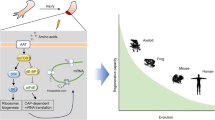
Abstract
AICP is a crucial process that maintaining tissue homeostasis and regeneration. In the past, cell death was perceived merely as a means to discard cells without functional consequences. However, during regeneration, effector caspases orchestrate apoptosis, releasing signals that activate stem cells, thereby compensating for tissue loss across various animal models. Despite significant progress, the activation of Wnt3a by caspase-3 remains a focal point of research gaps in AICP mechanisms, spanning from lower to higher regenerative animals. This inquiry into the molecular intricacies of caspase-3-induced Wnt3a activation contributes to a deeper understanding of the links between regeneration and cancer mechanisms. Our report provides current updates on AICP pathways, delineating research gaps and highlighting the potential for future investigations aimed at enhancing our comprehension of this intricate process.





Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Hall* PA, Coates PJ, Ansari B, Hopwood D (1994) Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci 107:3569–3577
Shibahara T, SATO N, WAGURI S et al (1995) The fate of Effete Epithelial cells at the Villas Tips of the human small intestine. Arch Histol Cytol 58:205–219
David L, Nelson DL, Cox MM et al (2000) Lehninger principles of biochemistry
Brill A, Torchinsky A, Carp H, Toder V (1999) The role of apoptosis in normal and abnormal embryonic development. J Assist Reprod Genet 16:512–519
Chera S, Ghila L, Dobretz K et al (2009) Apoptotic cells provide an unexpected source of Wnt3 signaling to drive hydra head regeneration. Dev Cell 17:279–289
Hwang JS, Kobayashi C, Agata K et al (2004) Detection of apoptosis during planarian regeneration by the expression of apoptosis-related genes and TUNEL assay. Gene 333:15–25
Tseng A-S, Levin M (2008) Tail regeneration in Xenopus laevis as a model for understanding tissue repair. J Dent Res 87:806–816
Sîrbulescu RF, Zupanc GKH (2010) Inhibition of caspase-3-mediated apoptosis improves spinal cord repair in a regeneration-competent vertebrate system. Neuroscience 171:599–612
Sandu C, Ryoo HD, Steller H (2010) Drosophila IAP antagonists form multimeric complexes to promote cell death. J Cell Biol 190:1039–1052
Li F, Huang Q, Chen J et al (2010) Apoptotic cells activate the phoenix rising pathway to promote wound healing and tissue regeneration. Sci Signal 3:ra13–ra13
Riwaldt S, Corydon TJ, Pantalone D et al (2021) Role of apoptosis in wound healing and apoptosis alterations in microgravity. Front Bioeng Biotechnol 9:679650
Bergmann A, Steller H (2010) Apoptosis, stem cells, and tissue regeneration. Sci Signal 3:re8–re8
Ding S, Schultz PG (2004) A role for chemistry in stem cell biology. Nat Biotechnol 22:833–840
Monaghan JR, Maden M (2012) Cellular plasticity during vertebrate appendage regeneration. New Perspect Regen 53–74
Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z (2019) Stem cells: past, present, and future. Stem Cell Res Ther 10:1–22
Smart N, Riley PR (2008) The stem cell movement. Circ Res 102:1155–1168
Abnave P, Ghigo E (2019) Role of the immune system in regeneration and its dynamic interplay with adult stem cells. In: Seminars in cell & developmental biology. Elsevier, pp 160–168
Huh JR, Guo M, Hay BA (2004) Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase dronc in a nonapoptotic role. Curr Biol 14:1262–1266
Ryoo HD, Gorenc T, Steller H (2004) Apoptotic cells can induce compensatory cell proliferation through the JNK and the wingless signaling pathways. Dev Cell 7:491–501
Pérez-Garijo A, Martín FA, Morata G (2004) Caspase inhibition during apoptosis causes. abnormal signalling and developmental aberrations in Drosophila
