Abstract
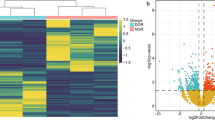
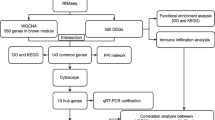
Cumulus granulosa cells (CGCs) play a crucial role in follicular development, but so far, no research has explored the impact of SARS-CoV-2 infection on ovarian function from the perspective of CGCs. In the present study, we compared the cycle outcomes between infected and uninfected female patients undergoing controlled ovarian stimulation, performed bulk RNA-sequencing of collected CGCs, and used bioinformatic methods to explore transcriptomic changes. The results showed that women with SARS-CoV-2 infection during stimulation had significantly lower number of oocytes retrieved and follicle-oocyte index, while subsequent fertilization and embryo development were similar. CGCs were not directly infected by SARS-CoV-2, but exhibited dramatic differences in gene expression (156 up-regulated and 65 down-regulated). Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analyses demonstrated a high enrichment in antiviral, immune and inflammatory responses with necroptosis. In addition, the pathways related to telomere organization and double strand break repair were significantly affected by infection in gene set enrichment analysis. Further weighted gene co-expression network analysis identified a key module associated with ovarian response traits, which was mainly enriched as a decrease of leukocyte chemotaxis and migration in CGCs. For the first time, our study describes how SARS-CoV-2 infection indirectly affects CGCs at the transcriptional level, which may impair oocyte-CGC crosstalk and consequently lead to poor ovarian response during fertility treatment.







Similar content being viewed by others
Data availability
The dataset analyzed during the current study has been submitted to the NCBI BioProject with accession number (PRJNA1003555).
References
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506
Singh M, Bansal V, Feschotte CA, Single-Cell RNA (2020) Expression map of human coronavirus entry factors. Cell Rep 32:108175
Albini A, Di Guardo G, Noonan DM, Lombardo M (2020) The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies. Intern Emerg Med 15:759–766
Jackson CB, Farzan M, Chen B, Choe H (2022) Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 23:3–20
Harrison AG, Lin T, Wang P (2020) Mechanisms of SARS-CoV-2 transmission and Pathogenesis. Trends Immunol 41:1100–1115
Nie X, Qian L, Sun R, Huang B, Dong X, Xiao Q et al Multi-organ proteomic landscape of COVID-19 autopsies. Cell 2021;184:775 – 91.e14.
Stein SR, Ramelli SC, Grazioli A, Chung JY, Singh M, Yinda CK et al (2022) SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 612:758–763
Rabaan AA, Smajlović S, Tombuloglu H, Ćordić S, Hajdarević A, Kudić N et al (2023) SARS-CoV-2 infection and multi-organ system damage: a review. Biomol Biomed 23:37–52
Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M, Lindskog C (2020) The protein expression profile of ACE2 in human tissues. Mol Syst Biol 16:e9610
Luongo FP, Dragoni F, Boccuto A, Paccagnini E, Gentile M, Canosi T et al (2022) SARS-CoV-2 infection of human ovarian cells: a potential negative impact on female fertility. Cells 11:1431
Essahib W, Verheyen G, Tournaye H, Van de Velde H (2020) SARS-CoV-2 host receptors ACE2 and CD147 (BSG) are present on human oocytes and blastocysts. J Assist Reprod Genet 37:2657–2660
Domińska K (2020) Involvement of ACE2/Ang-(1–7)/MAS1 Axis in the regulation of ovarian function in mammals. Int J Mol Sci ;21
Carp-Veliscu A, Mehedintu C, Frincu F, Bratila E, Rasu S, Iordache I et al (2022) The effects of SARS-CoV-2 infection on female fertility: a review of the literature. Int J Environ Res Public Health ;19
Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB (2015) Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril 103:303–316
Eppig JJ (2001) Oocyte control of ovarian follicular development and function in mammals. Reproduction 122:829–838
