Abstract
This study explores sustainable agricultural practices by examining the role of organic materials in enhancing native predatory mites for controlling spider mites in apple orchards. Developing techniques to conserve indigenous natural enemies is vital for sustainable agricultural production. Phytoseiid mites can control spider mites, which are among the most significant pests in apple production. To conserve phytoseiid mite populations, it is important to identify alternative prey and to determine their role in phytoseiid mite proliferation. We demonstrated that the concurrent use of specific organic fertilizers and coconut husks can increase prey Tyrophagus mites, thereby enhancing phytoseiid mite density. Our research was conducted using sticky traps at the Miyagi Prefectural Agriculture and Horticulture Research Center in Japan. The occurrence of Tyrophagus mites was significantly correlated with the occurrence of phytoseiid mites in 2 years. In laboratory experiments, the use of organic fertilizers increased the density of Tyrophagus mites by 83 × within 4 weeks. Several species of phytoseiid mites were able to lay between 0.25 and 1.03 eggs per day per female by preying on Tyrophagus larvae. A 2-year field survey revealed that the use of organic fertilizers more than doubled the density of phytoseiid mites on apple leaves, likely through promoting Tyrophagus mite proliferation on the ground. These results highlight the potential of organic fertilizers not only to enhance soil nutrients, but also to boost phytoseiid mite populations, thereby contributing to more sustainable apple production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Within the framework of integrated pest management (IPM), chemical pest control is a key component. In recent years, the intensive and frequent use of pesticides has led many pests to develop resistance to chemical agents (Sonoda et al. 2012; van Leeuwen et al. 2015). Biological control is important for effective control of such pests, and indigenous natural enemies are important for long-term control of pest densities on host plants (Li et al. 2021). Therefore, the development of techniques to conserve and enhance indigenous natural enemies is important to efficient IPM.
Among agricultural pests with resistance to pesticides, spider mites of the genus Tetranychus (Acari: Tetranychidae) are among the most economically damaging worldwide (Sun et al. 2012; Tehri 2014). Tetranychus mites’ infestation of apple leaves causes economic losses in apple production due to reduced fruit quality, poor growth of trees, and reduced yields (El Taj et al. 2011; Jakubowska et al. 2022; Warabieda 2015). Phytoseiidae mites have proven effective at controlling spider mites in many fruit tree-growing regions in Japan (Funayama et al. 2015; Funayama 2016; Funayama and Komatsu 2020; Ishii et al. 2018; Katayama et al. 2006; Kishimoto 2002; Wari et al. 2014).
To enhance the densities of phytoseiid mite species, it is important to identify alternative prey and to determine the rate of phytoseiid mite proliferation. Owing to the presence of arthropods in open fields, including mycophagous, humicivorous, and predatory arthropods (Yahya et al. 2020), phytoseiid mites might maintain population densities partly through predation on the ground, where alternative prey might include mites of the suborder Astigmata. Some astigmatid mites can serve as alternative prey in the rearing of some phytoseiid mite species (Massaro et al. 2016; Vangansbeke et al. 2014; Pirayeshfar et al. 2020). Although some astigmatid mites, especially of the genus Tyrophagus, are known to be pests of leafy greens (Cilbircioğlu and Çobanoğlu 2019; Kasuga and Amano 2006; Kirişik et al. 2018), their adverse effects on fruit tree crops, including apples, have rarely been documented (JSAEZ 2006). Because Tyrophagus mites are not considered pests in apple cultivation and have no known beneficial function, ecological knowledge about them in apple orchards is scarce. However, because they are ubiquitous and are often found in soil, litter, and bark (Swift and Goff 2001), they might serve as an important food source for phytoseiid mites in apple orchards. If we can encourage Tyrophagus mites in the field as an alternative food source, it may be possible to increase the density of some species of phytoseiid mites.
Tyrophagus mites are fungivores and humicivores. Some organic fertilizers are made from protein- and lipid-rich ingredients such as feather meal and fish meal, which can be a food resource for Tyrophagus mites (Erban et al. 2015; Masuda 2010), making organic fertilizers a potential promoter of multiplication of these mites in the field. In addition, coconut husks provide a habitat for some fungivores mites (Shen et al. 2021, 2023).
