Abstract
Mussel losses peak after relaying seed on culture plots. The present paper is an attempt to examine the role of shore crab predation and initial mussel density on mussel losses in mussel bottom culture using an intertidal culture plot as a case study. Because of their small size and loose attachment, mussels are particularly vulnerable to predation at this stage, as well as to handling stress and intraspecific competition. In the experimental field plots (1 × 1 m) in the intertidal Oosterschelde, three different densities (1, 5 and 10 kg m−2) of mussel seed are laid, with half of the experimental plots protected from predation by means of exclosures. Duration of the experiment was 5 weeks (August–September 2012) post-seeding. Protection was the major factor accounting for biomass production, followed by mussel density. Loss rates increased with mussel density, both in the exclosures and in the exposed plots. Losses in the exclosures with the lowest density were still 45 %. There are indications that handling stress prior to the start of the experiment played a major role in these losses. At the higher densities in the exclosures, losses increased to 72.1 % and were not significantly different between 5 and 10 kg m−2. About one-third of the total loss (32.6 %) was attributed to shore crab predation. The number of shore crabs observed on the plots did not differ between treatments. Byssal thread development during the experimental period was followed and was found to be a slow process that was insufficient to protect mussels from crab predation at the sheltered experimental location.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The seeding of mussel seed dredged from natural beds or collected from submerged seed mussel collectors (SMCs) in the water column is the most critical step in the mussel bottom culture cycle (Calderwood et al. 2014; Capelle et al. 2014). Most of the mussel seed does not survive the cycle from seeding to harvest. Average survival of SMC-seed or seed relayed from wild mussel beds in autumn over the culture cycle is 8 and 15 %, respectively (Capelle et al. 2016). A substantial part of the losses is associated with seeding (Theisen 1968; Capelle et al. 2014, 2016). Such losses are a significant factor in biomass production (Capelle et al. 2016).
Losses on culture plots associated with or following seeding are clearly density dependent (Gascoigne et al. 2005; Capelle et al. 2014, 2016). Seeding practice in bottom culture leads to a highly heterogeneous distribution of mussels on culture plots, with high mussel densities within the space occupied by mussels (Capelle et al. 2014). Mussels are gregarious organisms and aggregate in patches, thereby competing for food and space (Fréchette and Bourget 1985; Liu et al. 2012).
Other factors that account for such losses are damaged mussels, handling stress (Calderwood et al. 2014) and predation. The main predators of young mussels are starfish in the subtidal area (Gallagher et al. 2008), and birds and tidal-moving shore crabs in the intertidal zone (Hilgerloh et al. 1997; Silva et al. 2014).
The shore crab (Carcinus maenas) is a well-studied predator of juvenile mussels. The feeding rate of shore crabs depends on the predator–prey size ratio, where the predation rate shows a rapid decrease with increasing mussel size (Crothers 1968; Mascaró and Seed 2001; Murray et al. 2007; Kamermans et al. 2009). The impact of crab predation over the entire culture cycle has been estimated to range from 9.5 % (Murray et al. 2007) to 52 % mussel loss (Grosholz et al. 2011). These data are extrapolations of laboratory results on feeding rates to estimated field abundances. Exclusion of shore crabs from an intertidal culture plot (800 m2) via installation of fences improved the yield by a factor of 4–5 for the entire culture cycle (Davies et al. 1980).
Mussels display several defence mechanisms against predation. In response to chemical cues from crabs, mussels increase their shell thickness, which significantly increases the handling time for foraging crabs (Freeman 2007). Mussels also increase the production of byssal thread in the presence of predators, thereby being more firmly attached and leading to reduced predation rates. This defence is widely used and has been known with regard to Homarus sp., C. maenas and Cancer sp. feeding on Mytilus edulis (Côté 1995; Reimer and Tedengren 1997; Garner and Litvaitis 2013), Callinectes sapidus feeding on Ischadium recurvum (Brown et al. 2011) and Acanthocylus gayi feeding on Perumytilus purpuratus and Semimytilus algosus (Caro et al. 2008), where A. gayi was selecting the mussels with the weakest attachment. Since shore crabs display competitive behaviour for food, dense mussel aggregations increase the intraspecific interactions between the crabs that might lead to a decrease in the predator–prey interactions in the field (Sneddon et al. 1997; Smallegange and Van Der Meer 2007). Interference between crabs is also indicated by experiments of Kamermans et al. (2009) where the rate at which 20 mm mussel seed was consumed, decreased from 6 seeds/day/crab to 3 seeds/day/crab when two crabs were placed together in one cage. However, when this hypothesis was tested on cockles, it was observed that the aggregation level had no effect on predation success of shore crabs (Whitton et al. 2012). When mussels were presented as a group rather than as a sequence of single individuals, predation rates increased, as the shore crabs were able to select smaller-sized individuals of a group more easily (Burch and Seed 2000).
