Abstract
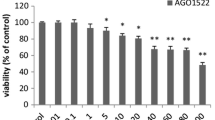
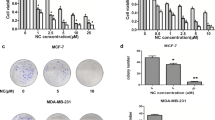
In this study, we aimed to investigate the antitumor effect of LYG-202, a newly synthesized piperazine-substituted derivative of flavonoid on human breast cancer cells and illustrate the potential mechanisms. LYG-202 induced apoptosis in MCF-7, MDA-MB-231 and MDA-MB-435 cells. LYG-202 triggered the activation of mitochondrial apoptotic pathway through multiple steps: increasing Bax/Bcl-2 ratio, decreasing mitochondrial membrane potential (ΔΨ m ), activating caspase-9 and caspase-3, inducing cleavage of poly(ADP-ribose) polymerase, cytochrome c release and apoptosis-inducing factor translocation. Furthermore, LYG-202 inhibited cell cycle progression at the G1/S transition via targeting Cyclin D, CDK4 and p21Waf1/Cip1. Additionally, LYG-202 increased the generation of intracellular ROS. N-Acetyl cysteine, an antioxidant, reversed LYG-202-induced apoptosis suggesting that LYG-202 induces apoptosis by accelerating ROS generation. Further, we found that LYG-202 deactivated the PI3K/Akt pathway, activated Bad phosphorylation, increased Cyclin D and Bcl-xL expression, and inhibited NF-κB nuclear translocation. Activation of PI3K/Akt pathway by IGF-1 attenuated LYG-202-induced apoptosis and cell cycle arrest. Our in vivo study showed that LYG-202 exhibited a potential antitumor effect in nude mice inoculated with MCF-7 tumor through similar mechanisms identified in cultured cells. In summary, our results demonstrated that LYG-202 induced apoptosis and cell cycle arrest via targeting PI3K/Akt pathway, indicating that LYG-202 is a potential anticancer agent for breast cancer.








Similar content being viewed by others
Abbreviations
- ROS:
-
Reactive oxygen species
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DMSO:
-
Dimethylsulfoxide
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PBS:
-
Phosphate buffered saline
- PI:
-
Propidium iodide
- PARP:
-
Poly(ADP-ribose) polymerase
- AIF:
-
Apoptosis-inducing factor
- CDK:
-
Cyclin-dependent kinase
- NAC:
-
N-Acetyl cysteine
- IGF-1:
-
Insulin-like growth factor-1
References
Luo M, Guan JL (2010) Focal adhesion kinase: a prominent determinant in breast cancer initiation, progression and metastasis. Cancer Lett 289:127–139
Lin CC, Kuo CL, Lee MH et al (2011) Wogonin triggers apoptosis in human osteosarcoma U-2 OS cells through the endoplasmic reticulum stress, mitochondrial dysfunction and caspase-3-dependent signaling pathways. Int J Oncol 39:217–224
Crompton M, Virji S, Doyle V, Johnson N, Ward JM (1999) The mitochondrial permeability transition pore. Biochem Soc Symp 66:167–179
Fleury C, Mignotte B, Vayssiere JL (2002) Mitochondrial reactive oxygen species in cell death signaling. Biochimie 84:131–141
Thannickal VJ, Fanburg BL (2000) Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279:L1005–L1028
Cory S, Huang DC, Adams JM (2003) The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22:8590–8607
Johansson M, Persson JL (2008) Cancer therapy: targeting cell cycle regulators. Anti-Cancer Agents Med Chem 8:723–731
Boonstra J, Post JA (2004) Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene 337:1–13
Sheppard K, Kinross KM, Solomon B, Pearson RB, Phillips WA (2012) Targeting PI3 kinase/AKT/mTOR signaling in cancer. Crit Rev Oncol 17:69–95
Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer 2:489–501
Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ (1995) Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell 80:285–291
Datta SR, Dudek H, Tao X et al (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231–241
Datta SR, Katsov A, Hu L et al (2000) 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell 6:41–51
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB (1999) NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401:82–85
Romashkova JA, Makarov SS (1999) NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401:86–90
Karin M (2006) Nuclear factor-kappaB in cancer development and progression. Nature 441:431–436
Karin M, Greten FR (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5:749–759
Yang L, Wang Q, Li D et al (2013) Wogonin enhances antitumor activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo through ROS-mediated downregulation of cFLIPL and IAP proteins. Apoptosis 18:618–626
Lucas CD, Allen KC, Dorward DA et al (2013) Flavones induce neutrophil apoptosis by down-regulation of Mcl-1 via a proteasomal-dependent pathway. FASEB J 27:1084–1094
Zeng S, Liu W, Nie FF et al (2009) LYG-202, a new flavonoid with a piperazine substitution, shows antitumor effects in vivo and in vitro. Biochem Biophys Res Commun 385:551–556
Liu W, Dai Q, Lu N et al (2011) LYG-202 inhibits the proliferation of human colorectal carcinoma HCT-116 cells through induction of G1/S cell cycle arrest and apoptosis via p53 and p21(WAF1/Cip1) expression. Biochem Cell Biol 89:287–298
Tao L, Fu R, Wang X et al (2014) LL-202, a newly synthesized flavonoid, inhibits tumor growth via inducing G(2)/M phase arrest and cell apoptosis in MCF-7 human breast cancer cells in vitro and in vivo. Toxicol Lett 228:1–12