Here, we conducted a 2-year survey using sticky traps placed on apple tree trunks to search for potential prey species, including Tyrophagus mites. We assessed the effectiveness of organic materials (organic fertilizer + coconut husks, hereafter OFCH) on the proliferation of Tyrophagus mites and the influence of these mites as a food source on the reproduction of various species of phytoseiid mites in controlled laboratory settings. Finally, we assessed the effect of OFCH on the density of phytoseiid mites in an apple orchard. We discuss the use of OFCH to conserve indigenous natural enemies for sustainable IPM in apple cultivation.
Materials and methods
Mite population survey using sticky traps
We surveyed mite populations in an apple orchard (~ 23,000 m2) at the Miyagi Prefectural Agriculture and Horticulture Research Center (MPAHRC; Natori, Miyagi, Japan; 38°10′N, 140°51′E) from 15 May to 23 October 2020 and from 16 May to 15 October 2022. Apple trees (Malus × domestica ‘Fuji’ grafted onto M. prunifolia var. ringo) planted in groups of 5 spaced 0.6 m apart were managed by joint cultivation (Shibata and Kawashima 2005) with the main trunks grafted at a height of about 2.2 m above ground level (Fig. S1). The trees were at least 5 years old in 2020 and were pruned to a height of about 3 m. The weather course during the survey period is shown in Fig. S2, and the chemical application history is shown in Table S1. Weeds were controlled with a hand-pushed mower, with the ground cover managed at 10–15 cm height.
To investigate the abundance of mites migrating from the ground to the trees, we conducted a periodic survey using sticky traps attached to the trunks (Fig. S3a). The traps consisted of rubber sponge mats, transparent sticky sheets, and binding bands (Fig. S3b). Although water often accumulated in the upper part of the sponge following rainfall and chemical application, it did not flow into the lower part of the trap through the gaps in the sponge, so there was little to no gap between the trunk and trap. Traps were replaced every 1 to 6 weeks, with a new trap immediately replacing the old one. Traps were brought back to the laboratory in cool bags at 5 °C and stored at − 30 °C until the survey. We counted astigmatid, tetranychid, and phytoseiid mites under a microscope. Some astigmatid mites were removed from the traps with hexane and stored at − 30 °C in 99.5% ethanol, and 82 specimens were identified to genus on the basis of morphological characteristics according to Okabe (2006) under a digital microscope (VHX-7000, Keyence, Tokyo, Japan) with × 500 magnification using a ZS200 objective lens under coaxial lighting.
All statistical analyses were performed in R software v. 4.3.0 (R Development Core Team, 2023). Correlations among mite groups were examined with a generalized linear mixed model (GLMM) in the lme4 package (Bates et al. 2011). The results of the surveys showed two peaks in the occurrence of phytoseiid mites in both years: peak 1, 29 May to 7 August 2020 and 30 May to 21 July 2022; peak 2, 21 August to 9 October 2020 and 3 August to 15 October 2022. We constructed models to analyze the factors that influenced mite abundance during each peak, with the number of phytoseiid mites trapped as the dependent variable with a negative binomial distribution, the numbers of astigmatid and tetranychid mites captured in the same trap as explanatory variables, and tree ID and survey date as random effects. Likelihood ratio tests were conducted using the Anova function in the car package to analyze the effects of the explanatory variables on the dependent variable (Fox et al. 2012).
