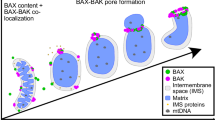
Abstract
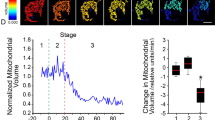
Recent advancements in live cell imaging technologies have identified the phenomenon of intracellular propagation of late apoptotic events, such as cytochrome c release and caspase activation. The mechanism, prevalence, and speed of apoptosis propagation remain unclear. Additionally, no studies have demonstrated propagation of the pro-apoptotic protein, BAX. To evaluate the role of BAX in intracellular apoptotic propagation, we used high speed live-cell imaging to visualize fluorescently tagged-BAX recruitment to mitochondria in four immortalized cell lines. We show that propagation of mitochondrial BAX recruitment occurs in parallel to cytochrome c and SMAC/Diablo release and is affected by cellular morphology, such that cells with processes are more likely to exhibit propagation. The initiation of propagation events is most prevalent in the distal tips of processes, while the rate of propagation is influenced by the 2-dimensional width of the process. Propagation was rarely observed in the cell soma, which exhibited near synchronous recruitment of BAX. Propagation velocity is not affected by mitochondrial volume in segments of processes, but is negatively affected by mitochondrial density. There was no evidence of a propagating wave of increased levels of intracellular calcium ions. Alternatively, we did observe a uniform increase in superoxide build-up in cellular mitochondria, which was released as a propagating wave simultaneously with the propagating recruitment of BAX to the mitochondrial outer membrane.







Similar content being viewed by others
Data availability
The datasets generated and analyzed for the current study are available from the corresponding author on reasonable request.
Code Availability
Not Applicable.
References
Cowan CM, Thai J, Krajewski S, Reed JC, Nicholson DW, Kaufmann SH, Roskams AJ (2001) Caspases 3 and 9 send a pro-apoptotic signal from synapse to cell body in olfactory neurons. J Neurosci 21:7099–7109
Pacher P, Hajnóczky G (2001) Propagation of the mitochondrial signal by mitochondrial waves. EMBO J 20:4107–4121
Lartigue L, Medina C, Schembri L et al (2008) An intracellular wave of cytochrome c propagates and precedes Bax redistribution during apoptosis. J Cell Sci 121:3515–3523
Bhola PD, Mattheyses AL, Simon SM (2009) Spatial and temporal dynamics of mitochondrial membrane permeability waves during apoptosis. Biophys J 97:2222–2231
Rehm M, Huber HJ, Hellwig CT, Anguissola S, Dussmann H, Prehn JHM (2009) Dynamics of outer mitochondrial membrane permeabilization during apoptosis. Cell Death Differ 16:613–623
Garcia-Perez C, Roy SS, Naghdi S, Lin X, Davies E, Hajnóczky G (2012) Bid-induced mitochondrial membrane permeabilization waves propagated by local reative oxygen species (ROS) signaling. Proc Natl Acad Sci USA 109:4497–4502
Longtine MS, Barton A, Chen B, Nelson DM (2012) Live-cell imaging shows apoptosis initiates locally and propagates as a wave throughout syncytiotrophoblasts in pimary cultures of human placental villous trophoblasts. Placenta 33:971–976
Cheng X, Ferrell JE Jr (2018) Apoptosis propagates through the cytoplasm as trigger waves. Science 361:607–612
Prise KM, Folkard M, Michael BD (2003) A review of the bystander effect and its implications for low-dose exposure. Rad Protect Dos 104:347–355
Maes ME, Schlamp CL, Nickells RW (2017) Live-cell imaging to measure BAX recruitment kinetics to mitochondria during apoptosis. PLoS ONE 12:e0184434
Nath B, Raza A, Sethi V, Dalal A, Ghosh SS, Biswas G (2018) Understanding flow dynamics, viability and metastatic potencu of cervical cancer (HeLa) cells through constricted microchannel. Sci Reports 8:17357
Li H, Zhu H, Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501; Slee EA, Keogh SA, Martin SJ (2000) Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ 7:556–565
Maes ME, Grosser JA, Fehrman RL, Schlamp CL, Nickells RW (2019) Completion of BAX recruitment correlates with mitochondrial fission during apoptosis. Sci Reports 9:16565
Semaan SJ, Nickells RW (2010) The apoptotic response in HCT116BAX-/- cancer cells becomes rapidly saturated with increasing expression of a GFP-BAX fusion protein. BMC Cancer 10:554
Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR (2000) The coordinate release of cytochrome c during apoptosis is rapid, complete, and kinetically invariant. Nat Cell Biol 2:156–162
Yang Y, Liu N, He Y et al (2018) Improved calcium sensor GCaMP-X overcomes the caclium channel perturbations induced by the calmodulin in GCaMP. Nat Commun 9:1504
Thompson AF, Crowe ME, Lieven CJ, Levin LA (2015) Induction of neuronal morphology in the 661W cone photoreceptor cell line with staurosporine. PLoS ONE 10:e0145270
Adams JM, Cory S (2007) The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26:1324–1337
Csordás G, Thomas AP, Hajnóczky G (1999) Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J 18:96–108
Grimm S (2012) The ER-mitochondria interface: the social network of cell death. Biochim. Biophys. Acta 1823: 327–334; Giorgi C, Missiroli S, Patergnani S, Duszynski J, Wieckowski MR, Pinton P (2015) Mitochondria-associated membranes: composition, molecular mechanisms, and physiopathological implications. Antioxid Redox Signal 22:995–1019
Acknowledgements
This work was supported by National Institute of Health grants R01 EY030123, P30 EY016665, and T32 GM081061, an unrestricted research grant from Research to Prevent Blindness, Inc., and the Frederick A. Davis Endowment from the Department of Ophthalmology and Visual Sciences at the University of Wisconsin-Madison.
Author information
Authors and Affiliations
Contributions
J.G. and M.M. collected data. J.G. analyzed data. J.G. and R.N. designed experiments, interpreted results, and wrote the manuscript. All authors approved of the final draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in the execution of this work. No patients or animals were used in these studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Live-cell imaging of the cell in Figure 1. Size bars are 5 µm (mov 4261 KB)
Example of the propagation of both BAX recruitment and SMAC/Diablo release. This cell was nucleofected with mCherry-BAX, SMAC/Diablo-GFP, and Mito-BFP and induced to die using 1 µM STS. BAX recruitment and SMAC release initiated 14.5 hrs post-STS on the left side of the image and propagated across the cellular chain over the course of approximately 15 minutes (mov 3916 KB)
Live-cell imaging of the cell in Figure 3. Size bars are 10 µm (mov 7104 KB)
Live-cell imaging of the cell in Figure 6. Size bars are 5 µm (mov 7708 KB)
Live-cell imaging of the cell in Figure 7. Size bars are 5 µm (mov 12484 KB)
Rights and permissions
About this article
Cite this article
Grosser, J.A., Maes, M.E. & Nickells, R.W. Characteristics of intracellular propagation of mitochondrial BAX recruitment during apoptosis. Apoptosis 26, 132–145 (2021). https://doi.org/10.1007/s10495-020-01654-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-020-01654-w