Gilchrist RB, Ritter LJ, Armstrong DT (2004) Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci ;82–83:431–46
Coticchio G, Dal Canto M, Mignini Renzini M, Guglielmo MC, Brambillasca F, Turchi D et al (2015) Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update 21:427–454
Barragan M, Guillén JJ, Martin-Palomino N, Rodriguez A, Vassena R (2021) Undetectable viral RNA in oocytes from SARS-CoV-2 positive women. Hum Reprod 36:390–394
Boudry L, Essahib W, Mateizel I, Van de Velde H, De Geyter D, Piérard D et al (2022) Undetectable viral RNA in follicular fluid, cumulus cells, and endometrial tissue samples in SARS-CoV-2-positive women. Fertil Steril 117:771–780
Kteily K, Pening D, Diaz Vidal P, Devos M, Dechene J, Op de Beeck A et al (2022) Risk of contamination of semen, vaginal secretions, follicular fluid and ovarian medulla with SARS-CoV-2 in patients undergoing ART. Hum Reprod 37:235–241
Recommendations on the management (2023) Of assisted reproductive technology under the Class B infectious disease policy for COVID-19 (first edition) [In Chinese]. Chin J Reprod Contracep 43:109–111
Alviggi C, Conforti A, Esteves SC, Vallone R, Venturella R, Staiano S et al (2018) Understanding ovarian hypo-response to Exogenous Gonadotropin in Ovarian Stimulation and its new proposed marker-the Follicle-To-Oocyte (FOI) index. Front Endocrinol (Lausanne) 9:589
Tian LF, Tan J, Zou Y, Su Q, Li Y, Xu DF et al (2019) Mild starting dosage ovarian stimulation combined with a modified prolonged GnRH-a protocol improved IVF/ICSI outcomes in normal ovarian responders. Arch Med Sci 15:1294–1300
Huang J, Xia L, Lin J, Liu B, Zhao Y, Xin C et al (2022) No effect of inactivated SARS-CoV-2 vaccination on in vitro fertilization outcomes: a propensity score-matched study. J Inflamm Res 15:839–849
Love MI, Huber W, Anders S (2014) Moderated estimation of Fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550
Gustavsson EK, Zhang D, Reynolds RH, Garcia-Ruiz S, Ryten M (2022) Ggtranscript: an R package for the visualization and interpretation of transcript isoforms using ggplot2. Bioinformatics 38:3844–3846
Li Z, Chen J, Zhao S, Li Y, Zhou J, Liang J et al (2021) Discovery and validation of novel biomarkers for detection of cervical cancer. Cancer Med 10:2063–2074
The Gene Ontology Resource (2019) 20 years and still GOing strong. Nucleic Acids Res 47:D330–d8
Kanehisa M, Goto S (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102:15545–15550
Yu G, Wang LG, Han Y, He QY (2012) clusterProfiler: an R package for comparing biological themes among gene clusters. Omics 16:284–287
Langfelder P, Horvath S (2008) WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9:559
von Mering C, Huynen M, Jaeggi D, Schmidt S, Bork P, Snel B (2003) STRING: a database of predicted functional associations between proteins. Nucleic Acids Res 31:258–261
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D et al (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504
Bentov Y, Beharier O, Moav-Zafrir A, Kabessa M, Godin M, Greenfield CS et al (2021) Ovarian follicular function is not altered by SARS-CoV-2 infection or BNT162b2 mRNA COVID-19 vaccination. Hum Reprod 36:2506–2513
Odeh-Natour R, Shapira M, Estrada D, Freimann S, Tal Y, Atzmon Y et al (2022) Does mRNA SARS-CoV-2 vaccine in the follicular fluid impact follicle and oocyte performance in IVF treatments? Am J Reprod Immunol 87:e13530
Youngster M, Avraham S, Yaakov O, Landau Rabbi M, Gat I, Yerushalmi G et al (2022) IVF under COVID-19: treatment outcomes of fresh ART cycles. Hum Reprod 37:947–953
Kabalkin Y, Bentov Y, Gil M, Beharier O, Jaber S, Moav-Zafrir A et al (2022) Mild COVID-19 was not Associated with impaired IVF outcomes or early pregnancy loss in IVF patients. J Clin Med ;11
Ata B, Vermeulen N, Mocanu E, Gianaroli L, Lundin K, Rautakallio-Hokkanen S et al (2022) SARS-CoV-2, fertility and assisted reproduction. Hum Reprod Update