Dietary suitability of organic materials for the increase of Tyrophagus putrescentiae
We used a strain of T. putrescentiae isolated from a Neoseiulus cucumeris product (AgriSect Co., Ltd., Ibaraki, Japan) and maintained on dry yeast (Nisshin Flour Milling Werna Co., Ltd., Tokyo, Japan). Soil collected from a vegetable field at the MPAHRC was sieved through a 2-mm mesh. We used a commercial granular organic fertilizer containing feather meal, fish meal, and palm ash (Agret 666, Asahi Industries Co., Ltd., Tokyo, Japan), which was ground in a mixer and sieved through a 2-mm mesh, and coconut husks (Bellabon, Fujick Co., Ltd, Tokyo, Japan) cut into 1-cm3 pieces. The soil, organic fertilizer, and husks were stored at 5 °C for at least 3 months to remove soil animals, according to Shen et al. (2021). Each trial had two treatments: 2.0 g of soil only (control) and 2.0 g of soil + 0.4 g of organic fertilizer + 1 cm3 of coconut husk (OFCH treatment). The samples were placed in 5-mL glass bottles (2 cm diameter, 4.5 cm high) with a 20-µm mesh on the top, and 10 adult female T. putrescentiae were added in 9 or 10 replicates. The mites were incubated at 25 °C, 16L/8D, ~ 100% relative humidity. After 4 weeks, the total number of mites of all stages was counted under a microscope. To measure the physicochemical properties of soil, organic fertilizer, and coconut husk, each mixer-ground sample passed through a 2-mm mesh was obtained. To measure pH, 20 g of soil, 20 g of organic fertilizer, and 1 g of husk were individually added to 50 ml of distilled water, permeated and extracted for 1 h, and then measured with a pH meter (HM-25R) without filtration. For EC measurements, 10 g of soil, 10 g of organic fertilizer, and 1 g of husk were used in the same manner as for pH measurements with an EC meter (RS-232C, both from DKK-TOA Co., Ltd., Tokyo, Japan). Ion concentrations were measured by ion chromatography analysis (Fritz 1987). Samples equivalent to 10 g of dry weight for soil and organic fertilizer and 1 g of dry weight for husk were permeated with 100 ml of distilled water for 1 h, filtered, and subjected to ion chromatography analysis to determine each ion consentration.The gas phase of each material was measured with a Digital Actual Volumenometer (DIK-1150, Daiki Rika Kogyo Co., Ltd., Saitama, Japan) by subjecting 100 ml of the air-dried samples.
A generalized linear model (GLM) was developed to determine the suitability of OFCH for the proliferation of T. putrescentiae. The dependent variable was the final total number of all stages with a Poisson distribution, and the explanatory variable was the presence/absence of OFCH. Likelihood ratio tests were conducted using the Anova function of the car package.
Fecundity of various species of phytoseiid mites fed on T. putrescentiae
We assessed the suitability of Tyrophagus mites as a food source on the reproduction of five species of phytoseiid mites naturally occurring in Japanese fruit orchards: Amblyseius eharai, Amblyseius tsugawai, Euseius sojaensis, Neoseiulus californicus, and Typhlodromus vulgaris (Table 1). Deutonymphs of female mites from stock cultures of each species were transferred singly into a Munger cell (Ehara and Gotoh 2009; with modification Kishimoto et al. 2014), and two adult males and sufficient tea pollen were added. The cells were placed in a plastic container and incubated at 25 °C, 16L:8D, 70%–90% relative humidity. After molting, the adult female was transferred into a new Munger cell to which 100 larvae of T. putrescentiae were added as a food source. Predation on T. putrescentiae and number of eggs laid were counted every 24 h for the next 7 days. After each count, the adult female was transferred on a paintbrush into another Munger cell containing 100 larvae of T. putrescentiae. The stock culture of T. putrescentiae was maintained on dry yeast.
The Steel–Dwass test (Aoki 2015) was used to compare the number of total laid eggs or consumed T. putrescentiae larvae among phytoseiid mite species.
Effects of organic material treatments on the density of phytoseiid mite population
We conducted a survey in an apple orchard at MPAHRC (Fig. S4). The trees (‘Fuji’ grafted on M. prunifolia var. ringo) were planted at a spacing of 2 m × 2 m and were at least 3 years old in 2022. They were pruned to a height of ~ 2.5 m. This experiment had two treatments: without fertilizer application (control) and with 2.5 kg/m2 of the granular organic fertilizer + 5.0 L/m2 of coconut husk (OFCH) applied on the ground surface on 7 April 2022 and on 17 March 2023. The materials used for OFCH were those described above. In both treatments, groundcover was managed at 10–15 cm height with a hand-pushed mower. On each tree, mite densities on 30 (in 2022) or 50 (in 2023) randomly selected leaves were surveyed from 30 May to 29 August 2022 and from 12 May to 29 August 2023, at 1–5 week intervals. The chemical application history is shown in Table S2.