Mussels attach to conspecific organisms using their byssus threads. The attachment strength of mussels increases with time and depends on the number of byssal threads that are produced (Kangeri et al. 2014). If shore crab predation rates are dependent on byssus attachment strength, mussels are most vulnerable during the short period after seeding, when the mussels redistribute (Capelle et al. 2014) and need to develop their attachment strength. Furthermore, damaged mussels or dead mussels, as a result of the seeding process (Calderwood et al. 2014) emit cues that enable shore crabs (which orientate themselves mainly via chemoreception) to locate a culture plot from a long distance shortly after seeding (Crothers 1968). Damaged or dead mussels are easy prey for shore crabs.
Losses peak on intertidal (Capelle et al. 2014) and subtidal plots (Capelle et al. 2016) shortly after seeding. The contribution of different loss factors to this loss is not known. It is hypothesised that density-dependent losses and shore crab predation are the major loss factors for mussel seed on intertidal culture plots.
We expect that shore crab predation will contribute significantly to losses shortly after seeding because of the small mussel size, weak byssal attachment and the high chemical attraction of damaged mussels. Therefore, the role of byssal attachment strength shortly after seeding as a protection mechanism against loss was also investigated.
Materials and methods
A field experiment was conducted in order to estimate losses during the first 5 weeks post-seeding. In this experiment, the effects of mussel density and protection against predation on mussel condition and survival were measured.
Site and experimental set-up
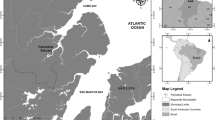
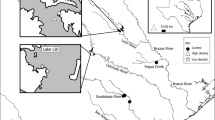
The field experiment was undertaken on an intertidal commercial mussel plot (51°N33.193′, 3°E53.327′) from the 15th of August 2012 to the 20th of September 2012. The site is located at a sheltered location within the Oosterschelde estuary in the Netherlands (Fig. 1). A section of this mussel plot was laid with mussel seed by a mussel farmer during high tide on the 14th of August 2012. Mussel seed used was harvested from seed mussel collectors (SMCs) from the western part of the Oosterschelde (Fig. 1) a day previously and was held overnight without water in the hold of a mussel vessel prior to seeding. A total of eighteen experimental units were set out in three rows, with six experimental units of 1 m × 1 m per row, with 1 m between each experimental unit. The experimental units were located approximately 10 m north-east of the area where the mussels were laid by the mussel farmer. Within each experimental unit of 1 m2, mussels that were collected from the culture plot were evenly distributed in three different biomass densities over the experimental plots a day after seeding: 1, 5 and 10 kg m−2, which corresponded to approximate mussel densities of 3000, 15,000 and 30,000 mussels m−2, respectively. Half of the experimental units from each density were protected by cages Fig. 2). The cages were constructed using wooden frames (1 m × 1 m × 0.5 m) that were wrapped with chicken wire mesh (size: 0.8 mm). All the treatments (density and exclosure) were randomly assigned to the experimental units in triplicate. During the course of the experiment, due to an unknown reason, one of the exclosures of an initial biomass of 1 kg m−2 of mussels was lost between the third and fourth week. At the start of the experiment, a random sub-sample (n = 424) was taken from the culture plot in order to measure the length and the byssal attachment strength of the mussels. For a period of 4 weeks, three core samples (d = 75 mm) were taken from each experimental unit haphazardly, once every week, and the collected mussels were pooled per experimental unit. In the fifth week of the experiment, all the remaining mussels were collected from each of the experimental units and were weighed for biomass, after which a random volumetric (100 ml) sub-sample was taken for analysis.
