohita (Hamilton, 1822). Aquaculture 255:536–541
DoF (2017) Yearbook of fisheries statistics of Bangladesh 2017-2018. Fisheries Resources Survey System (FRSS), Department of Fisheries. Bangladesh
Falk-Petersen IB (2005) Comparative organ differentiation during early life stages of marine fish. Fish Shellfish Immunol 19(5):397–412
FAO (2018) The state of food security and nutrition in the world: building climate resilience for food security and nutrition. Food and Agriculture Organization of the United Nations, Rome
Fitzsimmons SD, Perutz M (2006) Effects of egg incubation temperature on survival, prevalence and types of malformations in vertebral column of Atlantic cod (Gadus morhua) larvae. Bulletin- European Association of Fish Pathologists 26:80–86
Fu KK, Fu C, Qin YL, Bai Y, Fu SJ (2018) The thermal acclimation rate varied among physiological functions and temperature regimes in a common cyprinid fish. Aquaculture 495:393–301
Gao Y, Kim SG, Lee JY (2011) Effects of pH on fertilization and hatching rates of far eastern catfish Silurus asotus. Fish Aquatic Science 14(4):417–420
Geffen AJ, Fox CJ, Nash RDM (2006) Temperature-dependent developmental rates of cod Gadus morhua eggs. J Fish Biol 69:1060–1080
Georgakopoulou E, Katharios P, Divanach P, Koumoundouros G (2010) Effect of temperature on the development of skeletal deformities in gilthead seabream (Sparus aurata Linnaeus, 1758). Aquaculture 308:3–19
Hassell KL, Coutin PC, Nugegoda D (2008) Hypoxia impairs embryo development and survival in black bream (Acanthopagrus butcheri). Mar Pollut Bull 57:302–306
Johnston IA (2001) Genetic and environmental determinants of muscle growth. In Fish Physiology, (Johnston, I. A., ed.), San Diego, CA: Academic Press 18:141–186
Jonsson B, Jonsson N (2019) Phenotypic plasticity and epigenetics of fish: embryo temperature affects later-developing life-history traits. Aquatic Biol 28:21–32
Kaur R, Ram RN (2017) Temperature’s influence on the embryonic development of Labeo rohita (Hamilton, 1822). J Entom Zool Stud 5(2):1172–1176
Kausar R, Salim M (2006) Effect of water temperature on the growth performance and feed conversion ratio of Labeo rohita. Pak Vet J 26(3):105–108
Kim MS, Kim S, Jeon J, Kim KT, Lee HA, Lee HY, Park J, Seo E, Kim SB, Yeom SI, Lee YH, Choi D (2018) Global gene expression profiling for fruit organs and pathogen infections in the pepper, Capsicum annuum L. Sci Data 5:180103
Korwin-Kossakowski M (2008) The influence of temperature during the embryonic period on larval growth and development in carp, Cyprinus carpio L., and grass carp, Ctenopharyngodon idella (Val.): theoretical and practical aspects. Arch Polish Fish 16:231–314
Koumoundouros G, Divanach P, Kentouri M (1999) Ontogeny and allometric plasticity of Dentex dentex (Osteichthyes: Sparidae) in rearing conditions. Mar Biol 135:561–572
Kupren K, Mamcarz A, Kucharczyk D, Prusinska M, Krejszeff S (2008) Influence of water temperature on eggs incubation time and embryonic development of fish from genus Leuciscus. Pol J Nat Sci 23:461–481
Lein I, Holmefjord I, Rye M (1997) Effects of temperature on yolk sac larvae of Atlantic halibut (Hippoglossus hippoglossus L.). Aquaculture 157(1–2):121–133
Madsen L, Arnbjerg J, Dalsgaard I (2001) Radiological examination of the spinal column in farmed rainbow trout Oncorhynchus mykiss (Walbaum): experiments with Flavobacterium psychrophilum and oxytetracycline. Aquac Res 32:235–241
Morrongiello J, Beatty R, Bennett SJ, Crook JC, Ikedife DA, Kennard DNEN, Kerezsy M, Lintermans J, McNeil AM, Pusey BJ, Rayner T (2011) Climate change and its implications for Australia’s freshwater fish. Mar Freshw Res 62(9):1082–1098
Ottesen OH, Bolla S (1998) Combined effects of temperature and salinity on development and survival of Atlantic halibut larvae. Aquac Int 6:103–120
Pankhurst NW, King HR (2010) Temperature and salmonid reproduction: implications for aquaculture. J Fish Biol 76:69–85
Ponnuraj M, Murugesan AG, Sukumaran N (2002) Effect of temperature on incubation time, fertilization rate and survival of the spawn of Rohu, Labeo rohita. In: Venkataramani, B., Sukumaran, N. (Eds.), BRNS. DAE, Mumbai (Pub), Therm Ecol 430–433
Portner HO, Peck MA (2010) Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J Fish Biol 77:1745–1779