A GLMM was constructed to analyze the effect of OFCH treatments on the density of phytoseiid and tetranychid mites, with a negative binomial distribution for abundance, using ± OFCH as an explanatory variable, and survey year, survey date, survey plot, and tree ID as random effects. Likelihood ratio tests were conducted using the Anova function in the car package to analyze the effects of each explanatory variable.
Results
OFCH treatment increased the density of phytoseiid mites on apple leaves (Fig. 1a, b; χ2 = 8.52, df = 1, P = 0.004). Tetranychid mites were mainly Tetranychus kanzawai and were observed at low densities throughout the study period, with no differences in density between treatments (Fig. 1c, d; χ2 = 0.36, df = 1, P = 0.550). Astigmatid mites were never observed on apple leaves.
OFCH proved to be suitable food to allow T. putrescentiae to proliferate in the laboratory (χ2 = 10,994, df = 1, P < 0.001), with a density increase of 82.69 × ± 9.27 (mean ± SE) after 4 weeks’ incubation (Fig. 2). The organic fertilizers were rich in PO43− (3.12 g/kg dry sample) and K+ (38.57 g/kg dry sample), while the soil was richest in NO3− (2.03 g/kg dry sample; Table S3). Coconut husk had the highest gas phase ratio (95.92%).
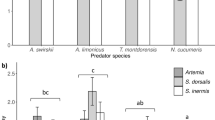
The larvae of T. putrescentiae were suitable prey for egg laying by A. eharai (1.03 ± 0.41 eggs/day), T. vulgaris (0.82 ± 0.15 eggs/day), and A. tsugawai (0.25 ± 0.19 eggs/day), but N. californicus and E. sojaensis laid no eggs (P < 0.05, Steel–Dwass test, Fig. 3a). Although all species were observed to prey on T. putrescentiae, predation was greater by the species that laid eggs (A. eharai, 80.54 ± 6.00 larvae/day; A. tsugawai, 56.29 ± 14.69 larvae/day; T. vulgaris, 49.45 ± 5.30 larvae/day; > > N. californicus, 12.66 ± 3.39 larvae/day; E. sojaensis, 7.56 ± 3.00 larvae/day; P < 0.05, Steel–Dwass test; Fig. 3b). All individuals of E. sojaensis died on average 2.17 ± 0.31 days after the start of the experiment.
Comparison of the suitability of Tyrophagus putrescentiae larvae for the reproduction of five species of phytoseiid mites: a mean egg numbers oviposited by five phytoseiid species and b mean numbers of T. putrescentiae larvae consumed by these species. Different letters denote significant difference (Steel–Dwass test, P < 0.05). Error bars represent standard error of means
Numbers of phytoseiid mites were significantly correlated with numbers of astigmatid mites in the first peak in each sticky trap survey, but not with tetranychid mites (Fig. 4; astigmatids, χ2 = 16.51, df = 1, P < 0.001; tetranychids, χ2 = 0.09, df = 1, P = 0.761). Conversely, they were significantly correlated with numbers of tetranychid mites in the second peak in each survey, but not with astigmatid mites (Fig. 4; tetranychids, χ2 = 16.51, df = 1, P < 0.001; astigmatids, χ2 = 0.09, df = 1, P = 0.761). Most astigmatid mites were in the genus Tyrophagus (Table 2). All tetranychid mites captured were T. kanzawai.
Discussion
The results suggest that Tyrophagus mites can function as prey of phytoseiid mites in apple orchards at times, and that artificial proliferation of Tyrophagus mites by OFCH application may increase the density of some phytoseiid mite species in apple production orchards.