Byssal attachment strengths of all the individual mussels, both within the sub-sample and within the intermediate core samples, were measured using a digital gauge (Sauter FK-10, 0–10 N). This device was attached to plastic tick tweezers, which were positioned around an individual mussel and pulled in a 180° direction from the point of attachment of the byssus threads until the mussel became loose. The force gauge displayed the maximum force (N) that was used during the experiment. The length of the individual mussel (L) was measured using a digital calliper (accurate to 0.1 mm), while the ash-free dry weight (AFDW) per sample was obtained by drying the sample at 80 °C and ashing it at 560 °C in a prepASH® 340 series until the difference in weight was observed to be less than 1 % per 30 min. Relative mussel loss rates (r, d −1) were calculated for each of the experimental units as (ln(N end) – ln(N start)) divided by the time of the experiment (days), where N is the number of mussels m−2. A condition index (CI, mg cm−3) was calculated for each experimental unit via AFDW/L 3 (Beukema 1976). Biomass development over the experiment was expressed as B end /B start, where B is biomass (kg m−2).
Eight video cameras (wide-angle waterproof surveillance cameras) were placed above the exposed plots on the 22nd of August and nine cameras on the 29th of August, each covering an area of 0.6–0.7 m2. Cameras recorded the activities on the plots continuously for 2.2 h, during high tide. The videos were used to estimate the average density of foraging shore crabs on the exposed plots in order to evaluate the relationship between the numbers of shore crabs and the mussel density, as well as to estimate the shore crab predation pressure. The crabs were counted and expressed as average number of individuals per m2 per hour. Predation pressure was expressed as the number of foraging crabs per m2 per hour. A crab was considered to be foraging when it stayed in one place for at least 30 s. Food processing movements were also observed in most such cases.
Average water temperature was 18 °C during the experimental period. Average wind speed was 4.0 m s−1 (Beaufort 3); the maximum wind speed during the experimental period was recorded as 9.2 m s−1 (Beaufort 5) on 1 day during the experiment (day 17 from the start). Wind direction was observed to range between 120° and 340°, with an average of 223° north. Experimental plots were completely dry only at water levels below about –1.50 m NAP (Amsterdam Ordnance Datum), which occurred for about 2 h out of every 24 h on average. No shore crabs were found in the exclosures at the end of the experiment.
In addition, we carried out a small predation experiment in the laboratory in which pre-aggregated mussels (four tanks) and loose mussels (four tanks) were exposed to an individual shore crab for a period of 8 h—the crabs had carapace width of 60–70 mm, and mussels ranged from 20 to 25 mm in shell length, from a different batch than used in the field experiment, and were fished from a subtidal commercial mussel plot. The experiment was repeated eight times over a period of 5 weeks using different crabs and mussels for each run. Average attachment strength of the pre-aggregated mussels was found to be 2.0 ± 1.0 N (n = 185), and mussels that were presented loose developed an attachment strength of 1.0 ± 0.7 N (n = 83) over the course of the 8 h for which the experiment lasted.
Data analysis
The field experiment was set up as a random design. Differences in loss rates, biomass development and CI between treatments at the end of the experimental period were analysed using a two-way analysis of variance (ANOVA), with the factors of initial biomass and protection as response variables. The analyses were followed by a post hoc Tukey HSD (honest significant difference). The quantity of crabs that was measured from the video observations was tested against the initial mussel biomass (as factor) via the Kruskal–Wallis test. The level of significance was 0.05 for each analysis. Normality and homogeneity of the variances of the residuals were evaluated for all models by following Zuur et al. (2010).
The development of the attachment strength of the mussels over time was first analysed via a repeated-measures ANOVA; however, because of the heterogeneity of residual variances, a linear mixed-effects model (LME) was applied using ‘lme’ from the nlme library (Pinheiro et al. 2014). In this model, the mussel density, protection and time (number of weeks) were taken as fixed effects, and time nested in subject as a random effect. Model selection was carried out according to Zuur et al. (2009), and non-significant (P > 0.05) interactions were removed in a reverse stepwise manner. Restricted maximum likelihood (REML) estimators were used to estimate whether random structures improved model fit. When the model was improved via a random structure, the optimal fixed-effect structure was determined (with the same random structure) using maximum likelihood (ML) estimators. The final model was presented using an REML estimation. Normalised residuals of this fit were visually checked for normality using a histogram and were plotted against fitted values and against each explanatory variable to validate the model. The residuals closely followed a normal distribution, and no violation of homogeneity was indicated at this point. When significant overall effects were found, pairwise comparisons were made by means of a Tukey HSD test.
The number of mussels consumed by each shore crab per 8 h in the laboratory experiment was analysed using a generalised linear model (GLM), with the response factor (Y) containing the proportion dead and live mussels per observation, and a quasi-binomial variance model to account for over-dispersion. All analyses were carried out using R v.3.1.2 (R Core Team 2013).