Herrero Y, Pascuali N, Velázquez C, Oubiña G, Hauk V, de Zúñiga I et al (2022) SARS-CoV-2 infection negatively affects ovarian function in ART patients. Biochim Biophys Acta Mol Basis Dis 1868:166295
Chico-Sordo L, Polonio AM, Córdova-Oriz I, Medrano M, Herraiz S, Bronet F et al (2022) Telomeres and oocyte maturation rate are not reduced by COVID-19 except in severe cases. Reproduction 164:259–267
Wang M, Yang Q, Ren X, Hu J, Li Z, Long R et al (2021) Investigating the impact of asymptomatic or mild SARS-CoV-2 infection on female fertility and in vitro fertilization outcomes: a retrospective cohort study. EClinicalMedicine 38:101013
Orvieto R, Segev-Zahav A, Aizer A (2021) Does COVID-19 infection influence patients’ performance during IVF-ET cycle? An observational study. Gynecol Endocrinol 37:895–897
Chen X, Shi H, Li C, Zhong W, Cui L, Zhang W et al (2023) The effect of SARS-CoV-2 infection on human embryo early development: a multicenter prospective cohort study. Sci China Life Sci 66:1697–1700
Tian F, Li S, Li N, Zhao H, Luo M, Zhang J et al (2023) Association of SARS-CoV-2 infection during controlled ovarian stimulation with oocyte- and embryo-related outcomes. JAMA Netw Open 6:e2323219
Sarapik A, Velthut A, Haller-Kikkatalo K, Faure GC, Béné MC, de Carvalho Bittencourt M et al (2012) Follicular proinflammatory cytokines and chemokines as markers of IVF success. Clin Dev Immunol 2012:606459
Gao H, Lin L, Haq IU, Zeng SM (2016) Inhibition of NF-κB promotes autophagy via JNK signaling pathway in porcine granulosa cells. Biochem Biophys Res Commun 473:311–316
Liu Y, Liu H, Li Z, Fan H, Yan X, Liu X et al (2021) The release of Peripheral Immune Inflammatory cytokines promote an inflammatory Cascade in PCOS patients via altering the Follicular Microenvironment. Front Immunol 12:685724
Fortin CS, Leader A, Mahutte N, Hamilton S, Léveillé MC, Villeneuve M et al (2019) Gene expression analysis of follicular cells revealed inflammation as a potential IVF failure cause. J Assist Reprod Genet 36:1195–1210
Bloom MS, Mok-Lin E, Fujimoto VY (2016) Bisphenol A and ovarian steroidogenesis. Fertil Steril 106:857–863
Galluzzi L, Kepp O, Chan FK, Kroemer G (2017) Necroptosis: mechanisms and relevance to Disease. Annu Rev Pathol 12:103–130
Du Y, Bagnjuk K, Lawson MS, Xu J, Mayerhofer A (2018) Acetylcholine and necroptosis are players in follicular development in primates. Sci Rep 8:6166
Blohberger J, Kunz L, Einwang D, Berg U, Berg D, Ojeda SR et al (2015) Readthrough acetylcholinesterase (AChE-R) and regulated necrosis: pharmacological targets for the regulation of ovarian functions? Cell Death Dis 6:e1685
Kang Y, Wang Q (2022) Potential therapeutic value of necroptosis inhibitor for the treatment of COVID-19. Eur J Med Res 27:283
Wang L, Xu X, Teng M, Zhao G, Lei A (2020) Coping with DNA double-strand breaks via ATM Signaling pathway in bovine oocytes. Int J Mol Sci ;21
Lopez SG, Luderer U (2004) Effects of cyclophosphamide and buthionine sulfoximine on ovarian glutathione and apoptosis. Free Radic Biol Med 36:1366–1377
Olsen MB, Huse C, de Sousa MML, Murphy SL, Sarno A, Obermann TS et al (2022) DNA repair mechanisms are activated in circulating lymphocytes of hospitalized Covid-19 patients. J Inflamm Res 15:6629–6644
Cao X, Li W, Wang T, Ran D, Davalos V, Planas-Serra L et al (2022) Accelerated biological aging in COVID-19 patients. Nat Commun 13:2135
Mongelli A, Barbi V, Gottardi Zamperla M, Atlante S, Forleo L, Nesta M et al (2021) Evidence for Biological Age Acceleration and Telomere Shortening in COVID-19 survivors. Int J Mol Sci ;22
Fattet AJ, Toupance S, Thornton SN, Monnin N, Guéant JL, Benetos A et al (2020) Telomere length in granulosa cells and leukocytes: a potential marker of female fertility? A systematic review of the literature. J Ovarian Res 13:96
Rincon-Arevalo H, Aue A, Ritter J, Szelinski F, Khadzhynov D, Zickler D et al (2022) Altered increase in STAT1 expression and phosphorylation in severe COVID-19. Eur J Immunol 52:138–148