Both field and laboratory experiments confirmed that Tyrophagus mites could act as a food source for naturally occurring phytoseiid mites. Numbers of Tyrophagus mites peaked from June to July, possibly because of rainfall during this period, which moistened the ground surface, providing suitable conditions for the growth of these mycophagous mites. The fecundity experiments in Munger cells imply that some generalists increased by feeding on the larvae of Tyrophagus mites, and their abundance peaked. Although the fecundity of A. eharai, A. tsugawai, and T. vulgaris was lower when fed on T. putrescentiae larvae than on tea pollen (Kishimoto et al. 2014), Tyrophagus mites could be an important food resource in some seasons, considering the significant correlation between Tyrophagus and phytoseiid mite abundances. The fecundity experiments also showed that some phytoseiid mite species could not reproduce by feeding on Tyrophagus mites, and we assume that their density was maintained on other food sources such as pollen. Nevertheless, some predation was observed, and N. californicus continued to survive (over 7 days), so T. putrescentiae larvae may be an emergency food source when food resources are scarce. Previous studies have reported that A. swirskii and N. barkeri can proliferate by predating on T. putrescentiae (Pirayeshfar et al. 2020; Zou et al. 2016). Besides the species examined in this study, other naturally occurring species could be enhanced by Tyrophagus mites. Since many other species of phytoseiid mites occur in apple orchards (e.g. Toyoshima et al. 2011), further studies are needed on species that are expected to increase with the density of Tyrophagus mites.
In the laboratory and field experiments, OFCH led to a proliferation of Tyrophagus mites and, possibly through it, increased the density of phytoseiid mites in the trees. OFCH used in this study were confirmed to be a suitable source for Tyrophagus mites, as reported by Coleman et al. (2017), who noted an increase in astigmatid mite populations in organic materials such as compost. The organic fertilizer used in this study contained 6% total N (Shen et al. 2021), but had lower concentration of N-containing ions than soil, suggesting that N was present mainly as organic matter. We infer that this organic matter is decomposed by soil microorganisms such as filamentous fungi, on which T. putrescentiae feed. The much larger empty gas spaces in the coconut husk than of the organic fertilizers and soil may provide an important ecological function for T. putrescentiae, which needs a safe refuge in the soil to avoid predation. From the manifestation of these synergetic effect in the field, we infer that the OFCH increased the occurrence of phytoseiid mites on apple leaves. Previous studies reported that organic materials increased the density of predatory mites, including phytoseiids, and decreased that of tetranychids on plants (Esteca et al. 2018, 2020). Also, soil-dwelling predatory mites have also been reported to prey on Tyrophagus mites (Saito and Takaku 2012, 2013). Therefore, it is possible that various generalist predatory mite species could proliferate by eating mycophagous or humicivorous mites, including Tyrophagus mites. Since tetranychid mites migrate from the ground cover to the canopy at certain times, it may be possible to control their densities by enhancing the predatory mites on the ground surface.
Among astigmatid mites, several species of Tyrophagus are agricultural and hygiene pests (Kasuga and Amano 2006; Malik et al. 2018), but these have rarely been found to cause damage in most fruit tree species and have not been recorded as common fruit pests (JSAEZ 2006). They are known to be allergens, but there have been no reports of allergies caused by them, at least in orchards. From our results, although the abundance of Tyrophagus mites peaked in early summer (June–July), the risk of their contamination of apple fruits during the late fall (November–December) harvest is presumed to be low because their survival rates are significantly reduced under hot and dry conditions (Hadi et al. 2020) of mid-summer (July–August). Thus, adverse effects on harvested fruits due to the proliferation of Tyrophagus mites in apple orchards is unlikely. However, if nearby fields are planted with crops attacked by the mites, strict attention should be paid to the risk of their movement into such fields.
We verified the effectiveness of OFCH in maintaining the predatory mite population over multiple years. However, the generality of this effect should be tested in more orchards. Future research should compare several organic materials suitable for Tyrophagus mite proliferation, also considering the fertilization effect. This would aid in determining the optimal rate of substitution of chemical fertilizers to preserve phytoseiid mite populations. Moreover, if the enhancement of phytoseiid mites can lead to a decreased need for acaricide applications, it will simultaneously lower the consumption of both chemical fertilizers and acaricides. This approach could become a significant technology for establishing an efficient, cost-effective, sustainable IPM system.
Data availability
The datasets generated during and/or analysed during this study are available from the corresponding author on reasonable request.