Results
Video observations in the first week (22nd of August) showed significantly more (F(1,11) = 8.33, P = 0.01) (5.4 ± 2.8) shore crabs foraging for mussels (11.8 % of all passing crabs) than in the second week (29th of August, 1.7 ± 1.8 shore crabs, 7.4 % of all passing crabs). No statistical association between the number of foraging crabs and the initial mussel density was observed. Since crabs migrate with the tide, the measurements obtained during high tide will probably reflect the peak in numbers within each day.
Loss rates differed significantly between exclosures and exposed plots (F(1,13) = 80.38, P < 0.001) and between levels of initial density (F(2, 13) = 8.71, P = 0.003, Fig. 3); protection and levels of initial density showed no significant interaction. Loss increased with (In) initial density. The differences in mussel loss between the exclosures and the exposed plots are expressed as percentages in Table 1.
Mussel loss rates over a 5-week field experiment at three different initial densities on exclosures and unprotected plots. Lines show a significant relationship for exclosures (r 2 = 0.57, F(1,6) = 10.37, P = 0.018, coefficients: a = 0.0092 ± 0.0029 (sem), b = −0.055 ± 0.027 (sem)) and unprotected plots (r 2 = 0.41, F(1,7) = 6.51, P = 0.038, coefficients: a = 0.025 ± 0.0096 (sem), b = −0.12 ± 0.090 (sem))
Biomass development, calculated as final mussel biomass/initial biomass over the 5-week experimental period, was affected by the factors of initial density (F(2,11) = 16.22, P < 0.001) and protection (F(1,11) = 99.19, P < 0.001). However, there was a significant interaction effect (F(2,11) = 4.25, P = 0.04) between the factors, indicating that the biomass development was higher on the protected plots (exclosures) than it was on the exposed plots (Fig. 4). In the exclosures, mussel biomass increased in 5 weeks with a factor of 1.5; this ratio was below 1 in all the plots with higher initial biomasses.
Condition indices of the mussels from all the treatments (Fig. 5) were subjected to a two-way analysis of variance test, with two levels of protection and three levels of initial density. However, the effects were not statistically different.
Attachment strength increased over time (Fig. 5) and was significantly affected by protection (Table 2). A regression tree model showed that protection did not matter before the fifth week. In other words, within the exclosures, byssal attachment strength of the mussels did not increase, whereas on the exposed plots, it increased in the fifth week. The initial biomass was found to have no effect on attachment strength. Results of the additional (controlled) laboratory experiment showed that the number of mussels consumed by each shore crab per 8 h (mean 10.15 ± 5.53) did not differ between the pre-aggregated or the loose mussels (GLM, P > 0.05). Average attachment strength of the pre-aggregated mussels was 2.0 ± 1.0 N (n = 185), and the mussels that were presented loose developed an attachment strength of 1.0 ± 0.7 N (n = 83) over the course of the 8 h that the experiment lasted. This shows that mussel attachment strength that developed in the first week in the field was not sufficient to protect them from crab predation (Fig. 6).
Discussion
The present study closely followed mussel performance on an intertidal commercial mussel plot for a 5-week period after seeding. Effects of initial mussel density and crab predation (through use of exclosures) on mussel survival were estimated. Overall losses were extremely high, particularly when compared to earlier tests as detailed below.
Potential loss factors
The present study confirmed earlier findings that mussel loss is very high shortly after seeding and that mussel loss increases with mussel density (Theisen 1968; Capelle et al. 2014, 2015). Differences between the exclosures and the exposed plots can be used to distinguish between the loss factors. In the exclosures, mussel loss was probably due to mortality associated with handling stress (Calderwood et al. 2014) and intraspecific competition. At the lowest density (1 kg m−2), where intraspecific completion was assumed to be low, mortality was still 45 % after 5 weeks. We could not find any studies on mussel bed performance as a function of density by means of exclosures. However, because 1 kg m−2 is a low density in common mussel culture practice (Capelle et al. 2014), this 45 % is regarded as background mortality that might show losses following handling stress. At higher densities of 5 and 10 kg m−2, mortality was observed to be 71 and 72 %, respectively, during the experiment. This mortality increase can be attributed to intraspecific competition at higher densities. The difference between mussel mortality in the exclosures and the losses on the exposed plots is due to dislodgement, and to predation by birds and shore crabs. This difference was in the range of 27–45 % (average 32.6 %; Table 1), which resulted in a final mussel biomass range of 0.3–0.4 kg m−2 on the exposed plots for each treatment.