Pérez-Garijo A, Martín FA, Struhl G, Morata G (2005) Dpp signaling and the induction of neoplastic tumors by caspase-inhibited apoptotic cells in Drosophila. Proc Natl Acad Sci 102:17664–17669
Fogarty CE, Bergmann A (2017) Killers creating new life: caspases drive apoptosis-induced proliferation in tissue repair and disease. Cell Death Differ 24:1390–1400
Morgan TH (1901) Regeneration and liability to injury. Sci (80-) 14:235–248
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453:314–321
Bely AE (2010) Evolutionary loss of animal regeneration: pattern and process. Integr Comp Biol 50:515–527
Vibert L, Daulny A, Jarriault S (2018) Wound healing, cellular regeneration and plasticity: the Elegans way. Int J Dev Biol 62:491
Zattara EE, Fernández-Álvarez FA, Hiebert TC et al (2019) A phylum-wide survey reveals multiple independent gains of head regeneration in Nemertea. Proc R Soc B 286:20182524
Bae YS, Kim J, Yi J et al (2020) Characterization of perionyx excavatus development and its head regeneration. Biology (Basel) 9:273
Klemm J, Stinchfield MJ, Harris RE (2021) Necrosis-induced apoptosis promotes regeneration in Drosophila wing imaginal discs. bioRxiv
Vlaskalin T, Wong CJ, Tsilfidis C (2004) Growth and apoptosis during larval forelimb development and adult forelimb regeneration in the newt (Notophthalmus viridescens). Dev Genes Evol 214:423–431
Galliot B, Chera S (2010) The Hydra model: disclosing an apoptosis-driven generator of wnt-based regeneration. Trends Cell Biol 20:514–523
Bolkent Ş, ÖZtay F, OKTAYOĞLU S et al (2016) A matter of regeneration and repair: caspases as the key molecules. Turkish J Biol 40:333–352
Spead O, Verreet T, Donelson CJ, Poulain FE (2018) Characterization of the caspase family in zebrafish. PLoS ONE 13:e0197966
Salvesen GS, Abrams JM (2004) Caspase activation–stepping on the gas or releasing the brakes? Lessons from humans and flies. Oncogene 23:2774–2784
Denecker G, Ovaere P, Vandenabeele P, Declercq W (2008) Caspase-14 reveals its secrets. J Cell Biol 180:451–458
Yun M, Yi Y-S (2020) Regulatory roles of ginseng on inflammatory caspases, executioners of inflammasome activation. J Ginseng Res 44:373–385
Ryoo HD, Bergmann A (2012) The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harb Perspect Biol 4:a008797
Tseng A-S, Adams DS, Qiu D et al (2007) Apoptosis is required during early stages of tail regeneration in Xenopus laevis. Dev Biol 301:62–69
Creagh EM, Conroy H, Martin SJ (2003) Caspase-activation pathways in apoptosis and immunity. Immunol Rev 193:10–21
Schuler M, Green DR (2001) Mechanisms of p53-dependent apoptosis. Biochem Soc Trans 29:684–688
Shen Y, White E (2001) p53-dependent apoptosis pathways
Kondo S, Senoo-Matsuda N, Hiromi Y, Miura M (2006) DRONC coordinates cell death and compensatory proliferation. Mol Cell Biol 26:7258–7268
Wells BS, Yoshida E, Johnston LA (2006) Compensatory proliferation in Drosophila imaginal discs requires Dronc-dependent p53 activity. Curr Biol 16:1606–1615
Fan Y, Bergmann A (2008) Apoptosis-induced compensatory proliferation. The cell is dead. Long live the cell! Trends Cell Biol 18:467–473
Pérez-Garijo A, Shlevkov E, Morata G (2009) The role of Dpp and Wg in compensatory proliferation and in the formation of hyperplastic overgrowths caused by apoptotic cells in the Drosophila wing disc
Warner SJ, Yashiro H, Longmore GD (2010) The Cdc42/Par6/aPKC polarity complex regulates apoptosis-induced compensatory proliferation in epithelia. Curr Biol 20:677–686
Trembley A (1744) X. translation of a letter from Mr. Abraham Trembley, FRS to the President, with observations upon several newly discover’d species of fresh-water Polypi. Philos Trans R Soc Lond 43:169–183
Chera S, Ghila L, Wenger Y, Galliot B (2011) Injury-induced activation of the MAPK/CREB pathway triggers apoptosis‐induced compensatory proliferation in hydra head regeneration. Dev Growth Differ 53:186–201