References
Aoki S (2015) Multiple comparisons by Steel-Dwass method. http://aoki2.si.gunma-u.ac.jp/R/src/Steel-Dwass.R. Accessed 9 April 2024
Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Scheipl F, Grothendieck G (2011) Package ‘lme4.’ Linear Mixed-Effects Models Using S4 Classes. R Package Version, 1
Cilbircioğlu C, Çobanoğlu S (2019) Phytophagous mite (Acari) species on garlic (Allium sativum L.) cultivation areas and storages of Kastamonu, Turkey. Persian J Acarol 8:211–224. https://doi.org/10.22073/pja.v8i3.46349
Coleman DC, Callaham MA, Crossley DA Jr (2017) Fundamentals of soil ecology. Georgia, Athens
Ehara S, Gotoh T (2009) Colored guide to the plant mites of Japan. Taito, Tokyo
El Taj HF, Mahmood S, Kabir MA, Hossain MA (2011) Influence of apple cultivars on the development and fecundity of the two-spotted spider mite, Tetranychus urticae (Koch). Int J Stress Manag 2:403–408
Erban T, Rybanska D, Hubert J (2015) Population growth of the generalist mite Tyrophagus putrescentiae (Acari: Acaridida) following adaptation to high-or low-fat and high-or low-protein diets and the effect of dietary switch. Environ Entomol 44:1599–1604. https://doi.org/10.1093/ee/nvv129
Esteca FDCN, Rodrigues LR, de Morães GJ, Júnior ID, Klingen I (2018) Mulching with coffee husk and pulp in strawberry affects edaphic predatory mite and spider mite densities. Exp Appl Acarol 76:161–183. https://doi.org/10.1007/s10493-018-0309-0
Esteca FDCN, Trandem N, Klingen I, Santos JC, Júnior ID, de Morães GJ (2020) Cereal straw mulching in strawberry—a facilitator of plant visits by edaphic predatory mites at night? Diversity 12:242. https://doi.org/10.3390/books978-3-0365-1853-4
Fox J, Weisberg S, Adler D, Bates D, Baud-Bovy G, Ellison S, Monette G (2012) Package ‘car.’ R Foundation for Statistical Computing, Vienna, p 16
Fritz JS (1987) Ion chromatography. Anal Chem 59:335–344. https://doi.org/10.1021/ac00131a002
Funayama K (2016) Influence of mowing on dynamics of native phytoseiid mites and Tetranychus urticae in apple orchards in northern Japan. Exp Appl Acarol 70:57–67. https://doi.org/10.1007/s10493-016-0064-z
Funayama K, Komatsu M (2020) Absence of mowing prevents resurgence of Tetranychus urticae and Panonychus ulmi (Acari: Tetranychidae) after broad-spectrum insecticide use in apple orchards. Appl Entomol Zool 55:379–384. https://doi.org/10.1007/s13355-020-00693-8
Funayama K, Komatsu M, Sonoda S, Takahashi I, Hara K (2015) Management of apple orchards to conserve generalist phytoseiid mites suppresses two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Exp Appl Acarol 65:43–54. https://doi.org/10.1007/s10493-014-9850-7
Hadi UK, Soviana S, Qamariah, N (2020) Diversity, distribution, and abundance of house dust mites on settlement region in Bogor. Atlantis Press. https://www.atlantis-press.com/article/125940413.pdf. Accessed 21 March 2024
Ishii H, Mikawa Y, Murase Y, Sonoda S, Hinomoto N, Kishimoto H, Toyoshima S, Toyama M (2018) Species composition and arthropod pest feeding of phytoseiid mites in a Japanese pear greenhouse. Appl Entomol Zool 53:463–474. https://doi.org/10.1007/s13355-018-0575-7
Jakubowska M, Dobosz R, Zawada D, Kowalska J (2022) A review of crop protection methods against the twospotted spider mite—Tetranychus urticae Koch (Acari: Tetranychidae)—with special reference to alternative methods. Agriculture 12:898. https://doi.org/10.3390/agriculture12070898
JSAEZ (Japanese Society of Applied Entomology and Zoology) (2006) Major Insect and Other Pests of Economic Plants in Japan, Revised edition. Tokyo, Japan
Kasuga S, Amano Y (2006) Infestation of Tyrophagus similis Volgin (Acari: Acaridae) on spinach during the seed germination period. J Acarol Soc Jpn 15:69–73. https://doi.org/10.2300/acari.15.69