Mussel loss on the exposed plots
Exclosures not only protected mussels against crab predation, but also against dislodgement and bird predation. Both bird predation and dislodgement are known factors contributing to loss on mussel beds (Nehls and Thiel 1993; Ens and Alting 1996; Hilgerloh et al. 1997). Herring gulls feed on small mussels (of around 20 mm), and oystercatchers feed on larger mussels (30–45 mm; Meire and Ervynck 1986). These birds are the main predators of mussel seed on intertidal flats (Hilgerloh et al. 1997). The experimental area was embedded in a culture plot on which the same mussel seed was seeded. On that culture plot, mussel farmers were present at low tides every day during the daytime and actively chased the birds away. This activity stopped after the first 3 weeks of the experiment because of the low number of birds observed in the area and subsequently on the culture plot. Exposure of the experimental area during low tides was relatively short and occurred, on average, only 2 h in every 24 h. Such circumstances led to speculation that bird predation might not be a significant loss factor on the exposed plots. The other major mussel predator in this area is starfish (Asterias rubens); however, their distribution is limited to the subtidal zone (Saier 2001). Also no starfish were observed on the video. It has been observed that the intertidal mussel beds are destroyed by dislodgement as a result of the effect of severe storms, but they remain relatively stable in sheltered locations (Nehls and Thiel 1993). The experimental site for the present study was located within a sheltered area in the Oosterschelde (Fig. 1), where the wind speed did not peak above 9.2 m s−1 throughout the duration of the experiment. The dominant direction of the wind during the 5 weeks of the experimental period was south-west. No mussel seed was found in the area around the (seeded) culture plot. These circumstances during the experimental period did reduce dislodgement risk and were important factors in the evaluation of the mussel loss. Thus, only predation by crabs remained as a factor to analyse the primary difference in mussel loss between the exclosures and the exposed plots.
Mussel loss caused by handling stress
In contrast to a similar experiment that was performed a year earlier on the same culture plot, with 60–85 % loss 4 weeks after relaying on exposed plots, mussel loss was observed to be higher at the exposed plots in the current study with 90–99 % loss (Capelle et al. 2014). Furthermore, in the present study, the mussel loss was also higher than the losses that are generally experienced after seeding (around 50 %) (Theisen 1968; Capelle et al. 2016). According to mussel farmers, such high mussel loss may be due to the possibility that the mussels were kept in the vessel for too long from the time of harvest to seeding, especially because the day was very hot and sunny, and it has been reported that such conditions might cause some kind of stress that decreases survival after relay (Calderwood et al. 2014). It might be a satisfactory explanation for the very high mussel losses (Table 1), because the condition index of the seed, a day after seeding, was high (8.08 mg cm−3). In the similar experiment that was performed a year earlier on the same culture plot with mussels from similar origin and size, condition index of mussels at start was only 5.00 mg cm−3 (Capelle et al. 2014) and condition index of mussels of a similar size transplanted in Denmark ranged between 3.8 and 5.2 mg cm−3 (Dolmer et al. 2012). These losses due to handling stress also explain the total losses (95.6 %), and the resulting loss levels were at the levels that are normally expected after seeding (about 50 %, Table 1).
It has been reported that handling stress also has an impact on byssal thread production shortly after seeding (Calderwood et al. 2014). However, in spite of similar stress experienced by the mussels in each treatment and on the entire culture plot, mussel losses found in the present study were higher than those observed under normal conditions. The results support the suggestion by Calderwood et al. (2014) that mussels should not be kept out of the water for more than 24 h.
Development of attachment strength
Byssal attachment strength increased to a mean strength of 4.9 ± 0.2 N (SE) n = 9 during the fifth week at the exposed plots, while it remained at the same level for the protected mussels (mean 2.5 ± 0.2 N (SE), n = 8). Previous studies showed that the presence of shore crabs (effluent) stimulates the creation of byssus (Reimer and Tedengren 1997; Leonard et al. 1999) at a speed that can result in 10 byssus threads after 6 h and, in the field, to attachment strengths (after 30 days) of about 16 N (Côté 1995), which was twice as high as it was when no crabs were present (Leonard et al. 1999). Attachment strength is related to the sum of individual byssal threads (Bell and Gosline 1996), and mussels that are more strongly attached are less likely to be taken by crabs (Lin 1991). However, the byssus production on the exposed plots over time did not increase in the period after seeding, which is when the majority of the losses occur (Capelle et al. 2016). During the first week in the field, the mussels developed an average attachment strength of 1.6 ± 0.1 N (SE). The results of the laboratory experiment indicated that a mussel attachment strength of around 2 N is not sufficient to protect a mussel from crab predation.