Cazet JF, Cho A, Juliano CE (2021) Generic injuries are sufficient to induce ectopic wnt organizers in Hydra. Elife 10:e60562
Chera S, Ghila L, Wenger Y, Galliot B (2011) Injury-induced activation of the MAPK/CREB pathway triggers apoptosis-induced compensatory proliferation in hydra head regeneration. Dev Growth Differ 53:186–201. https://doi.org/10.1111/j.1440-169X.2011.01250.x
Lee S, Remark LH, Josephson AM et al (2021) Notch-wnt signal crosstalk regulates proliferation and differentiation of osteoprogenitor cells during intramembranous bone healing. NPJ Regen Med 6:1–10
Hamilton AM, Balashova OA, Borodinsky LN (2021) Non-canonical hedgehog signaling regulates spinal cord and muscle regeneration in Xenopus laevis larvae. Elife 10:e61804
Rämet M, Lanot R, Zachary D, Manfruelli P (2002) JNK signaling pathway is required for efficient wound healing in Drosophila. Dev Biol 241:145–156
Tursch A, Bartsch N, Holstein TW (2020) MAPK signaling links the injury response to Wnt-regulated patterning in Hydra regeneration. BioRxiv
Saló E (2006) The power of regeneration and the stem-cell kingdom: freshwater planarians (Platyhelminthes). BioEssays 28:546–559
Owlarn S, Klenner F, Schmidt D et al (2017) Generic wound signals initiate regeneration in missing-tissue contexts. Nat Commun 8:2282
Li D, Taylor DH, van Wolfswinkel JC (2021) PIWI-mediated control of tissue-specific transposons is essential for somatic cell differentiation. Cell Rep 37
Carmell MA, Girard A, Van De Kant HJG et al (2007) MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev Cell 12:503–514
Houwing S, Kamminga LM, Berezikov E et al (2007) A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in zebrafish. Cell 129:69–82
Pellettieri J, Fitzgerald P, Watanabe S et al (2010) Cell death and tissue remodeling in planarian regeneration. Dev Biol 338:76–85
Almuedo-Castillo M, Crespo X, Seebeck F et al (2014) JNK controls the onset of mitosis in planarian stem cells and triggers apoptotic cell death required for regeneration and remodeling. PLoS Genet 10:e1004400
Beane WS, Morokuma J, Lemire JM, Levin M (2013) Bioelectric signaling regulates head and organ size during planarian regeneration. Development 140:313–322
Cebrià F, Adell T, Saló E (2018) Rebuilding a planarian: from early signaling to final shape. Int J Dev Biol 62:537–550
Shiroor DA, Bohr TE, Adler CE (2020) Injury delays stem cell apoptosis after radiation in planarians. Curr Biol 30:2166–2174
Arnold CP, Merryman MS, Harris-Arnold A et al (2016) Pathogenic shifts in endogenous microbiota impede tissue regeneration via distinct activation of TAK1/MKK/p38. Elife 5:e16793
Wang Q, Sun X, Xiao J et al (2022) Djptpn11 is indispensable for planarian regeneration by affecting early wound response genes expression and the wnt pathway. Biochimie 201:184–195
Bodó K, Kellermayer Z, László Z et al (2021) Injury-induced innate immune response during segment regeneration of the earthworm, Eisenia andrei. Int J Mol Sci 22:2363
Selvan Christyraj JD, Azhagesan A, Ganesan M et al (2020) Understanding the role of the Clitellum in the regeneration events of the Earthworm Eudrilus eugeniae. Cells Tissues Organs 208:134–141
Rajagopalan K, Christyraj JDS, Chelladurai KS et al (2022) Comparative analysis of the survival and regeneration potential of juvenile and matured earthworm, Eudrilus eugeniae, upon in vivo and in vitro maintenance. Vitr Cell Dev Biol 58:587–598
Hock FJ (2016) Drug discovery and evaluation: pharmacological assays. Springer
Anand SK, Sahu MR, Mondal AC (2021) Induction of oxidative stress and apoptosis in the injured brain: potential relevance to brain regeneration in zebrafish. Mol Biol Rep 48:5099–5108
Roy S, Bayly CI, Gareau Y et al (2001) Maintenance of caspase-3 proenzyme dormancy by an intrinsic safety catch regulatory tripeptide. Proc Natl Acad Sci 98:6132–6137
