Abstract
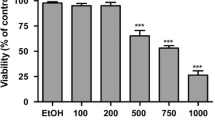
Chemotherapeutic regimens containing camptothecin (CPT), 5-fluorouracil, and oxaliplatin are used to treat advanced colorectal cancer. We previously reported that an indole derivative, 3-(2-bromoethyl)indole (BEI-9), inhibited the proliferation of colon cancer cells and suppressed NF-κB activation. Here, we show that a combination of BEI-9 with either CPT or tumor necrosis factor alpha (TNFα) enhances cell death. Using colorectal cancer cells, we examined the activation of NF-κB by drugs, the potential of BEI-9 for inhibiting drug-induced NF-κB activation, and the enhancement of cell death by combination treatments. Cells were treated with the chemotherapeutic drugs alone or in combination with BEI-9. NF-κB activation, cell cycle profiles, DNA-damage response, markers of cell death signaling and targets of NF-κB were evaluated to determine the effects of single and co-treatments. The combination of BEI-9 with CPT or TNFα inhibited NF-κB activation and reduced the expression of NF-κB-responsive genes, Bcl-xL and COX2. Compared to CPT or BEI-9 alone, sequential treatment of the cells with CPT and BEI-9 significantly enhanced caspase activation and cell death. Co-treatment with TNFα and BEI-9 also caused more cytotoxicity than TNFα or BEI-9 alone. Combined BEI-9 and TNFα enhanced cell death through caspase activation and cleavage of the switch-protein, RIP1 kinase. BEI-9 reduced the expression of COX2 both alone and in combination with CPT or TNF. We postulate that BEI-9 enhances the effects of these drugs on cancer cells by turning off or redirecting NF-κB signaling. Therefore, the combination of BEI-9 with drugs that activate NF-κB needs to be evaluated for clinical applications.





Similar content being viewed by others
References
Deng Y, Cai Y, Lin J, Jiang L, Hu H (2015) Survival of patients with KRAS wild-type metastatic colorectal cancer is identical after sequential treatment with cetuximab and bevacizumab regardless of the sequence—a retrospective single-center study. Gastroenterol Rep 3:339–343
Kirstein MM, Lange A, Prenzler A, Manns MP, Kubicka S, Vogel A (2014) Targeted therapies in metastatic colorectal cancer: a systematic review and assessment of currently available data. Oncologist 19:1156–1168
Samuel T, Fadlalla K, Gales DN, Putcha BD, Manne U (2014) Variable NF-kappaB pathway responses in colon cancer cells treated with chemotherapeutic drugs. BMC Cancer 14:599
Huang TT, Wuerzberger-Davis SM, Seufzer BJ et al (2000) NF-kappaB activation by camptothecin. A linkage between nuclear DNA damage and cytoplasmic signaling events. J Biol Chem 275:9501–9509
McCool KW, Miyamoto S (2012) DNA damage-dependent NF-kappaB activation: NEMO turns nuclear signaling inside out. Immunol Rev 246:311–326
Brach MA, Hass R, Sherman ML, Gunji H, Weichselbaum R, Kufe D (1991) Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. J Clin Invest 88:691–695
Criswell T, Leskov K, Miyamoto S, Luo G, Boothman DA (2003) Transcription factors activated in mammalian cells after clinically relevant doses of ionizing radiation. Oncogene 22:5813–5827
Prasad AV, Mohan N, Chandrasekar B, Meltz ML (1994) Activation of nuclear factor kappa B in human lymphoblastoid cells by low-dose ionizing radiation. Radiat Res 138:367–372
Fadlalla K, Elgendy R, Gilbreath E et al (2015) 3-(2-Bromoethyl)-indole inhibits the growth of cancer cells and NF-kappaB activation. Oncol Rep 34:495–503
Varfolomeev E, Vucic D. (2016) Intracellular regulation of TNF activity in health and disease. Cytokine
Sun J, Han J, Zhu Q, Li Z, Hu J (2012) Camptothecin fails to induce apoptosis in tumor necrosis factor-alpha-treated HaCaT cells. Pharmacology 89:58–63
Wu ZH, Shi Y, Tibbetts RS, Miyamoto S (2006) Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science 311:1141–1146
Grivennikov SI, Kuprash DV, Liu ZG, Nedospasov SA (2006) Intracellular signals and events activated by cytokines of the tumor necrosis factor superfamily: from simple paradigms to complex mechanisms. Int Rev Cytol 252:129–161
Baud V, Karin M (2001) Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 11:372–377
Aggarwal BB (2000) Tumour necrosis factors receptor associated signalling molecules and their role in activation of apoptosis, JNK and NF-kappaB. Ann Rheum Dis 59(Suppl 1):i6-16
Ray Chaudhuri A, Hashimoto Y, Herrador R et al (2012) Topoisomerase I poisoning results in PARP-mediated replication fork reversal. Nat Struct Mol Biol 19:417–423
