Abstract
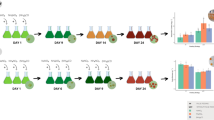
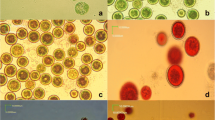
Mixotrophic cultivation of Haematococcus is efficient to increase astaxanthin productivity. However, application of this technique in open bioreactors is restricted because of bacterial contamination. In this study, mixotrophic cultivation of Haematococcus was carried out successfully in an outdoor raceway pond. The Haematococcus cells grew on light first, and then acetate/acetic acid was supplemented when nitrate was depleted from the culture media. Under such conditions, Haematococcus cells grew on intracellular nitrogen pool, while bacterial reproduction was limited. The average astaxanthin productivity reached 140 mg m−2day−1, which is about 1.2 times that of the simple phototrophic cultivation. This study explores a potential way to enhance astaxanthin productivity, and the results are helpful to the development of the Haematococcus industries.







Similar content being viewed by others
Abbreviations
- OD540 :
-
Optical density at wavelength of 540 nm
- Chl a :
-
Chlorophyll a
- Chl b :
-
Chlorophyll b
- T-Car:
-
Total carotenoids
- Asta:
-
Astaxanthin
- HL:
-
High light
- LL:
-
Low light
- CFU:
-
Bacterial colony-forming unit
References
Bing W, Aliza Z, Achim T et al (2003) Astaxanthin accumulation in Haematococcus pluvialis (Chlorophyceae) as an active photoprotective process under high irradiance. J Phycol 39:1116–1124
Bínová J, Tichý V, Lívanský K et al (1998) Bacterial contamination of microalgal biomass during outdoor production and downstream processing. Algol Stud 89:151–158
Borowitzka MA, Huisman JM, Osborn A (1991) Culture of the astaxanthin-producing green alga Haematococcus pluvialis 1. Effects of nutrients on growth and cell type. J Appl Phycol 3:295–304
Choi YE, Yun YS, Park JM (2002) Evaluation of factors promoting astaxanthin production by a unicellular green alga, Haematococcus pluvialis, with fractional factorial design. Biotechnol Prog 18:1170–1175
Cysewski GR, Lorenz RT (2004) Industrial production of microalgal cell-mass and secondary products - species of high potential: Haematococcus. In: Richmond A (ed) Handbook of Microalgal Culture: biotechnology and Applied Phycology. Blackwell, USA
Deschênes J-S, Boudreau A, Tremblay R (2015) Mixotrophic production of microalgae in pilot-scale photobioreactors: practicability and process considerations. Algal Res 10:80–86
Domı́nguez-Bocanegra AR, Guerrero Legarreta I, Martinez Jeronimo F et al (2004) Influence of environmental and nutritional factors in the production of astaxanthin from Haematococcus pluvialis. Bioresour Technol 92:209–214
Friedman M, Henika PR, Mandrell RE (2002) Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot 65:1545–1560
Goksan T, Ak I, Gokpinar S (2010) An alternative approach to the traditional mixotrophic cultures of Haematococcus pluvialis Flotow (Chlorophyceae). J Microbiol Biotechnol 20:1276
Guerin M, Huntley ME, Olaizola M (2003) Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol 21:210–216
Harker M, Tsavalos AJ, Young AJ (1996) Factors responsible for astaxanthin formation in the chlorophyte Haematococcus pluvialis. Bioresour Technol 55:207–214
Hu J, Nagarajan D, Zhang Q, Chang JS, Lee DJ (2018) Heterotrophic cultivation of microalgae for pigment production: a review. Biotechnol Adv 36:54–67
Industry experts report (2015) Global Astaxanthin Market – Sources, Technologies and Applications. http://industry-experts.com/verticals/healthcare-and-pharma/global-astaxanthin-market-sources-technologies-and-applications. Accessed 21 Aug 2018.