Sakata S, Yan Y, Satou Y et al (2007) Conserved function of caspase-8 in apoptosis during bony fish evolution. Gene 396:134–148
Gauron C, Rampon C, Bouzaffour M et al (2013) Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Sci Rep 3:1–9
Higuchi M, Honda T, Proske RJ, Yeh ETH (1998) Regulation of reactive oxygen species-induced apoptosis and necrosis by caspase 3-like proteases. Oncogene 17:2753–2760
Kulkarni AA, Conteh AM, Sorrell CA et al (2018) An In Vivo zebrafish model for interrogating ros-mediated pancreatic β-cell injury, response, and prevention. Oxid Med Cell Longev 2018
Goessling W, North TE, Loewer S et al (2009) Genetic interaction of PGE2 and wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136:1136–1147
Pipalia TG, Koth J, Roy SD et al (2016) Cellular dynamics of regeneration reveals role of two distinct Pax7 stem cell populations in larval zebrafish muscle repair. Dis Model Mech 9:671–684
Deng J, Yu L, Liu C et al (2009) Hexabromocyclododecane-induced developmental toxicity and apoptosis in zebrafish embryos. Aquat Toxicol 93:29–36
Issac PK, Lite C, Guru A et al (2021) Tryptophan-tagged peptide from serine threonine-protein kinase of Channa Striatus improves antioxidant defence in L6 myotubes and attenuates caspase 3–dependent apoptotic response in zebrafish larvae. Fish Physiol Biochem 47:293–311
Brock CK, Wallin ST, Ruiz OE et al (2019) Stem cell proliferation is induced by apoptotic bodies from dying cells during epithelial tissue maintenance. Nat Commun 10:1–11
Cindy XK, Son PH, Lauper J, Tseng KA-S (2018) A model for investigating developmental eye repair in Xenopus laevis. Exp Eye Res 169:38–47
Hamilton AM, Borodinsky LN (2020) Non-canonical Hedgehog signaling regulates spinal cord and muscle regeneration. bioRxiv
Yokoyama H, Ogino H, Stoick-Cooper CL et al (2007) Wnt/β-catenin signaling has an essential role in the initiation of limb regeneration. Dev Biol 306:170–178
Moon RT (1993) In pursuit of the functions of the wnt family of developmental regulators: insights from Xenopus laevis. BioEssays 15:91–97
Sugiura T, Tazaki A, Ueno N et al (2009) Xenopus Wnt-5a induces an ectopic larval tail at injured site, suggesting a crucial role for noncanonical wnt signal in tail regeneration. Mech Dev 126:56–67
Yin A, Winata CL, Korzh S et al (2010) Expression of components of wnt and hedgehog pathways in different tissue layers during lung development in Xenopus laevis. Gene Expr Patterns 10:338–344
Patel JH, Schattinger PA, Takayoshi EE, Wills AE (2022) Hif1α and wnt are required for posterior gene expression during Xenopus tropicalis tail regeneration. Dev Biol 483:157–168
Ankawa R, Goldberger N, Yosefzon Y et al (2021) Apoptotic cells represent a dynamic stem cell niche governing proliferation and tissue regeneration. Dev Cell 56:1900–1916
Brock AR, Seto M, Smith-Bolton RK (2017) Cap-n-collar promotes tissue regeneration by regulating ROS and JNK signaling in the Drosophila melanogaster wing imaginal disc. Genetics 206:1505–1520
Ahmed-de-Prado S, Diaz-Garcia S, Baonza A (2018) JNK and JAK/STAT signalling are required for inducing loss of cell fate specification during imaginal wing discs regeneration in Drosophila melanogaster. Dev Biol 441:31–41
Meserve JH, Duronio RJ (2018) Fate mapping during regeneration: cells that undergo compensatory proliferation in damaged Drosophila eye imaginal discs differentiate into multiple retinal accessory cell types. Dev Biol 444:43–49
Tian Y, Smith-Bolton RK (2021) Regulation of growth and cell fate during tissue regeneration by the two SWI/SNF chromatin-remodeling complexes of Drosophila. Genetics 217:iyaa028
Xu D, Wang Y, Willecke R et al (2006) The effector caspases drICE and dcp-1 have partially overlapping functions in the apoptotic pathway in Drosophila. Cell Death Differ 13:1697–1706
