Abstract
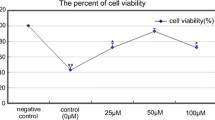
Dihydromyricetin (DMY) is a traditional herbal medicine, with a wide range of biological activities. Extreme hyperthermia (HT) can suppress the immune system; thus, protection of the immune system is beneficial in heat-related diseases, including heatstroke. In our study, we revealed the protective effect of DMY against HT-induced apoptosis and analysed the underlying molecular mechanisms. We incubated human myelomonocytic lymphoma U937 cells at 44 °C for 30 min with or without DMY and followed by further incubation for 6 h at 37 °C. Cell viability was determined by the CCK-8 assay. DMY did not cause any cytotoxic effects in U937 cells even at high doses. HT treatment alone induced significant apoptosis, which was detected by DNA fragmentation and Annexin V/PI double staining. Mitochondrial dysfunction was identified by loss of mitochondrial membrane potential (MMP) during heat stimulation. Apoptotic related proteins were involved, truncated Bid and caspase-3 were upregulated, and Mcl-1 and XIAP were downregulated. We also identified the related signalling pathways, such as the MAPK and PI3K/AKT pathways. However, changes in HT were dramatically reversed when the cells were pretreated with DMY before exposure to HT. Overall, MAPKs and PI3K/AKT signalling, mitochondrial dysfunction, and caspase-mediated pathways were involved in the protective effect of DMY against HT-induced apoptosis in U937 cells, which was totally reversed by DMY pretreatment. These findings indicate a new clinical therapeutic strategy for the protection of immune cells during heatstroke.








Similar content being viewed by others
References
Wang TY, Li Q, Bi KS (2018) Bioactive flavonoids in medicinal plants: structure, activity and biological fate. Asian J Pharm Sci 13:12–23. https://doi.org/10.1016/j.ajps.2017.08.004
Hou X, Zhang J, Ahmad H, Zhang H, Xu Z, Wang T (2014) Evaluation of antioxidant activities of ampelopsin and its protective effect in lipopolysaccharide-induced oxidative stress piglets. PLoS ONE 9:e108314. https://doi.org/10.1371/journal.pone.0108314
Liu TT, Zeng Y, Tang K, Chen X, Zhang W, Xu XL (2017) Dihydromyricetin ameliorates atherosclerosis in LDL receptor deficient mice. Atherosclerosis 262:39–50. https://doi.org/10.1016/j.atherosclerosis.2017.05.003
Ren ZX, Zhao YF, Cao T, Zhen XC (2016) Dihydromyricetin protects neurons in an MPTP-induced model of Parkinson’s disease by suppressing glycogen synthase kinase-3 beta activity. Acta Pharmacol Sin 37:1315–1324. https://doi.org/10.1038/aps.2016.42
Wang J, Yu Z, Yu Q, Kong Q, Zhang N, He L, Yi Y (2014) Study on neuroprotective capacity of key flavonoids of ampelopsis grossedentata. J Food Nutr Res 2:880–889. https://doi.org/10.12691/jfnr-2-12-4
Liu P, Zou D, Chen K, Zhou Q, Gao Y, Huang Y, Zhu J, Zhang Q, Mi M (2016) Dihydromyricetin improves hypobaric hypoxia-induced memory impairment via modulation of SIRT3 signaling. Mol Neurobiol 53:7200–7212. https://doi.org/10.1007/s12035-015-9627-y
Lin X, Lin CH, Zhao T, Zuo D, Ye Z, Liu L, Lin MT (2017) Quercetin protects against heat stroke-induced myocardial injury in male rats: antioxidative and antiinflammatory mechanisms. Chem Biol Interact 265:47–54. https://doi.org/10.1016/j.cbi.2017.01.006
Axelrod YK, Diringer MN (2008) Temperature management in acute neurologic disorders. Neurol Clin 26:585–603. https://doi.org/10.1016/j.ncl.2008.02.005
Fauci A, Braunwald E, Kasper D, Hauser S, Longo D, Jameson J, Loscalzo J (2008) Harrison’s principles of internal medicine, 17th edn. McGraw-Hill Professional, New York, pp 117–121
Tintinalli JE, Kelen GD, Stapczynski JS (2004) Emergency medicine: a comprehensive study guide, 6th edn. McGraw-Hill Professional, New York, p 1187
Brandom BW (2005) The genetics of malignant hyperthermia. Anesthesiol Clin N Am 23:615–619. https://doi.org/10.1016/j.atc.2005.08.006 (viii).