Jeon YC, Cho CW, Yun YS (2006) Combined effects of light intensity and acetate concentration on the growth of unicellular microalga Haematococcus pluvialis. Enzym Microb Technol 39:490–495
Kamalanathan M, Chaisutyakorn P, Gleadow R et al (2018) A comparison of photoautotrophic, heterotrophic, and mixotrophic growth for biomass production by the green alga Scenedesmus sp. (Chlorophyceae). Phycologia 57:309–317
Kobayashi M, Kakizono T, Yamaguchi K et al (1992) Growth and astaxanthin formation of Haematococcus pluvialis in heterotrophic and mixotrophic conditions. J Ferment Bioeng 74:17–20
Kobayashi M, Kurimura Y, Kakizono T et al (1997a) Morphological changes in the life cycle of the green alga Haematococcus pluvialis. J Ferment Bioeng 84:94–97
Kobayashi M, Kurimura Y, Tsuji Y (1997b) Light-independent, astaxanthin production by the green microalga Haematococcus pluvialis under salt stress. Biotechnol Lett 19:507–509
Lam TP, Lee T-M, Chen C-Y, Chang JS (2018) Strategies to control biological contaminants during microalgal cultivation in open ponds. Bioresour Technol 252:180–187
Lavín PL, Lourenço SO (2005) An evaluation of the accumulation of intracellular inorganic nitrogen pools by marine microalgae in batch cultures. Braz J Oceanogr 53:55–67
Lee YK (2004) Algal nutrition: heterotrophic carbon nutrition. In: Richmond A (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell, USA
Li XF, Xu H, Wu QY (2007) Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol Bioeng 98:764–771
Li YG, Miao FP, Geng YH et al (2012) Accurate quantification of astaxanthin from Haematococcus crude extract spectrophotometrically. Chin J Oceanol Limnol 30:627–637
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Lim KC, Yusoff FM, Shariff M et al (2017) Astaxanthin as feed supplement in aquatic animals. Rev Aquac 0:1–36
Lorenz R, Cysewski G (2000) Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol 18:160–167
Miao FP, Lu DY, Li YG et al (2006) Characterization of astaxanthin esters in Haematococcus pluvialis by liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. Anal Biochem 352:176–181
Middelburg J, Nieuwenhuize J (2000) Nitrogen uptake by heterotrophic bacteria and phytoplankton in the nitrate-rich Thames estuary. Mar Ecol Progress 203:13–21
Orosa M, Franqueira D, Cid A et al (2001) Carotenoid accumulation in Haematococcus pluvialis in mixotrophic growth. Biotechnol Lett 23:373–378
Orosa M, Franqueira D, Cid A, Abalde J (2005) Analysis and enhancement of astaxanthin accumulation in Haematococcus pluvialis. Bioresour Technol 96:373–378
Perez-Garcia O, Escalante FME, de Bashan LE et al (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45:11–36
Poddar N, Sen R, Martin GJO (2018) Glycerol and nitrate utilisation by marine microalgae Nannochloropsis salina and Chlorella sp. and associated bacteria during mixotrophic and heterotrophic growth. Algal Res 33:298–309
Solovchenko A, Khozin-Goldberg I, Recht L, Boussiba S (2011) Stress-induced changes in optical properties, pigment and fatty acid content of Nannochloropsis sp.: implications for non-destructive assay of total fatty acids. Mar Biotechnol 13:527–535
Wang ZJ, Wen XB, Xu Y et al (2018) Maximizing CO2 biofixation and lipid productivity of oleaginous microalga Graesiella sp. WBG1 via CO2-regulated pH in indoor and outdoor open reactors. Sci Total Environ 619-620:827–833
Wen XB, Du K, Wang ZJ et al (2016) Effective cultivation of microalgae for biofuel production: a pilot-scale evaluation of a novel oleaginous microalga Graesiella sp. WBG-1. Biotechnol Biofuels 9:123
Xu H, Miao XL, Wu QY (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507
Acknowledgments
This work is supported by the Science and Technology Service Network Initiative of the Chinese Academy of Sciences (KFJ-SW-STS-163). We thank Dr. Deborah Ballantine for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wen, X., Wang, Z., Ding, Y. et al. Enhancing the production of astaxanthin by mixotrophic cultivation of Haematococcus pluvialis in open raceway ponds. Aquacult Int 28, 625–638 (2020). https://doi.org/10.1007/s10499-019-00483-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-019-00483-2