Byssal attachment strength, which was measured both in established and in transplanted (after 30 days) mussel aggregations at exposed rocky shores in the study conducted by Leonard et al. (1999), was much higher (about 10–35 N) than it was in the present study. The byssal attachment strength measured after re-attachment under laboratory conditions (Lee et al. 1990; Dolmer and Svane 1994; Babarro and Reiriz 2010) was much lower than that which was reported in the findings by Leonard et al. (1999), and was lower than values measured in the present study. The experimental site at which the present experiment was carried out was a sheltered area of the Oosterschelde. Site conditions are strong determinants of attachment strength (Bell and Gosline 1996), and formation of byssus is costly (8 % of carbon and nitrogen in the total production in summer (Hawkins and Bayne 1985). Sheltered site conditions might, therefore, be more relevant for explaining the development of attachment strength than the presence of shore crabs, and may also play a role in protection against shore crab predation.
Crab predation
It is estimated that about one-third (average 32.6 %; Table 1) of mussel loss in the 1-month period after seeding can be attributed to crab predation. These results are in line with the Plass-Johnson et al. (2010), who attributed 35 % of the mussel losses to predation and with estimations of Grosholz et al. (2011) who extrapolated results from Beal and Kraus (2002) and from (Beal 2006) where predation losses varied between 13 and 55 %. Murray et al. (2007) estimated that losses as a result of shore crab predation are 9.5 % over 12 months in the Menai Street in Wales, which is substantially lower than in the present study. However, predation rates were based on mean number of crabs per square metre on mussel beds in Wales which was lower than in the present study with on average 0.5 crabs m−2 in August (Murray et al. 2007; Kamermans et al. 2009). A peak in foraging crabs can be expected closely after relaying. However, detailed observations from the first weeks after relaying are scarce, but the present study indicates such an effect. Crab abundance was much lower in the second week after seeding than it was in the first week after seeding, probably due to the attraction of the crabs to the smell of dead or damaged mussels during and shortly after seeding. Losses in mussel bottom culture are determined by high losses following seeding (Capelle et al. 2016). Mussels in week 1 and week 2 were between 16 and 18 mm in size. Murray et al. (2007) reported that the number of mussels consumed decreased as the mussel size increased. An individual shore crab can consume 21–36 mussels of 20 mm (minimum size presented) per 24 h depending on the size of the crab (Murray et al. 2007, Kamermans et al. 2009). It is assumed that for mussels of between 16 and 18 mm, the average food intake is around 1.25 mussels per hour, and a similar food intake was observed in the small predation experiment and in Kamermans et al. (2009); thus, the amount of crabs observed would have eaten about 286–1676 (mean ± SD) mussels/m2 within the first 2 weeks: 730 (±355) mussels/m2 in the first week and 216 (±229) mussels/m2 in the second week. At the exposed plots with the highest density, 7000 mussels/m2 were lost in a period of 5 weeks. With the estimated food intake, it would have taken between four and 24 weeks to consume such a high number of mussels with regard to the number of crabs that was observed. The amount of variation shows that such estimations can only be regarded as a rough estimate; however, it does show that the number of shore crabs observed could explain the observed mussel losses.
Conclusions
Based on the present analysis, it can be concluded that mussel losses were high (mean 95.6 %) in a short period (about 1 month) after seeding on an intertidal culture plot, particularly when compared to previous studies. We observed a high and unexpected background mortality, which was the largest relative proportion (45 %) of all losses. Handling stress caused by the culture process could have played a major role in this loss. Moreover, (18 %) higher losses were observed at the higher stocking densities (5–10 kg m−2) than at the low mussel density (1 kg m−2). Remaining losses (about one-third; 32.6 %) were attributed to crab predation. No correlation was found between the number of shore crabs and the density of mussels. The development in attachment strength of the mussels at the sheltered location was observed to be a slow process and was thus unlikely to serve as protection against predation.