Pan D, Li W, Miao H et al (2014) LW-214, a newly synthesized flavonoid, induces intrinsic apoptosis pathway by down-regulating Trx-1 in MCF-7 human breast cells. Biochem Pharmacol 87:598–610
Qiang L, Yang Y, You QD et al (2008) Inhibition of glioblastoma growth and angiogenesis by gambogic acid: an in vitro and in vivo study. Biochem Pharmacol 75:1083–1092
Chen Y, Lu N, Ling Y et al (2010) LYG-202, a newly synthesized flavonoid, exhibits potent anti-angiogenic activity in vitro and in vivo. J Pharm Sci 112:37–45
Huang C, Cao J, Huang KJ et al (2006) Inhibition of STAT3 activity with AG490 decreases the invasion of human pancreatic cancer cells in vitro. Cancer Sci 97:1417–1423
Clarke R, Dickson RB, Brunner N (1990) The process of malignant progression in human breast cancer. Ann Oncol 1:401–407
Soule HD, McGrath CM (1980) Estrogen responsive proliferation of clonal human breast carcinoma cells in athymic mice. Cancer Lett 10:177–189
Zhang X, Chen J, Graham SH et al (2002) Intranuclear localization of apoptosis-inducing factor (AIF) and large scale DNA fragmentation after traumatic brain injury in rats and in neuronal cultures exposed to peroxynitrite. J Neurochem 82:181–191
Das A, Gopalakrishnan B, Voss OH, Doseff AI, Villamena FA (2012) Inhibition of ROS-induced apoptosis in endothelial cells by nitrone spin traps via induction of phase II enzymes and suppression of mitochondria-dependent pro-apoptotic signaling. Biochem Pharmacol 84:486–497
Qiu P, Guan H, Dong P et al (2011) The p53-, Bax- and p21-dependent inhibition of colon cancer cell growth by 5-hydroxy polymethoxyflavones. Mol Nutr Food Res 55:613–622
She QB, Solit DB, Ye Q, O’Reilly KE, Lobo J, Rosen N (2005) The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell 8:287–297
Millis RM, Alvin ZV, Zhao A, Haddad GE (2012) Effects of IGF-1 on I(K) and I(K1) channels via PI3K/Akt signaling in neonatal cardiac myocytes. Int J Cell Biol 2012:712153
Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N (2001) Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev 15:1406–1418
Yun J, Lv YG, Yao Q, Wang L, Li YP, Yi J (2012) Wortmannin inhibits proliferation and induces apoptosis of MCF-7 breast cancer cells. Eur J Gynaecol Oncol 33:367–369
Chen CY, Hsu YL, Chen YY, Hung JY, Huang MS, Kuo PL (2007) Isokotomolide A, a new butanolide extracted from the leaves of Cinnamomum kotoense, arrests cell cycle progression and induces apoptosis through the induction of p53/p21 and the initiation of mitochondrial system in human non-small cell lung cancer A549 cells. Eur J Pharmacol 574:94–102
Newman DJ, Cragg GM, Snader KM (2003) Natural products as sources of new drugs over the period 1981–2002. J Nat Prod 66:1022–1037
Gabai VL, Meriin AB, Yaglom JA, Volloch VZ, Sherman MY (1998) Role of Hsp70 in regulation of stress-kinase JNK: implications in apoptosis and aging. FEBS Lett 438:1–4
Zamzami N, Marchetti P, Castedo M et al (1995) Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med 182:367–377
Antonsson B, Conti F, Ciavatta A et al (1997) Inhibition of Bax channel-forming activity by Bcl-2. Science 277:370–372
Indran IR, Tufo G, Pervaiz S, Brenner C (2011) Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim Biophys Acta 1807:735–745
Stridh H, Kimland M, Jones DP, Orrenius S, Hampton MB (1998) Cytochrome c release and caspase activation in hydrogen peroxide- and tributyltin-induced apoptosis. FEBS Lett 429:351–355
Yang L, Zhang HW, Hu R et al (2009) Wogonin induces G1 phase arrest through inhibiting Cdk4 and cyclin D1 concomitant with an elevation in p21Cip1 in human cervical carcinoma HeLa cells. Biochem Cell Biol 87:933–942
Gupta S (2001) Molecular steps of death receptor and mitochondrial pathways of apoptosis. Life Sci 69:2957–2964
Malumbres M (2005) Revisiting the “Cdk-centric” view of the mammalian cell cycle. Cell Cycle 4:206–210
Liu DX, Greene LA (2001) Neuronal apoptosis at the G1/S cell cycle checkpoint. Cell Tissue Res 305:217–228
Yu SM, Kim SJ (2014) Withaferin A-caused production of intracellular reactive oxygen species modulates apoptosis via PI3K/Akt and JNKinase in rabbit articular chondrocytes. J Korean Med Sci 29:1042–1053
Kaufmann M, Jonat W, Hilfrich J et al (2007) Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: the ARNO 95 Study. J Clin Oncol 25:2664–2670
Staal SP (1987) Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA 84:5034–5037
Zhou BP, Hung MC (2002) Novel targets of Akt, p21(Cipl/WAF1), and MDM2. Semin Oncol 29:62–70
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (No. JKY2011051), the National Natural Science Foundation of China (No. 21072232), the Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (No. JKGZ201101), the National Science & Technology Major Project (No. 2012ZX09304-001) and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT-IRT1193).
Conflict of interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yue Zhao and Xiaoping Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhao, Y., Wang, X., Sun, Y. et al. LYG-202 exerts antitumor effect on PI3K/Akt signaling pathway in human breast cancer cells. Apoptosis 20, 1253–1269 (2015). https://doi.org/10.1007/s10495-015-1145-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-015-1145-x