Abstract
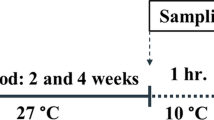
Shrimp farming in biofloc technology system (BFT) has been considered a sustainable alternative to conventional production, reducing environmental impacts due to zero water exchange. Besides increasing productivity, as microorganisms represent additional feed for shrimp, BTF may prevent diseases. White spot disease is the most devastating viral disease of farmed shrimp, causing large socio-economic impacts. Our aim was to compare survival rates and transcription profiles of target genes in different tissues of juvenile specimens of the Pacific white shrimp, Litopenaeus vannamei, reared either in BFT or in clear seawater (CSW), after challenging with white spot syndrome virus (WSSV). Shrimp survival rate was higher among animals kept in CSW, which also presented a lower viral load after 48 h post-infection (p.i.). We could also address possible individual patterns in the sole survivor at 72 h p.i. in biofloc, probably a less vulnerable shrimp to WSSV. There was no significant difference regarding the transcription profile of target genes between systems before WSSV infection; gene transcription was apparently modulated by WSSV infection. Changes in gene profile were more frequently observed in the hepatopancreas. Out of the eleven selected target genes, those coding for Calreticulin, β-tubulin, and Prophenoloxidase showed the most significant upregulation, followed by QM, SOD, and Ubiquitin. Although BFT is considered more biosecure, shrimp kept in this system are not immune to disease, especially against highly virulent pathogens, such as WSSV.





Similar content being viewed by others
References
Cardona E, Gueguen Y, Magré K, Lorgeoux B, Piquemal D, Pierrat F, Noguier F, Saulnier D (2016) Bacterial community characterization of water and intestine of the shrimp Litopenaeus stylirostris in a biofloc system. BMC Microbiol 16:157–165
Cerenius L, Söderhäll K (2004) The prophenoloxidase-activating system in invertebrates. Immunol Rev 198:116–126
Chen G, Zhang C, Li C, Wang C, Xu Z, Yan P (2011) Haemocyte protein expression profiling of scallop Chlamys farreri response to acute viral necrosis virus (AVNV) infection. Dev Comp Immunol 35:1135–1145
Chiu CH, Guu YK, Liu CH, Pan TM, Cheng W (2007) Immune responses and gene expression in white shrimp, Litopenaeus vannamei, induced by Lactobacillus plantarum. Fish Shellfish Immunol 23:364–377
Corteel M, Dantas-Lima JJ, Wille M, Alday-Sanz V, Pensaert MB, Sorgeloos P, Nauwynck HJ (2009) Molt stage and cuticle damage influence white spot syndrome virus immersion infection in penaeid shrimp. Vet Microbiol 137:209–216
Cottin D, Shillito B, Chertemps T, Tanguy A, Léger N, Ravaux J (2010) Identification of differentially expressed genes in the hydrothermal vent shrimp Rimicaris exoculata exposed to heat stress. Mar Genomics 3:71–78
Crab R, Defoirdt T, Bossier P, Verstraete W (2012) Biofloc technology in aquaculture: beneficial effects and future challenges. Aquaculture 356:351–356
Cuéllar-Anjel J, Corteel M, Galli L, Alday-Sanz V, Hasson KW (2014) Principal shrimp infectious diseases, diagnosis and management. In: The shrimp book (ed. by V. Alday-Sanz), pp.517–621. Nottingham University Press, Nottingham
Dhar AK, Bowers RM, Licon KS, Veazey G, Read B (2009) Validation of reference genes for quantitative measurement of immune gene expression in shrimp. Mol Immunol 46:1688–1695
Duan Y, Liu P, Li J, Wang Y, Li J, Chen P (2014) Molecular responses of calreticulin gene to Vibrio anguillarum and WSSV challenge in the ridgetail white prawn Exopalaemon carinicauda. Fish Shellfish Immunol 36:164–171
Esparza-Leal HM, Escobedo-Bonilla CM, Casillas-Hernández R, Álvarez-Ruíz P, Portillo-Clark G, Valerio-García RC, Hernández-López J, Méndez-Lozano J, Vibanco-Pérez N, Magallón-Barajas FJ (2009) Detection of white spot syndrome virus in filtered shrimp-farm water fractions and experimental evaluation of its infectivity in Penaeus (Litopenaeus) vannamei. Aquaculture 292:16–22
Flegel TW (2007) Update on viral accommodation, a model for host-viral interaction in shrimp and other arthropods. Dev Comp Immunol 31:217–231
Flegel TW (2014). Importance of host-viral interactions in the control of shrimp disease outbreaks. In: The shrimp book (ed. by V. Alday-Sanz), pp.623–654. Nottingham University Press, Nottingham
Gao W, Tian L, Huang T, Yao M, Xu Q, Guo TL (2016) Molecular cloning and expression of the calreticulin gene of the Pacific white shrimp, Litopenaeus vannamei, in response to acute hypo-osmotic stress. Aquaculture 454:265–271