References
Babarro JMF, Reiriz MJF (2010) Secretion of byssal threads in Mytilus galloprovincialis: quantitative and qualitative values after spawning stress. J Comp Physiol B Biochem Syst Environ Physiol 180:95–104
Beal BF (2006) Relative importance of predation and intraspecific competition in regulating growth and survival of juveniles of the soft-shell clam, Mya arenaria L., at several spatial scales. J Exp Mar Biol Ecol 336(1):1–17
Beal BF, Kraus MG (2002) Interactive effects of initial size, stocking density, and type of predator deterrent netting on survival and growth of cultured juveniles of the soft-shell clam, Mya arenaria L., in eastern Maine. Aquaculture 208(1):81–111
Bell E, Gosline J (1996) Mechanical design of mussel byssus: material yield enhances attachment strength. J Exp Biol 199(4):1005–1017
Beukema JJ (1976) Biomass and species richness on the macro-benthic animals living on the tidal flats of the Dutch Wadden Sea. Neth J Sea Res 10:236–261
Brown KM, Aronhime B, Wang X (2011) Predatory blue crabs induce byssal thread production in hooked mussels. Invertebr Biol 130:43–48
Burch A, Seed R (2000) Foraging behaviour of Carcinus maenas on Mytilus edulis: the importance of prey presentation. J Mar Biol Assoc UK 80:799–810
Calderwood J, O’Connor NE, Sigwart J et al (2014) Determining optimal duration of seed translocation periods for benthic mussel (Mytilus edulis) cultivation using physiological and behavioural measures of stress. Aquaculture 434:288–295
Capelle JJ, Wijsman JWM, Schellekens T et al (2014) Spatial organisation and biomass development after relaying of mussel seed. J Sea Res 85:395–403
Capelle JJ, Wijsman JWM, Van Stralen MR, et al (2015) Effect of seeding density on biomass production in mussel bottom culture. Manuscript submitted for publication
Capelle JJ, Wijsman JW, van Stralen MR et al (2016) Effect of seeding density on biomass production in mussel bottom culture. J Sea Res 110:8–15
Caro AU, Escobar J, Bozinovic F et al (2008) Phenotypic variability in byssus thread production of intertidal mussels induced by predators with different feeding strategies. Mar Ecol Prog Ser 372:127–134
Côté IM (1995) Effects of predatory crab effluent on byssus production in mussels. J Exp Mar Biol Ecol 188:233–241
Crothers JH (1968) The biology of the shore crab Carcinus maenas (L.). 2. The life of the adult crab. Field Stud 2:579–614
Davies GP, Dare PJ, Edwards DB (1980) Fenced enclosures for the protection of seed mussels (Mytilus edulis L.) from predation by shore crabs (Carcinus maenas L.). Fisheries Research Technical Report 56
Dolmer P, Svane I (1994) Attachment and orientation of Mytilus edulis L. in flowing water. Ophelia 40:63–74
Dolmer P, Christensen HT, Hansen BW et al (2012) Area-intensive bottom culture of blue mussels Mytilus edulis in a micro-tidal estuary. Aquac Environ Interact 3:81–91
Ens BJ, Alting D (1996) The effect of an experimentally created mussel bed on bird densities and food intake of the oystercatcher Haematopus ostralegus. Ardea 84:493–507
Fréchette M, Bourget E (1985) Food-limited growth of Mytilus edulis L. in relation to the benthic boundary layer. Can J Fish Aquat Sci 42:1166–1170
Freeman AS (2007) Specificity of induced defenses in Mytilus edulis and asymmetrical predator deterrence. Mar Ecol Prog Ser 334:145–153
Gallagher T, Richardson CA, Seed R et al (2008) The seasonal movement and abundance of the starfish, Asterias rubens in relation to mussel farming practice: a case study from the Menai Strait, UK. J Shellfish Res 27:1209–1215
Garner YL, Litvaitis MK (2013) Effects of injured conspecifics and predators on byssogenesis, attachment strength and movement in the blue mussel, Mytilus edulis. J Exp Mar Biol Ecol 448:136–140
Gascoigne JC, Beadman HA, Saurel C et al (2005) Density dependence, spatial scale and patterning in sessile biota. Oecologia 145:371–381
Grosholz E, Lovell S, Besedin E et al (2011) Modeling the impacts of the European green crab on commercial shellfisheries. Ecol Appl 21:915–924
Hawkins A, Bayne B (1985) Seasonal variation in the relative utilization of carbon and nitrogen by the mussel Mytilus edulis: budgets, conversion efficiencies and maintenance requirements. Mar Ecol Progr Ser Oldendorf 25(2):181–188