Portner HO, Berdal B, Blust R, Brix O, Colosimo A, Wachter BD, Giuliani A, Johansen T, Fischer T, Knust R, Lannig G, Naevdal G, Nedenes A, Nyhammer G, Sartoris FJ, Serendero I, Sirabella P, Thorkildsen S, Zakhartsev M (2001) Climate induced temperature effects on growth performance, fecundity and recruitment in marine fish: developing a hypothesis for cause and effect relationships in Atlantic cod (Gadus morhua) and common eelpout (Zoarces viviparus). Cont Shelf Res 21:1975–1997
Puvaneswari S, Marimuthu K, Karuppasamy R, Haniffa MA (2009) Early embryonic and larval development of Indian catfish, Heteropneustes fossilis. EurAsian J Biosci 3:84–96
Rahman ML, Zahangir MM, Kitahashi T, Shahjahan M, Ando H (2019) Effects of high and low temperature on expression of GnIH, GnIH receptor, GH and PRL genes in the male grass puffer during breeding season. Gen Comp Endocrinol 282:113200
Rechulicz J, Ostaszewska T, Wojda R (2002) Water temperature effects on incubation of ide (Leuciscusidus L.) eggs and selected parameters of the larvae. Acta Sci Polon 1:35–46
Rombough PJ (1997) The effects of temperature on embryonic and larval development. In: Wood CM, McDonald DG (eds) Global warming: implications for freshwater and marine fish. Cambridge University Press, Cambridge, pp 177–223
Sadler J, Pankhurst PM, King HR (2001) High prevalence of skeletal deformity and reduced gill surface area in triploid Atlantic salmon (Salmo salar L.). Aquaculture 198:369–386
Sato M, Kondo T, Yoshinaka R, Ikeda S (1983) Effect of water temperature on the skeletal deformity in ascorbic acid-deficient rainbow trout. Bull Japan Soc Sci Fish 49:443–446
Schulte PM, Healy TM, Fangue NA (2011) Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr Comp Biol 51:691–702
Scott GR, Johnston IA (2012) Temperature during embryonic development has persistent effects on thermal acclimation capacity in zebrafish. Proc Natl Acad Sci 109(35):14247–14252
Shahjahan M, Kitahashi T, Ogawa S, Parhar IS (2013) Temperature differentially regulates the two kisspeptin systems in the brain of zebrafish. Gen Comp Endocrinol 193:79–85
Shahjahan M, Kitahashi T, Ando H (2017) Temperature affects sexual maturation through the control of kisspeptin, kisspeptin receptor, GnRH and GTH subunit gene expression in the grass puffer during the spawning season. Gen Comp Endocrinol 243:138–145
Shahjahan M, Al-Emran M, Islam SMM, Baten SMA, Rashid H, Haque MM (2020) Prolonged photoperiod inhibits growth and reproductive functions of rohu Labeo rohita. Aquacult Rep 16:100272
Steinarsson A, Bjornsson B (1999) The effects of temperature and size on growth and mortality of cod larvae. J Fish Biol 55:100–109
Strussmann C, Conover ADO, Somoza GM, Miranda LA (2010) Implications of climate change for the reproductive capacity and survival of new world silversides (family Atherinopsidae). J Fish Biol 77:1818–1834
Sund T, Falk-Petersen IB (2005) Effects of incubation temperature on development and yolk sac conversion efficiencies of spotted wolfish (Anarhichas minor Olafsen) embryos until hatch. Aquac Res 36:1133–1143
Thépot V, Jerry DR (2015) The effect of temperature on the embryonic development of barramundi, the Australian strain of Lates calcarifer (Bloch) using current hatchery practices. Aquacult Rep 2:132–138
Wang LH, Tsai CL (2000) Effects of temperature on the deformity and sex differentiation of Tilapia, Oreochromis mossambicus. J Exp Zool 286:534–537
Watanabe WO, Lee CS, Ellis SC, Ellis EP (1995) Hatchery study of the effects of temperature on eggs and yolksac larvae of the Nassau grouper Epinephelus striatus. Aquaculture 136(1–2):141–147
Zahangir MM, Haque F, Mustakim GM, Khatun H, Islam MS (2015) Effect of water pH on the early developmental responses in zebrafish (Danio rerio). Prog Agricul 26:85–89
Funding
Grants for Advanced Research in Education, Ministry of Education, People’s Republic of Bangladesh is received for the grant (2017/503/MoE) to the corresponding author (Md. Shahjahan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
The animal care and use committee of BAU approved the procedures followed in this experiment (Approval Number: BAU-FoF/2018/004).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ashaf-Ud-Doulah, M., Islam, S.M.M., Zahangir, M.M. et al. Increased water temperature interrupts embryonic and larval development of Indian major carp rohu Labeo rohita. Aquacult Int 29, 711–722 (2021). https://doi.org/10.1007/s10499-021-00649-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-021-00649-x