Dabkeviciene D, Jonusiene V, Zitkute V et al (2015) The role of interleukin-8 (CXCL8) and CXCR2 in acquired chemoresistance of human colorectal carcinoma cells HCT116. Medical Oncol 32:258
Jamieson T, Clarke M, Steele CW et al (2012) Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest 122:3127–3144
Lee YS, Choi I, Ning Y et al (2012) Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer 106:1833–1841
Li L, Xu L, Yan J et al (2015) CXCR2-CXCL1 axis is correlated with neutrophil infiltration and predicts a poor prognosis in hepatocellular carcinoma. J Exp Clin Cancer Res 34:129
Saintigny P, Massarelli E, Lin S et al (2013) CXCR2 expression in tumor cells is a poor prognostic factor and promotes invasion and metastasis in lung adenocarcinoma. Cancer Res 73:571–582
Magnusson C, Vaux DL (1999) Signalling by CD95 and TNF receptors: not only life and death. Immunol Cell Biol 77:41–46
Chu WM (2013) Tumor necrosis factor. Cancer Lett 328:222–225
Lin Y, Devin A, Rodriguez Y, Liu ZG (1999) Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev 13:2514–2526
He L, Kim BY, Kim KA et al (2007) NF-kappaB inhibition enhances caspase-3 degradation of Akt1 and apoptosis in response to camptothecin. Cell Signal 19:1713–1721
Togano T, Sasaki M, Watanabe M et al (2009) Induction of oncogene addiction shift to NF-kappaB by camptothecin in solid tumor cells. Biochem Biophys Res Commun 390:60–64
Capone ML, Tacconelli S, Sciulli MG, Patrignani P (2003) Clinical pharmacology of selective COX-2 inhibitors. Int J Immunopathol Pharmacol 16:49–58
Patrignani P, Patrono C (2015) Cyclooxygenase inhibitors: from pharmacology to clinical read-outs. Biochim Biophys Acta 1851:422–432
Pordanjani SM, Hosseinimehr SJ (2016) The Role of NF-kB Inhibitors in cell response to radiation. Curr Med Chem 23:3951–3963
Pilones KA, Vanpouille-Box C, Demaria S (2015) Combination of radiotherapy and immune checkpoint inhibitors. Semin Radiat Oncol 25:28–33
Esposito A, Criscitiello C, Curigliano G (2015) Immune checkpoint inhibitors with radiotherapy and locoregional treatment: synergism and potential clinical implications. Curr Opin Oncol 27:445–451
Derer A, Frey B, Fietkau R, Gaipl US (2016) Immune-modulating properties of ionizing radiation: rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Cancer Immunol Immunother 65:779–786
Demaria S, Coleman CN, Formenti SC (2016) Radiotherapy: changing the game in immunotherapy. Trends Cancer 2:286–294
Acknowledgements
This study was supported by grants from the National Institutes of Health through National Institute of General Medical Sciences NIGMS grant #SC3109314, National Institute of Minority Health and Health Disparities NIMHD grant #U54MD008149, and RCMI Core Facility Grant #G12MD007585 to Tuskegee University. The reported content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10495_2017_1427_MOESM1_ESM.pptx
Supplementary Fig. 1: A. Photomicrographs of SW620 cells treated with BEI (1, 2, or 5 µM), TNFα, or with BEI plus TNF combinations as shown. Arrows point to cells showing membrane blebbing phenotypes suggestive of apoptosis. B. Phase contrast (upper row) and fluorescent images (lower row) of SW620 cells treated as shown. The spots in the lower row show pseudo-colored images of caspase-positive (FITC-VAD-FMK reactive) cells, which fluoresce green. In this experiment, CPT+BEI treatment was simultaneous, and did not result in enhanced cell death. Supplementary Fig. 2: A. Morphology of LoVo cells treated for 24 hr with vehicle, BEI, TNF, or a combination of the two drugs. B. A caspase 3/7 activation fluorescent assay that shows the caspase-activating effect of the TNFα – BEI combination. C. Morphology of Colo205 cells treated as shown. D. Cell cycle analysis of Colo205 cells treated as in panel C. The bars indicate the percentages of cells at each phase of the cell cycle shown on the X-axis. The percentage of sub-G1 cells increases with BEI and with the combination BEI and TNFα. Supplemental Fig. 3: Annexin -V/PI staining profiles of HCT116 cells treated as shown for 24 hr. The percentages of Annexin V-positive or Annexin -V/PI double-positive cells are increased by combination treatment compared to single agent treatments. (PPTX 18005 KB)
Rights and permissions
About this article
Cite this article
Chowdhury, R., Gales, D., Valenzuela, P. et al. Bromoethylindole (BEI-9) redirects NF-κB signaling induced by camptothecin and TNFα to promote cell death in colon cancer cells. Apoptosis 22, 1553–1563 (2017). https://doi.org/10.1007/s10495-017-1427-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-017-1427-6