Hilgerloh G, Herlyn M, Michaelis H (1997) The influence of predation by herring gulls Larus argentatus and oystercatchers Haematopus ostralegus on a newly established mussel Mytilus edulis bed in autumn and winter. Helgol Mar Res 51:173–189
Kamermans P, Blankendaal M, Perdon J (2009) Predation of shore crabs (Carcinus maenas L.) and starfish (Asterias rubens L.) on blue mussel (Mytilus edulis L.) seed from wild sources and spat collectors. Aquaculture 290:256–262
Kangeri AK, Jansen JM, Barkman BR et al (2014) Perturbation induced changes in substrate use by the blue mussel, Mytilus edulis, in sedimentary systems. J Sea Res 85:233–240
Lee CY, Lim SSL, Owen MD (1990) The rate and strength of byssal reattachment by blue mussels (Mytilus edulis L.). Can J Zool 68:2005–2009
Leonard GH, Bertness MD, Yund PO (1999) Crab predation, waterborne cues, and inducible defenses in the blue mussel, Mytilus edulis. Ecology 80:1–14
Lin J (1991) Predator-prey interactions between blue crabs and ribbed mussels living in clumps. Estuar Coast Shelf Sci 32(1):61–69
Liu Q-X, Weerman EJ, Herman PMJ, et al (2012) Alternative mechanisms alter the emergent properties of self-organization in mussel beds. In: Proceedings of the royal society B: biological sciences
Mascaró M, Seed R (2001) Foraging behavior of juvenile Carcinus maenas (L.) and Cancer pagurus (L.). Mar Biol 139(6):1135–1145
Meire PM, Ervynck A (1986) Are oystercatchers (Haematopus ostralegus) selecting the most profitable mussels (Mytilus edulis)? Anim Behav 34(5):1427–1435
Murray LG, Seed R, Jones T (2007) Predicting the impacts of Carcinus maenas predation on cultivated Mytilus edulis beds. J Shellfish Res 26:1089–1098
Nehls G, Thiel M (1993) Large-scale distribution patterns of the mussel Mytilus edulis in the Wadden Sea of Schleswig-Holstein: Do storms structure the ecosystem? Neth J Sea Res 31:181–187
Pinheiro J, Bates D, DebRoy S, et al (2014) nlme: linear and nonlinear Mixed effects models. R package version 3.1-117. http://CRAN.R-project.org/package=nlme
Plass-Johnson JG, McQuaid CD, Porri F (2010) Top-down effects on intertidal mussel populations: assessing two predator guilds in a South African marine protected area. Mar Ecol Prog Ser 411:149–159
Reimer O, Tedengren M (1997) Predator induced changes in byssal attachment, aggregation and migration in the blue mussel, Mytulis edulis. Mar Freshw Behav Physiol 4:251–266
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Saier B (2001) Direct and indirect effects of seastars Asterias rubens on mussel beds (Mytilus edulis) in the Wadden Sea. J Sea Res 46:29–42
Silva ACF, Boaventura DM, Thompson RC et al (2014) Spatial and temporal patterns of subtidal and intertidal crabs excursions. J Sea Res 85:343–348
Smallegange IM, Van Der Meer J (2007) Interference from a game theoretical perspective: shore crabs suffer most from equal competitors. Behav Ecol 18:215–221
Sneddon LU, Huntingford FA, Taylor AC (1997) The influence of resource value on the agonistic behaviour of the shore crab, Carcinus maenas (L.). Mar Fresh Behav Psychol 30:225–237
Theisen BF (1968) Growth and mortality of culture mussels in the Danish Wadden Sea. Medd Danm Fiskeri og Havunders NS 6:47–78
Whitton TA, Jenkins SR, Richardson CA et al (2012) Aggregated prey and predation rates: juvenile shore crabs (Carcinus maenas) foraging on post-larval cockles (Cerastoderma edule). J Exp Mar Biol Ecol 432–433:29–36
Zuur A, Ieno EN, Walker N et al (2009) Mixed effects models and extensions in ecology with R. Springer, NewYork
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1(1):3–14
Acknowledgments
The authors wish to thank Robbie, Peter, Leon and Sjaak Praet from the company ‘N.L. Praet & zn’ for their support, the use of their lease site and for the mussel seed. Comments from Neil Bourne and an anonymous reviewer improved the quality of this paper. This work was part of the PRODUS project under contract of the Producers Organisation of the Dutch Mussel Culture and the Ministry of Economic Affairs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Capelle, J.J., Scheiberlich, G., Wijsman, J.W.M. et al. The role of shore crabs and mussel density in mussel losses at a commercial intertidal mussel plot after seeding. Aquacult Int 24, 1459–1472 (2016). https://doi.org/10.1007/s10499-016-0005-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-0005-1