Meier P, Silke J, Leevers SJ, Evan GI (2000) The Drosophila caspase DRONC is regulated by DIAP1. EMBO J 19:598–611
Brodsky MH, Nordstrom W, Tsang G et al (2000) Drosophila p53 binds a damage response element at the reaper locus. Cell 101:103–113
Bergmann A (2010) The role of ubiquitylation for the control of cell death in Drosophila. Cell Death Differ 17:61–67
Fan Y, Wang S, Hernandez J et al (2014) Genetic models of apoptosis-induced proliferation decipher activation of JNK and identify a requirement of EGFR signaling for tissue regenerative responses in Drosophila. PLoS Genet 10:e1004131
Herman PE, Papatheodorou A, Bryant SA et al (2018) Highly conserved molecular pathways, including wnt signaling, promote functional recovery from spinal cord injury in lampreys. Sci Rep 8:1–15
Chen S-H, Lu C-H, Tsai M-J (2020) TCTP is essential for cell proliferation and survival during CNS development. Cells 9:133
Jin H, Zhang X, Su J et al (2015) RNA interference–mediated knockdown of translationally controlled tumor protein induces apoptosis, and inhibits growth and invasion in glioma cells. Mol Med Rep 12:6617–6625
Jung J, Kim HY, Maeng J et al (2014) Interaction of translationally controlled tumor protein with Apaf-1 is involved in the development of chemoresistance in HeLa cells. BMC Cancer 14:1–13
Rho SB, Lee JH, Park MS et al (2011) Anti-apoptotic protein TCTP controls the stability of the tumor suppressor p53. FEBS Lett 585:29–35
Haupt S, Berger M, Goldberg Z, Haupt Y (2003) Apoptosis-the p53 network. J Cell Sci 116:4077–4085
Schuler M, Bossy-Wetzel E, Goldstein JC et al (2000) p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J Biol Chem 275:7337–7342
Shiloh Y (2006) The ATM-mediated DNA-damage response: taking shape. Trends Biochem Sci 31:402–410
Zannini L, Delia D, Buscemi G (2014) CHK2 kinase in the DNA damage response and beyond. J Mol Cell Biol 6:442–457
Cheng Q, Chen J (2010) Mechanism of p53 stabilization by ATM after DNA damage. Cell Cycle 9:472–478
Yu J, Zhang L (2008) PUMA, a potent killer with or without p53. Oncogene 27:S71–S83
Shibue T, Takeda K, Oda E et al (2003) Integral role of Noxa in p53-mediated apoptotic response. Genes Dev 17:2233–2238
Amaral JD, Xavier JM, Steer CJ, Rodrigues CM (2010) The role of p53 in apoptosis. Discov Med 9:145–152
Sax JK, Fei P, Murphy ME et al (2002) BID regulation by p53 contributes to chemosensitivity. Nat Cell Biol 4:842–849
Liu C, Vojnovic D, Kochevar IE, Jurkunas UV (2016) UV-A irradiation activates Nrf2-regulated antioxidant defense and induces p53/caspase3-dependent apoptosis in corneal endothelial cells. Invest Ophthalmol Vis Sci 57:2319–2327
Jänicke RU, Sohn D, Schulze-Osthoff K (2008) The dark side of a tumor suppressor: anti-apoptotic p53. Cell Death Differ 15:959–976
Charni M, Aloni-Grinstein R, Molchadsky A, Rotter V (2017) p53 on the crossroad between regeneration and cancer. Cell Death Differ 24:8–14
Dichtel-Danjoy M-L, Ma D, Dourlen P et al (2013) Drosophila p53 isoforms differentially regulate apoptosis and apoptosis-induced proliferation. Cell Death Differ 20:108–116
Clem RJ, Fechheimer M, Miller LK (1991) Prevention of apoptosis by a baculovirus gene during infection of insect cells. Sci (80-) 254:1388–1390
Lee S-R, Hong S-T, Choi K-W (2020) Regulation of epithelial integrity and organ growth by Tctp and Coracle in Drosophila. PLoS Genet 16:e1008885
Koziol МJ, Gurdon JB (2013) TCTP in Development and Cancer. Науковий журнал МОЗ України 152–163
Nagano-Ito M, Ichikawa S (2012) Biological effects of mammalian translationally controlled tumor protein (TCTP) on cell death, proliferation, and tumorigenesis. Biochem Res Int 2012
Telerman A, Amson R (2009) The molecular programme of tumour reversion: the steps beyond malignant transformation. Nat Rev Cancer 9:206–216
Amson R, Pece S, Lespagnol A et al (2012) Reciprocal repression between P53 and TCTP. Nat Med 18:91–99