Winkler ES, Bailey AL, Kafai NM, Nair S, McCune BT, Yu J et al (2020) SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol 21:1327–1335
Duffy DM, Ko C, Jo M, Brannstrom M, Curry TE (2019) Ovulation: parallels with inflammatory processes. Endocr Rev 40:369–416
Abdulrahman Alrabiah N, Evans ACO, Fahey AG, Cantwell N, Lonergan P, McCormack J et al (2021) Immunological aspects of ovarian follicle ovulation and corpus luteum formation in cattle. Reproduction 162:209–225
Kuwabara Y, Katayama A, Tomiyama R, Piao H, Kurihara S, Ono S et al (2015) Gonadotropin regulation and role of ovarian osteopontin in the periovulatory period. J Endocrinol 224:49–59
Lochab AK, Extavour CG (2017) Bone morphogenetic protein (BMP) signaling in animal reproductive system development and function. Dev Biol 427:258–269
Ogura-Nose S, Yoshino O, Osuga Y, Shi J, Hiroi H, Yano T et al (2012) Anti-mullerian hormone (AMH) is induced by bone morphogenetic protein (BMP) cytokines in human granulosa cells. Eur J Obstet Gynecol Reprod Biol 164:44–47
Sugiura K, Su YQ, Eppig JJ (2010) Does bone morphogenetic protein 6 (BMP6) affect female fertility in the mouse? Biol Reprod 83:997–1004
Shao W, Guo T, Toussaint NC, Xue P, Wagner U, Li L et al (2019) Comparative analysis of mRNA and protein degradation in prostate tissues indicates high stability of proteins. Nat Commun 10:2524
Shirvani H, Jafari H, Moravveji SS, Abbasi Faranghizadeh F, Talebi M, Ghanavi J et al (2022) Non-coding RNA in SARS-CoV-2: progress toward therapeutic significance. Int J Biol Macromol 222:1538–1550
Li CX, Chen J, Lv SK, Li JH, Li LL, Hu X, Whole-Transcriptome RNA (2021) Sequencing reveals significant differentially expressed mRNAs, miRNAs, and lncRNAs and related regulating Biological pathways in the Peripheral blood of COVID-19 patients. Mediators Inflamm 2021:6635925
Ahmed S, Paramasivam P, Kamath M, Sharma A, Rome S, Murugesan R (2021) Genetic Exchange of Lung-Derived Exosome to Brain causing neuronal changes on COVID-19 infection. Mol Neurobiol 58:5356–5368
Rudiansyah M, Terefe EM, Opulencia MJC, Abdelbasset WK, Bokov DO, El-Sehrawy AA et al (2022) Type 2 alveolar epithelial cell-derived circulating extracellular vesicle-encapsulated surfactant protein C as a mediator of cardiac inflammation in COVID-19. Inflamm Res 71:1003–1009
Russell DL, Gilchrist RB, Brown HM, Thompson JG (2016) Bidirectional communication between cumulus cells and the oocyte: old hands and new players? Theriogenology 86:62–68
Pournaghi M, Khodavirdilou R, Saadatlou M, Nasimi F, Yousefi S, Mobarak H et al (2021) Effect of melatonin on exosomal dynamics in bovine cumulus cells. Process Biochem 106:78–87
Acknowledgements
We are grateful to the LC-Bio Technology for assisting in sequencing and bioinformatic analysis.
Funding
This work was supported by the National Natural Science Foundation of China (82260315) and Natural Science Foundation of Jiangxi Province (20224BAB216025).
Author information
Authors and Affiliations
Contributions
J.H., L.T. and H.K. conceptualized and designed the study. X.W., J.C., and P.L. collected patient sample. L.X., D.X., K.Z. and Q.X. were responsible for clinical follow-up and data curation. X.W. and Z.F. performed the study experiments. Y.L., J.W. and Y.S. performed the statistical analyses. J.H. conducted computational bioinformatics analysis and wrote the original draft. J.T., H.K. and L.T. supervised the project administration and edited the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Reproductive Medicine Ethics Committee of Jiangxi Maternal and Child Health Hospital. All patients provided written informed contents for participation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, J., Fang, Z., Wu, X. et al. Transcriptomic responses of cumulus granulosa cells to SARS-CoV-2 infection during controlled ovarian stimulation. Apoptosis 29, 649–662 (2024). https://doi.org/10.1007/s10495-024-01942-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-024-01942-9