Schneiderbanger D, Johannsen S, Roewer N, Schuster F (2014) Management of malignant hyperthermia: diagnosis and treatment. Ther Clin Risk Manag 10:355–362. https://doi.org/10.2147/TCRM.S47632
Herlich A (2013) Perioperative temperature elevation: not all hyperthermia is malignant hyperthermia. Paediatr Anaesth 23:842–850. https://doi.org/10.1111/pan.12244
Howe AS, Boden BP (2007) Heat-related illness in athletes. Am J Sports Med 35:1384–1395. https://doi.org/10.1177/0363546507305013
Dieing A, Ahlers O, Hildebrandt B, Kerner T, Tamm I, Possinger K, Wust P (2007) The effect of induced hyperthermia on the immune system. Prog Brain Res 162:137–152. https://doi.org/10.1016/S0079-6123(06)62008-6
Sellins KS, Cohen JJ (1987) Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol 139:3199–3206
Hertveldt K, Pphilippe J, Thierens H, Conrnelissen M, Vral A, Ridder L (1997) Flow cytometry as a quantitative and sensitive method to evaluate low dose radiation induced apoptosis in vitro in human peripheral blood lymphocytes. Int J Radiat Biol 71:429–433. https://doi.org/10.1080/095530097144049
Gottlieb RA (2000) Mitochondria: execution central. FEBS Lett 482:6–12. https://doi.org/10.1016/S0014-5793(00)02010-X
Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22:153–183. https://doi.org/10.1210/edrv.22.2.0428
Nicholson KM, Anderson NG (2002) The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal 14:381–395. https://doi.org/10.1016/S0898-6568(01)00271-6
Li H, Li Q, Liu Z, Yang K, Chen Z, Cheng Q, Wu L (2017) The versatile effects of dihydromyricetin in health. Evid Based Complement Altern Med 2017:1053617. https://doi.org/10.1155/2017/1053617
Zhang Q, Liu J, Liu B, Xia J, Chen N, Chen X, Cao Y, Zhang C, Lu C, Li M, Zhu R (2014) Dihydromyricetin promotes hepatocellular carcinoma regression via a p53 activation-dependent mechanism. Sci Rep 4:4628. https://doi.org/10.1038/srep04628
Zhou Y, Shu F, Liang X, Chang H, Shi L, Peng X, Zhu J, Mi M (2014) Ampelopsin induces cell growth inhibition and apoptosis in breast cancer cells through ROS generation and endoplasmic reticulum stress pathway. PLoS ONE 9:e89021. https://doi.org/10.1371/journal.pone.0089021
Han M, Im DS (2008) Effects of mitochondrial inhibitors on cell viability in U937 monocytes under glucose deprivation. Arch Pharm Res 31:749–757. https://doi.org/10.1007/s12272-001-1222-5
Ly JD, Grubb DR, Lawen A (2003) The mitochondrial membrane potential (∆ψm) in apoptosis; an update. Apoptosis 8:115–128. https://doi.org/10.1023/A:1022945107762
Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA (2011) Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques 50:98–115. https://doi.org/10.2144/000113610
Thomas LW, Lam C, Edwards SW (2010) Mcl-1; the molecular regulation of protein function. FEBS Lett 584:2981–2989. https://doi.org/10.1016/j.febslet.2010.05.061
Deveraux QL, Takahashi R, Salvesen GS, Reed JC (1997) X-linked IAP is a direct inhibitor of cell-death proteases. Nature 388:300–304. https://doi.org/10.1038/40901
Zhao QL, Fujiwara Y, Kondo T (2006) Mechanism of cell death induction by nitroxide and hyperthermia. Free Radic Biol Med 40:1131–1143. https://doi.org/10.1016/j.freeradbiomed.2005.10.064
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116:205–219. https://doi.org/10.1016/S0092-8674(04)00046-7
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326–1331. https://doi.org/10.1126/science.270.5240.1326
Datta SR, Brunet A, Greenberg ME (1999) Cellular survival: a play in three Akts. Genes Dev 13:2905–2927. https://doi.org/10.1101/gad.13.22.2905
Wu J, Liu T, Rios Z, Mei Q, Lin X, Cao S (2017) Heat shock proteins and cancer. Trends Pharmacol Sci 38:226–256. https://doi.org/10.1016/j.tips.2016.11.009
Mymrikov EV, Seit-Nebi AS, Gusev NB (2011) Large potentials of small heat shock proteins. Physiol Rev 91:1123–1159. https://doi.org/10.1152/physrev.00023.2010
Landry J, Lambert H, Zhou M, Lavoie JN, Hickey E, Weber LA, Anderson CW (1992) Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem 267:794–803. https://doi.org/10.1074/jbc.M109.082644
Sreekanth GP, Chuncharunee A, Sirimontaporn A, Panaampon J, Noisakran S, Yenchitsomanus PT, Limjindaporn T (2016) SB203580 modulates p38 MAPK signaling and dengue virus-induced liver injury by reducing MAPKAPK2, HSP27, and ATF2 phosphorylation. PLoS ONE 11:e0149486. https://doi.org/10.1371/journal.pone.0149486
Nakashima M, Adachi S, Yasuda I, Yamauchi T, Kawaguchi J, Itani M, Yoshioka T, Matsushima-Nishiwaki R, Hirose Y, Kozawa O, Moriwaki H (2011) Phosphorylation status of heat shock protein 27 plays a key role in gemcitabine-induced apoptosis of pancreatic cancer cells. Cancer Lett 313:218–225. https://doi.org/10.1016/j.canlet.2011.09.008
Matsushima-Nishiwaki R, Takai S, Adachi S, Minamitani C, Yasuda E, Noda T, Kato K, Toyoda H, Kaneoka Y, Yamaguchi A, Kumada T, Kozawa O (2008) Phosphorylated heat shock protein 27 represses growth of hepatocellular carcinoma via inhibition of extracellular signal-regulated kinase. J Biol Chem 283:18852–18860. https://doi.org/10.1074/jbc.M801301200
Yasuda E, Kumada T, Takai S, Ishisaki A, Noda T, Matsushima-Nishiwaki R, Yoshimi N, Kato K, Toyoda H, Kaneoka Y, Yamaguchi A, Kozawa O (2005) Attenuated phosphorylation of heat shock protein 27 correlates with tumor progression in patients with hepatocellular carcinoma. Biochem Biophys Res Commun 337:337–342. https://doi.org/10.1016/j.bbrc.2005.08.273
Acknowledgements
This work was supported by JSPS KAKENHI Grant No. 18K10044.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interests regarding the publication of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feng, QW., Cui, ZG., Jin, YJ. et al. Protective effect of dihydromyricetin on hyperthermia-induced apoptosis in human myelomonocytic lymphoma cells. Apoptosis 24, 290–300 (2019). https://doi.org/10.1007/s10495-019-01518-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-019-01518-y