Subramanian ER, Gopi Daisy N, Sudalaimani DK et al (2017) Function of translationally controlled tumor protein (TCTP) in Eudrilus eugeniae regeneration. PLoS ONE 12:e0175319
Giri J, Basu M, Roy S et al (2022) Translationally controlled tumor protein–mediated stabilization of host antiapoptotic protein MCL-1 is critical for establishment of infection by Intramacrophage Parasite Leishmania donovani. J Immunol 208:2540–2548
Zhang Q, Cheng Z, Shi L, Mao G (2022) Mir-145-5p inhibits the proliferation of glioma stem cells by targeting translationally controlled tumor protein. J Cancer 13:1490
Sirois I, Raymond MA, Brassard N et al (2011) Caspase-3-dependent export of TCTP: a novel pathway for antiapoptotic intercellular communication. Cell Death Differ 18:549–562
Rajagopalan K, Christyraj JDS, Chelladurai KS et al (2023) Understanding the multi-functional role of TCTP in the regeneration process of Earthworm, Perionyx excavatus. Tissue Eng Regen Med 1–14
Joruiz SM, Bourdon J-C (2016) p53 isoforms: key regulators of the cell fate decision. Cold Spring Harb Perspect Med 6:a026039
Mollereau B, Ma D (2014) The p53 control of apoptosis and proliferation: lessons from Drosophila. Apoptosis 19:1421–1429
Yosefzon Y, Soteriou D, Feldman A et al (2018) Caspase-3 regulates YAP-dependent cell proliferation and organ size. Mol Cell 70:573–587
Missirlis F (2021) Regulation and biological function of metal ions in Drosophila. Curr Opin Insect Sci 47:18–24
Eron SJ, MacPherson DJ, Dagbay KB, Hardy JA (2018) Multiple mechanisms of zinc-mediated inhibition for the apoptotic caspases-3,-6,-7, and-8. ACS Chem Biol 13:1279–1290
Ramchandani D, Berisa M, Tavarez DA et al (2021) Copper depletion modulates mitochondrial oxidative phosphorylation to impair triple negative breast cancer metastasis. Nat Commun 12:7311
Kalishwaralal K, Abhishek A, Keerthana CK et al (2023) Selenium metabolic pathway in ferroptotic cell death. Ferroptosis in Health and Disease. Springer, pp 369–382
Lee Y-S, Kalimuthu K, Park YS et al (2020) BAX-dependent mitochondrial pathway mediates the crosstalk between ferroptosis and apoptosis. Apoptosis 25:625–631
Das A, Ash D, Fouda AY et al (2022) Cysteine oxidation of copper transporter CTR1 drives VEGFR2 signalling and angiogenesis. Nat Cell Biol 24:35–50
Prabhu KS (2023) The selenoprotein P–LRP5/6–WNT3A complex promotes tumorigenesis in sporadic colorectal cancer. J Clin Invest 133
Mao P, Smith L, Xie W, Wang M (2013) Dying endothelial cells stimulate proliferation of malignant glioma cells via a caspase 3–mediated pathway. Oncol Lett 5:1615–1620
Acknowledgements
Authors thank ‘International Research Centre (IRC) of Sathyabama Institute of Science and Technology, Chennai’ for providing support to carry out the research work. Also, we express our gratitude to Dr. Ramakrishnan Muthusamy, Bamboo Research Institute, Nanjing Forestry University, Nanjing, China for helping us to create the figures using biorender (www.biorender.com).
Funding
This work was supported by the DST-SHRI-INDIA (Ref. No. DST/TDT/SHRI- 24/2021 (G)).
Author information
Authors and Affiliations
Contributions
J.D.S.C: conceptualization, investigation, editing and wrote the manuscript. K.R: conceptualization, editing and wrote the manuscript. K.S.C: critical reviewing and visualization. K.K: conceptualization, editing, critical reviewing and wrote the manuscript. P.D: visualization.M.D: formal analysis. N.B: formal analysis. K.M: formal analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajagopalan, K., Selvan Christyraj, J., Chelladurai, K.S. et al. Understanding the molecular mechanism of regeneration through apoptosis-induced compensatory proliferation studies - updates and future aspects. Apoptosis (2024). https://doi.org/10.1007/s10495-024-01958-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s10495-024-01958-1