Katayama H, Masui S, Tsuchiya M, Tatara A, Doi M, Kaneko S, Saito T (2006) Density suppression of the citrus red mite Panonychus citri (Acari: Tetranychidae) due to the occurrence of Neoseiulus californicus (McGregor) (Acari: Phytoseiidae) on Satsuma mandarin. Appl Entomol Zool 41:679–684. https://doi.org/10.1303/aez.2006.679
Kirişik M, Topuz E, Çobanoğlu S (2018) Tyrophagus neiswanderi (Acari: Acaridae) as a pest of greenhouse spinach in Antalya, Turkey. J Agric Sci 24:517–522. https://doi.org/10.15832/ankutbd.349154
Kishimoto H (2002) Species composition and seasonal occurrence of spider mites (Acari: Tetranychidae) and their predators in Japanese pear orchards with different agrochemical spraying programs. Appl Entomol Zool 37:603–615. https://doi.org/10.1303/aez.2002.603
Kishimoto H, Ohira Y, Adachi I (2014) Effect of different plant pollens on the development and oviposition of seven native phytoseiid species (Acari: Phytoseiidae) in Japan. Appl Entomol Zool 49:19–25. https://doi.org/10.1007/s13355-013-0218-y
Li YY, Liu MX, Yuan JG, Okonkwo TT, Chen HQ, Liu H (2021) Evaluation of a philic egg-consumption predatory thrips Scolothrips takahashii for control of the citrus red mite Panonychus citri. Crop Prot 140:105421. https://doi.org/10.1016/j.cropro.2020.105421
Malik A, Gulati R, Duhan K, Poonia A (2018) Tyrophagus putrescentiae (Schrank) (Acari: Acaridae) as a pest of grains: a review. J Entomol Zool Stud 6:e2550
Massaro M, Martin JPI, de Morães GJ (2016) Factitious food for mass production of predaceous phytoseiid mites (Acari: Phytoseiidae) commonly found in Brazil. Exp Appl Acarol 70:411–420. https://doi.org/10.1007/s10493-016-0087-5
Masuda T (2010) Some fertilizers of animal matter as food for Tyrophagus similis Volgin. Ann Rept Plant Prot North Japan 61:189–191. https://doi.org/10.11455/kitanihon.2010.61_189
Okabe K (2007) Identification of Astigmatid mites (II). Plant Prot 61:64–77
Pirayeshfar F, Safavi SA, Sarraf Moayeri HR, Messelink GJ (2020) The potential of highly nutritious frozen stages of Tyrophagus putrescentiae as a supplemental food source for the predatory mite Amblyseius swirskii. Biocontrol Sci Technol 30:403–417. https://doi.org/10.1080/09583157.2020.1722798
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/. Accessed 4 April 2024
Saito M, Takaku G (2012) Predation of Tyrophagus similis Volgin (Acari: Acaridae) by female Hypoaspis (Euandrolaelaps) yamauchii Ishikawa (Acari: Laelapidae) at constant temperatures. J Acarol Soc Jpn 21:15–20. https://doi.org/10.2300/acari.21.15
Saito M, Takaku G (2013) Predation of Tyrophagus similis Volgin (Acari: Acaridae) by indigenous predatory mites (Acari: Gamasina) found in spinach fields. J Acarol Soc Jpn 22:37–43. https://doi.org/10.2300/acari.22.37
Shen H, Shiratori Y, Ohta S, Masuda Y, Isobe K, Senoo K (2021) Mitigating N2O emissions from agricultural soils with fungivorous mites. ISME J 15:2427–2439. https://doi.org/10.1038/s41396-021-00948-4
Shen H, Nagamine T, Shiratori Y, Senoo K (2023) Enhanced mite grazing leads to pattern shifts in soil N2O emissions after organic fertilizer application. Soil Biol Biochem 181:109027. https://doi.org/10.1016/j.soilbio.2023.109027
Shibata K, Kawashima K (2005) Method for culturing tree with grafted tree body. https://patents.google.com/patent/JP2005304495A/en. Accessed 24 April 2024
Sonoda S, Kohara Y, Siqingerile TS, Kishimoto H, Hinomoto N (2012) Phytoseiid mite species composition in Japanese peach orchards estimated using quantitative sequencing. Exp Appl Acarol 56:9–22. https://doi.org/10.1007/s10493-011-9485-x