Han F, Zhang X (2007) Characterization of a ras-related nuclear protein (Ran protein) up-regulated in shrimp antiviral immunity. Fish Shellfish Immunol 23:937–944
Hauton C (2012) The scope of the crustacean immune system for disease control. J Invertebr Pathol 110:251–260
Hsieh SL, Ruan YH, Li YC, Hsieh PS, Hu CH, Kuo CM (2008) Immune and physiological responses in Pacific white shrimp (Penaeus vannamei) to Vibrio alginolyticus. Aquaculture 275:335–341
Kim S-K, Pang Z, Seo H-C, Cho Y-R, Samocha T, Jang I-K (2014) Effect of bioflocs on growth and immune activity of Pacific white shrimp, postlarvae. Aquac Res 45(2):362-371
Lightner DV, Redman RM, Pantoja CR, Tang KFJ, Noble BL, Schofield P, Mohney LL, Nuna LM, Navarro SA (2012) Historic emergence, impact and current status of shrimp pathogens in the Americas. J Invertebr Pathol 110:174–183
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Lo CF, Leu JH, Ho CH, Chen CH, Peng SE, Chen YT, Chou CM, Yeh PY, Huang CJ, Chou HY, Wang CH, Ou GH (1996) Detection of baculovirus associated with white spot syndrome (WSBV) in penaeid shrimps using polymerase chain reaction. Dis Aquat Org 25:133–141
Maciel MLT (2002) Contribuição para o desenvolvimento de uma proposta de monitoramento e certificação sanitária em cultivo de camarão marinho no Estado de Santa Catarina. Master Dissertation, Aquaculture Program. Universidade Federal de Santa Catarina - UFSC
Moser JR (2011) Biomarcadores moleculares no camarão branco, Litopenaeus vannamei (Crustacea: Decapoda), submetido a estresse ambiental e infectado pelo vírus da síndrome da mancha branca (White Spot Syndrome Virus, WSSV). Ph.D. Thesis, Biotechnology and Bioscience Graduate Program. Universidade Federal de Santa Catarina - UFSC
Müller IC (2009) Genes diferencialmente expressos em camarões de cultivo Litopenaeus vannamei infectados pelo vírus da Síndrome da Mancha Branca e genotipagem de isolados geográficos brasileiros do vírus. Ph.D. Thesis. Biotechnology and Bioscience Graduate Program. Universidade Federal de Santa Catarina - UFSC
Murphy FA, Gibbs EPJ, Horzinek MC, Studdert MJ (1999) Veterinary virology, 3rd edn. Academic Press, USA
Ng TH, Chang SH, Wu MH, Wang HC (2013) Shrimp hemocytes release extracellular traps that kill bacteria. Dev Comp Immunol 41:644–651
Qiao G, Xu DH, Wang Z, Jang IK, Qi Z, Zhang M, Kim SK (2015) Comparison of immune response of Pacific white shrimp, Litopenaeus vannamei, after multiple and single infections with WSSV and Vibrio anguillarum. Fish Shellfish Immunol 44:257–264
Rivera IN, Lipp EK, Gil A, Choopun N, Huq A, Colwell RR (2003) Method of DNA extraction and application of multiplex polymerase chain reaction to detect toxigenic Vibrio cholerae O1 and O139 from aquatic ecosystems. Environ Microbiol 5:599–606
Shekhar MS, Azad IS, Ravichandran P (2006) Comparison of dot blot and PCR diagnostic techniques for detection of white spot syndrome virus in different tissues of Penaeus monodon. Aquaculture 261(4):1122–1127
Smith VJ, Roulston C, Dyrynda E (2014) The shrimp immune system. In: The shrimp book (ed. by V. Alday-Sanz), pp.89–148. Nottingham University Press, Nottingham
Vidya R, Gireesh-Babu P, Prasad KP (2013) White spot syndrome virus manipulates ubiquitin gene expression in Penaeus monodon. Indian J Virol 24:82–84
Wang YC, Chang PS, Chen HY (2007) Tissue expressions of nine genes important to immune defense of the Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 23:1161–1177
Xu J, Wu S, Zhang X (2008) Novel function of QM protein of shrimp (Penaeus japonicus) in regulation of phenol oxidase activity by interaction with hemocyanin. Cell Physiol Biochem 21:473–480
Acknowledgments
We thank the Laboratory of Marine Shrimp–LCM (Aquaculture Department, Federal University of Santa Catarina, Brazil) and staff members for providing shrimp used in this study, as well as the biofloc.
Funding
This research was funded by the National Council of Technological and Scientific Development (CNPq), Brazil, under the grant number Proc.no. 407211/2012-8 to MRFM. KOO was recipient of a PIBIC/CNPq undergraduate student scholarship. CSV was recipient of a CAPES scholarship from the Aquaculture Graduate Program at UFSC. FNV and MRFM are recipients of a CNPq productivity research grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Souza Valente, C., Oliveira Ortiz, K., Depperschmidt, R. et al. Transcription of defense related genes in Pacific white shrimp, Litopenaeus vannamei, kept in biofloc and in clear seawater and challenged with the white spot syndrome virus. Aquacult Int 28, 293–307 (2020). https://doi.org/10.1007/s10499-019-00461-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-019-00461-8