Sun JT, Lian C, Navajas M, Hong XY (2012) Microsatellites reveal a strong subdivision of genetic structure in Chinese populations of the mite Tetranychus urticae Koch (Acari: Tetranychidae). BMC Genet 13:1–13
Swift SF, Goff ML (2001) Mite (Acari) Communities Associated with’Ohi’a, Metrosideros polymorpha (Myrtaceae), at Hono O Na Pali and Kui’a Natural Area Reserves on Kaua’i Island, Hawaiian Islands. Pac Sci 55:23–40
Tehri K (2014) A review on reproductive strategies in two spotted spider mite, Tetranychus urticae Koch 1836 (Acari: Tetranychidae). J Entomol Zool Stud 2:35–39
Toyoshima S, Yaginuma K, Ihara F, Arai T, Takanashi M (2011) The succession of phytophagous and phytoseiid species in a newly planted apple orchard without insecticide application. J Acarol Soc Jpn 20:77–86. https://doi.org/10.2300/acari.20.77
Van Leeuwen T, Tirry L, Yamamoto A, Nauen R, Dermauw W (2015) The economic importance of acaricides in the control of phytophagous mites and an update on recent acaricide mode of action research. Pestic Biochem Physiol 121:12–21. https://doi.org/10.1016/j.pestbp.2014.12.009
Vangansbeke D, Nguyen DT, Audenaert J, Verhoeven R, Gobin B, Tirry L, De Clercq P (2014) Performance of the predatory mite Amblydromalus limonicus on factitious foods. Biocontrol 59:67–77. https://doi.org/10.1007/s10526-013-9548-5
Warabieda W (2015) Effect of two-spotted spider mite population (Tetranychus urticae Koch) on growth parameters and yield of the summer apple cv. Katja. Hort Sci 2015:167–175. https://doi.org/10.17221/259/2014-HORTSCI
Wari D, Yamashita J, Kataoka Y, Kohara Y, Hinomoto N, Kishimoto H, Toyoshima S, Sonoda S (2014) Population survey of phytoseiid mites and spider mites on peach leaves and wild plants in Japanese peach orchard. Exp Appl Acarol 63:313–332. https://doi.org/10.1007/s10493-014-9788-9
Yahya M, Afzal M, Majeed MZ, Sarwar I, Shehzad K, Luqman M, Sher MS (2020) Differential impact of land-use, season and soil characteristics on the abundance of edaphic springtails (Insecta: Collembola) and mites (Arachnida: Acari). Pak J Zool 52:1483. https://doi.org/10.17582/journal.pjz/20190817120809
Zou Z, Min Q, Xiao S, Xin T, Xia B (2016) Effect of photoperiod on development and demographic parameters of Neoseiulus barkeri (Acari: Phytoseiidae) fed on Tyrophagus putrescentiae (Acari: Acaridae). Exp Appl Acarol 70:45–56. https://doi.org/10.1007/s10493-016-0065-y
Acknowledgements
We thank Y. Suyama, Y. Fukasawa, D. Takahashi, and other members of the Laboratory of Forest Ecology, Tohoku University, for helpful discussions and technical advice. We are also grateful to M. Hori and S. Ando for advice on revisions to the fine details and S. Yano for advice on behavioral characteristics of spider mites. We thank Mr. Abe and Ms. Takahashi for measuring the physicochemical properties of materials used in this study.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Materials were prepared by YK, HK, RS, and MT. Field data were collected by YK, TO, and TS. Laboratory data were collected by YK and RS. Data were analysed by YK. The first draft of the manuscript was written by YK, and all authors commented on it. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical approval
No approval of research ethics committees was required to accomplish the goals of this study, because experimental work was conducted with an unregulated invertebrate species.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Komagata, Y., Oe, T., Sekine, T. et al. Phytoseiid mites benefited from organic fertilization by increasing the population of Tyrophagus mites in apple orchards. Exp Appl Acarol (2024). https://doi.org/10.1007/s10493-024-00948-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10493-024-00948-x